Abstract
Background
Vancomycin is often required to treat serious infections in children, including methicillin resistant Staphylococcus aureus (MRSA) infections. The pharmacodynamic index that best predicts efficacy with vancomycin use for MRSA infection in adults is the 24 hour area under the curve over the minimum inhibitory concentration (AUC/MIC). While multiple pediatric population pharmacokinetic (PK) vancomycin models have been published, few use Bayesian optimization. The current standard of care remains measuring vancomycin serum trough concentrations as a surrogate marker of AUC.
Materials and Methods
A prospective validation of a pediatric population PK model of vancomycin was performed. Hospitalized children < 18 years of age receiving vancomycin at Cincinnati Children's Hospital Medical Center (CCHMC) were invited to participate. Peak, trough, and random vancomycin serum concentrations were used for Bayesian estimation of individual PK parameters. Model covariates included age, weight, and serum creatinine. To evaluate the predictive performance of the model, precision and bias were measured and compared using the 95% confidence interval.
Results
15 subjects were enrolled; 13 subjects had vancomycin serum concentrations drawn per protocol. Of those 13 subjects, the median age was 6 years and 54% were male. Significant medical conditions included cancer (54%), lung disease (23%), neurologic disorders (23%), and prior transplantation (15%). The initial serum creatinine was normal (median 0.33, IQR 0.23-0.4 mg/dL), and none had underlying renal dysfunction. Equivalence of bias and precision between the original model validation and the CCHMC validation were found.
Conclusions
Pediatric population PK models for vancomycin with Bayesian estimation can be used to reliably predict vancomycin exposure in children. Using AUC instead of trough serum concentrations alone can provide an opportunity to maximally optimize vancomycin administration in children.
Vancomycin remains a mainstay for treatment of children with serious infections, in part due to an increase in multi-drug resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA). In children with serious infections, it has been suggested that a vancomycin starting dose of 60 mg/kg/day divided every 6 hours should be used to achieve predose trough concentrations of 15-20 μg/mL.1-3 This recommendation is based on the finding that children prescribed this regimen are more likely to achieve a 24 hour vancomycin concentration area under the curve (AUC) over the minimum inhibitory concentration of vancomycin for the isolated bacteria (MIC) ≥ 400 in S. aureus isolates with an MIC ≤ 1 μg/mL.3-6 AUC/MIC is the pharmacodynamic index that best predicts efficacy of vancomycin in the treatment of MRSA in adults.3 While at least 25 population pharmacokinetic models have been published in the literature, including models using pediatric patients7, the current standard of care involves measuring vancomycin serum trough concentrations alone as a surrogate marker of AUC 4.
Two recent studies using Monte Carlo Simulation using pediatric population pharmacokinetic models have suggested that vancomycin troughs of 7-10 μg/mL and 8-9 μg/mL, respectively, should be sufficient to reach an AUC/MIC ≥ 400 when the vancomycin MIC for S. aureus is ≤ 1 μg/mL.8; 9 The concept of using trough values alone is even more complicated in clinical practice, with one study reporting that only 40% of 96 children receiving 60 mg/kg/day of vancomycin achieved an AUC/MIC > 400.10 These deviations from expectations laid out in national guidelines emphasize the importance of using more accurate and precise methods to measure vancomycin exposure in children, such as predicting the AUC using population pharmacokinetic model based estimation instead of extrapolating exposure based on trough values.11 This allows for more precise measurements of vancomycin exposure against the MIC of the bacteria causing infection, providing for more accurate dose adjustments to optimize vancomycin exposure.
The purpose of the study was to determine if a previously published pediatric pharmacokinetic model for vancomycin could reliably predict vancomycin AUC with sparse sampling in children at Cincinnati Children's Hospital Medical Center (CCHMC).12
Materials and Methods
Hospitalized children < 18 years of age receiving vancomycin therapy and with no history of renal insufficiency were invited to participate. Trough serum vancomycin concentrations were obtained at the discretion of the admitting physician, typically before the fifth dose when the dose was administered every 6 hours or before the fourth dose when the dose was administered every 8 hours. Also, as the standard of clinical care, trough concentrations were also often obtained after dose adjustments. Enrolled subjects had two additional vancomycin concentrations drawn, a peak obtained one hour after the vancomycin infusion was complete and a random concentration obtained 3 hours after infusion was complete in a child receiving doses every 6 hours and 4 hours after infusion was complete in a child receiving doses every 8 hours. The time of administration of each vancomycin dose and the time each concentration was drawn were documented. Medical records for each subject were also reviewed for demographic data and past medical history.
A previously published two-compartment population pharmacokinetic model of vancomycin for pediatric patients by Lamarre et al was validated in our population using Bayesian estimation in MW/Pharm (Version 3.60, Mediware, Groningen, The Netherlands).12; 13 The model covariates included age, weight, and serum creatinine. Population parameters and their distributions were defined as means (± standard deviation) and are shown in Table 1. For the Bayesian estimation, all vancomycin doses, times of administration, and serum vancomycin concentrations were entered into the appropriate sections of the MW/Pharm software. To evaluate the predictive performance of the model, precision (a measure of the size of the prediction error) and bias (the degree to which error is either too high or too low) were measured using the mean squared prediction error and the mean prediction error, respectively, for each observed concentration.14 The values between the original model validation and the CCHMC validation using a posthoc Bayesian individualized approach were compared using the 95% confidence interval.
Table 1.
Population Pharmacokinetic Model Parameters for Vancomycin in Pediatric Subjects
| Parameter | Vancomycin Population Model12 (mean, ±SDa) | Range of Individual Estimates |
|---|---|---|
| CLnrb (L/h/70kg BWc) | 0.018 (0.008) | 0.018-0.018 |
| Frd | 0.460 (0.207) | 0.280-0.678 |
| V1e (L/kg LBMc) | 0.27 (0.07) | 0.22-0.26 |
| k12f (/h) | 1.00 (0.43) | 0.41-0.87 |
| k21 (/h) | 0.59 (0.25) | 0.13-0.84 |
| Vancomycin assay error pattern (mg/L) | 0.5 + 0.05*Cg | |
| Clearance Equations | ||
| CLh | CLnr * (BW/70) + Fr * CLcri | |
| CLcr | (0.55 * Htj)/Scrk * 88.5 | |
SD, standard deviation
CLnr, nonrenal clearance
BW, body weight; LBMc, lean body mass corrected19
Fr, fraction renally eliminated
V1, volume of distribution of central compartment
k12 and k21, inter-compartment rate constants
C, plasma concentration
CL, total body clearance
CLcr, creatinine clearance estimated by the Schwartz formula20
Ht, height in cm
Scr, serum creatinine in mg/dL
Our goal was to recruit 15 subjects. Sample size was based on the precision criteria method described by investigators at the FDA.15Based on the reported variabilty in vancomycin clearance (45%) and volume of distribution (43%) reported by Lamarre et al (2000) we estimated that fifteen subjects with a minimum of two and ideally three vancomycin concentrations measurements would allow robust estimation of the PK parameters of interest .
Descriptive statistics were used to characterize the study population. Inferential statistics performed included Mann-Whitney U-test for continuous variables, with data reported as medians and 25th and 75th interquartile ranges (IQR). Categorical variables were compared using the Fisher's exact test. These tests were performed using SigmaPlot (SPSS Science, San Rafael CA).
This study was approved by the Institutional Review Board at Cincinnati Children's Hospital Medical Center. Informed consent was obtained for all subjects, as well as assent for subjects aged 11 – 17 years.
Results
A total of 15 subjects participated in the validation of the model; of these, thirteen (87%) subjects had a total of 49 serum vancomycin concentrations drawn per protocol and were included in the validation analysis. Two subjects did not have a vancomycin peak concentration obtained and thus were excluded from the validation analysis. Of the 13 subjects included in the validation analysis, the median age was 6 years and 54% were male. Significant medical conditions among these children included cancer (54%), lung disease (23%), neurologic disorders (23%), and prior transplantation (15%). The initial serum creatinine was normal for the 13 subjects (median 0.33, IQR 0.23-0.4 mg/dL), and none had underlying renal dysfunction (Table 2). Although the recommended starting dose for vancomycin in children with MRSA infection is 60 mg/kg/day, the median starting dose in our validation study was 45 mg/kg/day (Table 2). This may be related to the high proportion of children with a history of cancer or transplantation. These children are often exposed to other potentially nephrotoxic medications which may have lead clinicians to start vancomycin at a lower dose. A comparison of the population model predicted and posthoc Bayesian predicted vancomycin serum concentration-time profiles as well as the observed concentrations for a sample subject are shown in Figure 1. Bias for peak serum concentrations was −0.66 μg/mL in the Lamarre study and −1.05 μg/mL in the CCHMC population, while precision was 4.41 μg/mL in the Lamarre study and 4.53 μg/mL in the CCHMC population. Bias for random/trough concentrations was 0.45 μg/mL in the Lamarre study and −0.27 μg/mL in the CCHMC population, while precision was 2.01 μg/mL in the Lamarre study and 2.16 μg/mL in the CCHMC population. The 95% confidence intervals for bias and precision between the CCHMC population and the Lamarre study overlapped suggesting equivalence between the two validation studies (Table 3).
Table 2.
Demographics and Underlying Conditions of Children in Lamarre and CCHMC Validation Study
| Lamarre Study12 n=78 | Validation Study n=13 | |
|---|---|---|
| Demographics | ||
| Age (year, median, IQR) | 7 (range 0.01-18) | 6.1 (3.1,6.4) |
| Gender (n, % male) | 40 (51) | 7 (54) |
| Weight, Baseline Creatinine, Vancomycin Data | ||
| Weight (kg, median (IQR)) | 25 (range 0.93-74) | 22 (13.3,31.6) |
| Serum Cr (mg/dL, median (IQR)) | 0.54 (SD ± 0.28) | 0.33 (0.23,0.4) |
| Initial vancomycin dose (mg/kg/day, median (IQR)) | NA | 45 (45-45) |
| Presence of underlying conditions (n, %) | ||
| Cancer | NA | 7 (54) |
| Prior transplant | NA | 2 (15) |
| Renal disease | NA | 1 (8) |
| Cardiac disease | NA | 0 (0) |
| GI disease | NA | 1 (8) |
| Genetic/metabolic disorder | NA | 1 (7) |
| Neurologic disorder | NA | 3 (23) |
| Lung disease | NA | 3 (23) |
NA – not available
Figure 1.
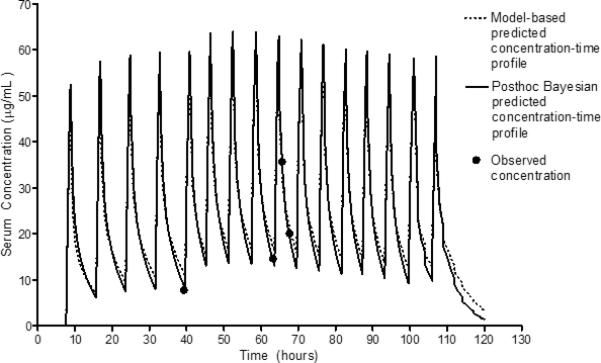
Comparison of Population Model Predicted versus Bayesian Individualized Concentration-Time Profiles.
Table 3.
Precision and bias (95% confidence interval) of the population PK model
| Bias (μg/mL) | Precision (μg/mL) | |||
|---|---|---|---|---|
| Peak | Random/Trough | Peak | Random/Trough | |
| Current study | −1.05 (−2.10, −0.01) | −0.27 (−0.75, 0.20) | 4.53 (0.49, 8.57) | 2.16 (0.46, 3.86) |
| Lamarre et al12 | −0.66 (−3.33, 2.01) | 0.45 (−0.84, 1.74) | 4.41 (2.59, 6.23) | 2.01 (1.07, 3.08) |
Discussion
The current study sought to validate the use of a previously published pediatric pharmacokinetic model of vancomycin to reliably predict AUC in children. Using a heterogeneous group of 13 children with normal baseline renal function, we found an equivalence of bias and precision in predicting vancomycin trough and peak serum concentrations in our population as the original study population. We feel confident our method of sparse sampling can be used to identify individual AUC estimates using a pediatric population PK model with Bayesian estimation and allow for better dose optimization in children. 16
While calculating AUC does require experience using a population PK model, it is a more accurate and precise method to estimate vancomycin exposure. As previous studies have shown, using trough values alone are not always reliable in estimating vancomycin exposure 8; 9 plus it does not provide a realistic description of the actual concentration-time profile. In addition, MICs of MRSA isolates are more commonly > 1 μg/mL17, but this appears to be geographically dependent18, which can further complicate using national or international guidelines for goal vancomycin trough values instead of taking into account the MIC of the organism for a particular patient.
If true vancomycin exposure is overestimated based on the interpretation of trough values, this may lead to ineffective killing of the bacteria causing infection. Likewise, underestimating true vancomycin exposure based on trough values may inadvertently lead the physician to increase vancomycin dosing and thus increase the risk of renal toxicity for the patient.3; 4 Both scenarios can negatively affect clinical outcomes. The findings in this study should encourage other institutions to validate a population PK model-based approach at their institution with their patient population and then use this method to better understand vancomycin exposure in each individual patient to allow for dose optimization. As at our institution, this type of model-based dose optimization could be provided by a clinical pharmacology or pharmacy consult service. With the availability of individuals knowledgeable in pharmacokinetics and pharmacodynamics this would be a great resource to learn and implement pharmacokinetic modeling into clinical practice, as described her in children who are receiving vancomycin for severe MRSA infection.
Limitations to the present study are related to the generalizability of our validation results as a significant proportion of the children had underlying diseases, particularly cancer. A study by Chang found that children with a malignancy had increased vancomycin clearance compared to those without, but had a similar volume of distribution.9 This could lead to less precision and a negative bias in predicting trough concentrations and ultimately lead to a lower predicted AUC. With that said, we found there was no significant difference in precision and bias between the Lamarre validation data and our validation results.12 Therefore we feel that this pediatric population PK model for vancomycin with Bayesian estimation can be used in a general pediatric population to reliably predict AUC.
Conclusions
In conclusion, using a previously published pediatric pharmacokinetic model for vancomyin to predict AUC using Bayesian estimation is a valid way to more reliably predict vancomycin exposure in children. This can allow for more personalized dosing adjustments to optimize vancomycin administration in children with serious infections, especially those with infections caused by MRSA.
Acknowledgements
This work was supported with federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development under a fellowship training grant (NIH 5 T32 HD069054).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Kim DI, Im MS, Choi JH, Lee J, Choi EH, Lee HJ. Therapeutic monitoring of vancomycin according to initial dosing regimen in pediatric patients. Korean journal of pediatrics. 2010;53:1000–5. doi: 10.3345/kjp.2010.53.12.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frymoyer A, Guglielmo BJ, Wilson SD, Scarpace SB, Benet LZ, Hersh AL. Impact of a hospitalwide increase in empiric pediatric vancomycin dosing on initial trough concentrations. Pharmacotherapy. 2011;31:871–6. doi: 10.1592/phco.31.9.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant staphylococcus aureus infections in adults and children: Executive summary. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;52:285–92. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 4.Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the american society of health-system pharmacists, the infectious diseases society of america, and the society of infectious diseases pharmacists. Pharmacotherapy. 2009;29:1275–9. doi: 10.1592/phco.29.11.1275. [DOI] [PubMed] [Google Scholar]
- 5.Frymoyer A, Hersh AL, Benet LZ, Guglielmo BJ. Current recommended dosing of vancomycin for children with invasive methicillin-resistant staphylococcus aureus infections is inadequate. The Pediatric infectious disease journal. 2009;28:398–402. doi: 10.1097/INF.0b013e3181906e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frymoyer A, Hersh AL, Coralic Z, Benet LZ, Joseph Guglielmo B. Prediction of vancomycin pharmacodynamics in children with invasive methicillin-resistant staphylococcus aureus infections: A monte carlo simulation. Clinical therapeutics. 2010;32:534–42. doi: 10.1016/j.clinthera.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marsot A, Boulamery A, Bruguerolle B, Simon N. Vancomycin: A review of population pharmacokinetic analyses. Clinical pharmacokinetics. 2012;51:1–13. doi: 10.2165/11596390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Frymoyer A, Guglielmo BJ, Hersh AL. Desired vancomycin trough serum concentration for treating invasive methicillin-resistant staphylococcal infections. The Pediatric infectious disease journal. 2013 doi: 10.1097/INF.0b013e318299f75c. [DOI] [PubMed] [Google Scholar]
- 9.Le J, Bradley JS, Murray W, et al. Improved vancomycin dosing in children using area under the curve exposure. The Pediatric infectious disease journal. 2013;32:e155–63. doi: 10.1097/INF.0b013e318286378e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chhim RF AS, Lee KR. Vancomycin dosing practices, trough concentrations, and predicted area under the curve in children with suspected invasive staphylococcal infections. Journal of the Pediatric Infectious Disease Society. 2013;2:259–262. doi: 10.1093/jpids/pis083. [DOI] [PubMed] [Google Scholar]
- 11.Le J, Ngu B, Bradley JS, et al. Vancomycin monitoring in children using bayesian estimation. Therapeutic drug monitoring. 2014 doi: 10.1097/FTD.0000000000000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamarre P, Lebel D, Ducharme MP. A population pharmacokinetic model for vancomycin in pediatric patients and its predictive value in a naive population. Antimicrobial agents and chemotherapy. 2000;44:278–82. doi: 10.1128/aac.44.2.278-282.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Proost JH, Meijer DK. Mw/pharm, an integrated software package for drug dosage regimen calculation and therapeutic drug monitoring. Computers in biology and medicine. 1992;22:155–63. doi: 10.1016/0010-4825(92)90011-b. [DOI] [PubMed] [Google Scholar]
- 14.Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. Journal of pharmacokinetics and biopharmaceutics. 1981;9:503–12. doi: 10.1007/BF01060893. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Jadhav PR, Lala M, Gobburu JV. Clarification on Precision Criteria to Derive Sample Size When Designing Pediatric Pharmacokinetic Studies. Journal of clinical pharmacology. 2012;52:1601–6. doi: 10.1177/0091270011422812. [DOI] [PubMed] [Google Scholar]
- 16.Jelliffe R, Neely M, Schumitzky A, et al. Nonparametric population modeling and bayesian analysis. Pharmacological research : the official journal of the Italian Pharmacological Society. 2011;64:426. doi: 10.1016/j.phrs.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Jimenez-Truque N, Thomsen I, Saye E, Creech CB. Should higher vancomycin trough levels be targeted for invasive community-acquired methicillin-resistant staphylococcus aureus infections in children? Pediatr Infect Dis J. 2010;29:368–70. doi: 10.1097/INF.0b013e3181c52a04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldman JL, Harrison CJ, Myers AL, Jackson MA, Selvarangan R. No evidence of vancomycin minimal inhibitory concentration creep or heteroresistance identified in pediatric staphylococcus aureus blood isolates. The Pediatric infectious disease journal. 2014;33:216–8. doi: 10.1097/01.inf.0000436281.18687.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang D. Influence of malignancy on the pharmacokinetics of vancomycin in infants and children. The Pediatric infectious disease journal. 1995;14:667–73. doi: 10.1097/00006454-199508000-00004. [DOI] [PubMed] [Google Scholar]


