Abstract
Objective
Posttraumatic stress disorder (PTSD) is associated with indicators of poor physical health and sleep disturbance. This study investigated the relationship between PTSD and metabolic risk factors and examined the role of sleep duration in a sample of medically healthy and medication-free adults.
Methods
Participants with PTSD (n = 44, mean age = 30.6 years) and control participants free of lifetime psychiatric history (n = 50, mean age = 30.3 years) recorded sleep using sleep diary for 10 nights and actigraphy for seven nights. We assessed metabolic risk factors including fasting triglycerides, total cholesterol, LDL cholesterol, and HDL cholesterol, as well as abdominal fat using dual-energy X-ray absorptiometry.
Results
PTSD was associated with shorter sleep duration (based on self-report but not actigraphy) and higher metabolic risks (controlling for body fat percentage), including increased triglycerides (p = .03), total cholesterol (p < .001), LDL cholesterol (p = .006), VLDL cholesterol (p = .002), and cholesterol: HDL ratio (p = .024). Additionally, sleep duration was associated with metabolic risks in PTSD (significant correlations ranged from r = −.20 to r = −.40) but did not fully account for the association between PTSD with metabolic measures.
Conclusions
Metabolic risk factors are associated with PTSD even in early adulthood, highlighting the need for early intervention. Future longitudinal research should assess whether sleep disturbance in PTSD is a mechanism that contributes to heightened metabolic risk in order to elucidate the pathway from PTSD to higher rates of medical disorders such as obesity, diabetes, and heart disease.
Keywords: posttraumatic stress disorder, cholesterol, triglycerides, lipoproteins, visceral adiposity, sleep duration
Accruing evidence suggests that posttraumatic stress disorder (PTSD) is associated with poor metabolic health. For example, several studies have reported dyslipidemia in veteran samples with PTSD (1–5). In a study of over 300,000 young veterans who accessed Department of Veterans Affairs (VA) health care, diagnosis of PTSD was associated with a significantly increased risk for hypertension and dyslipidemia (6). In addition, converging preliminary evidence including a recent meta-analysis of six studies (7) reported an association between PTSD and metabolic syndrome (a cluster of at least three risk factors that are associated with the development of diabetes and cardiovascular disease) (8). For example, Violanti and colleagues observed that police officers with severe PTSD symptoms were three times more likely to have metabolic syndrome, although age attenuated this association (9). Jin and colleagues observed in an outpatient sample of patients requiring ongoing antipsychotic treatment that the risk of metabolic syndrome was higher in PTSD compared to other diagnostic groups (e.g., schizophrenia) (10). In a series of studies, Heppner and colleagues observed that 43% of an older military veteran sample (mean age 52, with 70% of veterans having served in Vietnam) met criteria for metabolic syndrome, that those with more severe PTSD exhibited a higher likelihood of metabolic syndrome, and that antipsychotic use was not uniquely associated with metabolic risk (11, 12). Finally, Weiss and colleagues observed in a sample of 245 civilians of low socioeconomic status recruited from hospital general medical clinics that after controlling for demographics, smoking history, antipsychotic use, depression and exercise, current PTSD was the only significant predictor of metabolic syndrome (13). Body mass index and medical illness were not controlled.
Hence, the majority of the initial evidence suggests that PTSD is associated with a higher prevalence of metabolic risk factors. Despite this evidence, several questions remain in regard to PTSD and metabolic risk factors. First, there is some contradictory evidence. For example, one study did not observe a relationship between PTSD and lipid elevations in a civilian sample (14). Second, there has been a dearth of research on samples of young to middle-aged adults, who are more likely to be free of potentially complicating co-occurring medical issues and medications. Third, the mechanisms through which PTSD disturbs metabolism remain unclear.
Based on these knowledge gaps, we evaluated the association of PTSD and metabolic risk factors in a young, healthy, unmedicated sample. We also evaluated the role of sleep disturbance as a mechanism of increased metabolic risk. In individuals with PTSD, sleep disturbance is the most frequently reported symptom, even when nightmares are excluded (15), and as many as 90% of individuals with PTSD report insomnia and nightmares (16, 17). This sleep disturbance may contribute to metabolic risk, given the substantial evidence in healthy samples from both epidemiological and experimental laboratory studies supporting a critical role of sleep in metabolic regulation (e.g., 18, 19). For example, in a cross-sectional study, self-reported sleep quality was associated with metabolic syndrome, including measures related to obesity and insulin resistance (20) and another study found that sleep disturbance prospectively predicted the development of metabolic syndrome (21). Moreover, a recent study of over 800 individuals indicated that sleep duration of less than six hours per night was associated with an increase in the odds of having metabolic syndrome after adjusting for possible confounders (22). Finally, Kim and colleagues observed that short sleep was associated with visceral obesity as measured by computed tomography (23).
In the present study, metabolic risk factors and habitual sleep duration as measured by sleep diary and actigraphy were assessed in individuals with PTSD and healthy control participants. First, we assessed group differences in metabolic risk factors including triglycerides, total cholesterol, low density lipoprotein (LDL) cholesterol, very low density lipoprotein (VLDL) cholesterol, high density lipoprotein (HDL) cholesterol, cholesterol: HDL ratio, and truncal fat (a measure of visceral obesity), adjusting for overall body fat percentage. Then, we examined whether sleep duration would be associated with metabolic risk factors in both groups. We predicted that individuals in the PTSD group would exhibit higher metabolic risk factors and that sleep duration would account for metabolic risks in both groups.
Methods
Participants
Participants included 94 adults recruited from newspaper advertisements, web based postings, flyers in community-based outpatient clinics, and from the clinical PTSD Program at the San Francisco VA Medical Center. Participants enrolled between November 2005 and October 2008. Participants were relatively young, healthy, and medication-free in order to examine PTSD without the potential confounds of aging, physical illness, and medication. The majority of participants were civilian (89%). The sample included 44 individuals with current chronic PTSD (50% female) and 50 control subjects without PTSD (54% female), with a mean age of 30.44 (SD = 7.39; range 20 to 50 years).
Chronic PTSD was defined by DSM-IV PTSD criteria (24) or by a Clinician-Administered PTSD Scale (CAPS; 25) score of >40 for at least 3 months. Control participants were negative for lifetime PTSD, had a CAPS score of <20, and were free from lifetime major depressive disorder and panic disorder. Female subjects were studied during the follicular phase of their menstrual cycle. Exclusion criteria for both groups included neurologic disorder or systemic illness affecting CNS function; pregnancy; use of psychiatric, anticonvulsant, antihypertensive, sympathomimetic, estrogen replacement therapy, or steroid medication; lifetime history of any psychiatric disorder with psychotic features; bipolar disorder; obsessive-compulsive disorder; alcohol abuse or dependence within the past two years; and substance abuse or dependence in the past year.
Measures
Clinician-Administered PTSD Scale (CAPS)
Current PTSD was assessed with the CAPS (25). The CAPS has excellent test-retest reliability (r=0.92–0.99) and internal consistency (alpha=0.80–0.90; 26).
Structured Clinical Interview for DSM-IV (SCID)
Diagnoses other than PTSD were assessed with the SCID (27). The SCID has been shown to have good reliability (e.g., 28).
All diagnoses were made by trained clinical interviewers who calibrated their CAPS and SCID assessments at weekly case consensus meetings, supervised by an experienced Ph.D.-level clinical psychologist.
Metabolic Risk Factors
Lipids including triglycerides, total cholesterol, VLDL cholesterol, and HDL cholesterol were assessed and then assayed by a clinical laboratory contracted with the University of California, San Francisco. LDL cholesterol was calculated. The sum of LDL cholesterol, VLDL cholesterol, and HDL cholesterol comprised total cholesterol. Truncal fat was measured using dual-energy X-ray absorptiometry (DEXA) scan and truncal fat percentage was computed as DEXA-measured truncal fat divided by DEXA-measured total fat. In addition, body fat percentage was calculated from the DEXA measurements and was used as a covariate in the analyses. Six control participants did not complete the lipids assessment or DEXA body scan, and an additional four participants did not complete the DEXA scan only.
Sleep diary
Average sleep duration was computed from 10 days of sleep diary. Total sleep time (TST) was computed by subtracting reported sleep onset latency and wake after sleep onset from reported time in bed. The sleep diary has been shown to provide a reliable estimate (29) and is considered the gold standard subjective measure of sleep (30). A few participants did not complete all 10 nights of sleep diary. The mean number of nights for the PTSD group was 9.91 (SD = 0.47) and the mean number of nights for the control group was 9.70 (1.13). There was no significant difference between groups in the number of nights completed.
Actigraphy
Participants had their sleep-wake schedule monitored for seven nights with wrist actigraphy (Micro Motionlogger; Ambulatory Monitoring, Inc., Ardsley, NY). Actigraphy is an important objective estimate of sleep (31). Actigraphs were initialized and downloaded with the ActMe program (Ambulatory Monitoring, Inc., Ardsley, NY) using the ZCM sampling mode in one minute epochs. The Cole-Kripke algorithm was used in ActionW Version 2.7 (Ambulatory Monitoring, Inc., Ardsley, NY) software to estimate the sleep parameter of TST. Six participants in the control group did not have actigraphy data due to equipment malfunction (0 in the PTSD group), and a few participants in each group did not complete all seven nights. The mean number of nights for the PTSD group was 6.73 (SD = 0.87) and the mean number of nights for the control group was 6.52 (SD = 1.32). There was no significant difference between groups in the number of nights of actigraphy data.
Procedure
All research was approved by the Committee on Human Research at the University of California, San Francisco and at the San Francisco Veterans Affairs Medical Center. All participants provided written informed consent and appropriate institutional review boards approved the research protocol. Participants who were likely to be eligible after a telephone screen visited the laboratory for administration of the CAPS and SCID. Eligible participants then recorded their sleep at home using sleep diary for 10 nights and actigraphy for seven nights. Participants then visited the hospital for height and weight measurements, measurement of total and abdominal fat by DEXA scan, and venipuncture for measurement of triglycerides, total cholesterol, LDL cholesterol, VLDL cholesterol, and HDL cholesterol. The data in the present study were collected as a part of a larger research project which examined neuroendocrine factors mediating sleep disturbances in PTSD. The data and analyses presented in this paper do not overlap with previously-published articles from this study (32–34).
Statistical Analysis
We first examined potential demographic differences. Specifically, we examined potential differences in age, years of education, CAPS score, and body fat percentage using t-tests and gender, race, marital status, veteran status, and current depression status using chi-squared tests. We also examined potential group differences in sleep duration using a MANOVA. The MANOVA compared the group with PTSD to the healthy control group on the dependent variables of diary-measured total sleep time and actigraphy-measured total sleep time.
We conducted statistical analyses to test two study hypotheses—that individuals in the PTSD group would exhibit higher metabolic risk factors, and that sleep duration would account for metabolic risks in both the PTSD and healthy control groups. To test the first hypothesis, we conducted a multivariate analysis of covariance (MANCOVA) to examine group differences in metabolic risk factors. To test the second hypothesis, we conducted three sets of analyses to examine associations between the metabolic risk factors and sleep in those with and without PTSD.
For the first analysis, we conducted a multivariate analysis of covariance (MANCOVA) to examine whether there would be group differences in metabolic risk factors including triglycerides, total cholesterol, LDL cholesterol, VLDL cholesterol, HDL cholesterol, cholesterol: HDL ratio, and truncal fat percentage. DEXA-measured body fat percentage was included as a covariate in order to distinguish the effect of body fat on metabolic risk factors from psychiatric group status effects.
The second set of analyses examined associations between these risk factors and sleep in those with and without PTSD. Total sleep time was employed as the sleep outcome measure on the basis of previous research linking sleep duration to metabolic regulation, (e.g., 36, 37). Average total sleep was examined using sleep diary and actigraphy; the correlation between the two measures of sleep was r = .49 (95% C.I. .31 – .64, p < .001). In the first set of analyses, we examined partial correlations between sleep duration (as measured by sleep diary and actigraphy) and the metabolic risk factors, controlling for body fat percentage. We first examined these correlations in the full group. Next, these correlations were examined in the PTSD group and then in the control group to determine which group was driving any potential relationships. In the final set of analyses, on an exploratory basis (given the cross-sectional nature of the data), we tested mediation in path models conducted separately for each outcome, considering both diary and actigraphy TST in each model (see Figure 1 for a schematic diagram).
Figure 1.
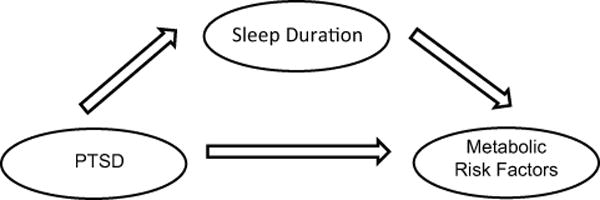
Diagram of path analyses.
Results
Participant Characteristics
There were no significant differences between groups in age, gender, or years of education. The PTSD group had fewer Caucasians, more individuals who were divorced or separated, more veterans, and a (marginally significant) higher body fat percentage (p = .05) (see Table 1). The mean CAPS score for the PTSD group was 54.79 (SD = 15.84) and the mean CAPS score for control participants who experienced a DSM-IV Criterion A event (n = 12) was 0.00. Eighteen percent of PTSD participants (n = 8) met criteria for a current Major Depressive Episode.
Table 1.
Characteristics of Healthy, Unmedicated PTSD Participants and Age-Matched Controls
| Group | ||||||
|---|---|---|---|---|---|---|
| Variable | PTSD group (n = 44) |
Control group (n = 50) |
Test Statistic | p value | ||
|
| ||||||
| M or n | SD or % | M or n | SD or % | |||
| Age (SD) | 30.55 | 6.57 | 30.34 | 8.11 | t(92) = −.13 | p = 0.89 |
| Gender (%) | χ2(1) = .15 | p = 0.70 | ||||
| Male | 22 | 50 | 23 | 46 | ||
| Female | 22 | 50 | 27 | 54 | ||
| Years of education (SD) | 14.86 | 2.20 | 15.40 | 2.04 | t(91) = 1.22 | p = 0.23 |
| Race (%) | χ2(4) = 11.29* | p = 0.024 | ||||
| African American | 5 | 11 | 1 | 2 | ||
| Asian American | 3 | 7 | 7 | 14 | ||
| Caucasian | 27 | 61 | 40 | 80 | ||
| Other | 7 | 16 | 1 | 2 | ||
| Unknown | 2 | 5 | 1 | 2 | ||
| Marital Status (%) | χ2(3) = 8.94* | p = 0.030 | ||||
| Single | 34 | 77 | 42 | 84 | ||
| Married/Partnered | 2 | 5 | 7 | 14 | ||
| Divorced | 5 | 11 | 1 | 2 | ||
| Separated | 3 | 7 | 0 | 0 | ||
| Veteran Status | χ2(1) = 12.72*** | p < .001 | ||||
| Civilian | 34 | 77 | 50 | 100 | ||
| Veteran | 10 | 23 | 0 | 0 | ||
| Body Fat Percentage | 30.77 | 8.40 | 26.60 | 10.63 | t(85) = −2.03* | p = .045 |
| CAPS | 54.79 | 15.84 | 0.00 | 0 | t(53) = −11.90*** | p < .001 |
| Current Depression | χ2(1) = 9.94** | p = .002 | ||||
| Absent | 36 | 82 | 50 | 100 | ||
| Present | 8 | 18 | 0 | 0 | ||
Note. Mean values presented. CAPS = Clinician-Administered PTSD Scale.
p < .05,
p < .01,
p < .001. Three participants who described their race as Hispanic comprised the participants in the ‘Unknown’ race category.
Sleep Duration
We examined baseline sleep characteristics of participants. A multivariate analysis of variance (MANOVA) was conducted on the sleep variables of total sleep time based on sleep diary and actigraphy with group (PTSD, control) as the between-subjects variable. A significant group effect was observed on the omnibus test (F(2, 82) = 6.24, p =.003). A significant multivariate effect was also observed on diary-measured TST, with the PTSD group reporting less total sleep time than the control group (see Table 2). No difference was observed on actigraphy-measured TST.
Table 2.
Sleep Duration and Metabolic Risk Factor Values
| Group | |||||||
|---|---|---|---|---|---|---|---|
| Variable | PTSD group | Control group | Test Statistic (with body fat percentage as covariate in metabolic analyses) |
Test Statistic (with body fat percentage and sleep diary TST as covariates) |
Test Statistic (with body fat percentage and actigraphy TST as covariate) |
||
|
| |||||||
| M | SD | M | SD | ||||
| Diary-measured TST | 6.59 | 1.27 | 7.43 | 0.80 | F(1,83) = 12.25, p = .001 | ||
| Actigraphy-measured TST | 6.94 | 1.41 | 7.32 | 0.98 | F(1,83) = 1.32 | ||
| Triglycerides (mg/dL) | 137.51 | 73.85 | 90.24 | 40.34 | F(1, 80) = 9.53, p = .003 | F(1,79) = 4.43, p = .038 | F(1,71) = 5.40, p = .023 |
| Cholesterol (mg/dL) | 170.26 | 33.75 | 138.89 | 31.93 | F(1,80) = 14.32, p < .001 | F(1,79) = 10.42, p = .002 | F(1,71) = 12.91, p = .001 |
| VLDL cholesterol (mg/dL) | 26.17 | 11.73 | 17.96 | 8.07 | F(1,80) = 10.01, p = .002 | F(1,79) = 4.70, p = .033 | F(1,71) = 5.73, p = .091 |
| LDL cholesterol (mg/dL) | 95.19 | 29.86 | 74.98 | 26.48 | F(1,80) = 7.93, p = .006 | F(1,79) = 6.24, p = .015 | F(1,71) = 6.58, p = .012 |
| HDL cholesterol (mg/dL) | 47.30 | 12.63 | 45.96 | 11.74 | F(1,80) = 0.89 | F(1,79) = 1.11 | F(1,71) = 2.28 |
| Cholesterol: HDL ratio | 8.1 | 3.85 | 3.15 | 0.88 | F(1,80) = 5.26, p = .024 | F(1,79) = 3.48 | F(1,71) = 2.60 |
| Truncal fat percentage | 53.00 | 7.10 | 52.00 | 6.16 | F(1,80) = 0.76 | F(1,79) = .04 | F(1,71) = .16 |
Metabolic Risk Factors
A multivariate analysis of covariance (MANCOVA) was conducted on metabolic risk factors (triglycerides, total cholesterol, LDL cholesterol, VLDL cholesterol, HDL cholesterol, cholesterol: HDL ratio, and truncal fat percentage) with group (PTSD, control) as the between-subjects variable and DEXA-measured body fat percentage as a covariate. A significant group effect was observed on the omnibus test (F(6,75) = 4.06, p < .001). In addition, significant multivariate effects were observed on the following dependent measures: triglycerides, total cholesterol, LDL cholesterol, VLDL cholesterol, and cholesterol: HDL ratio. No differences were observed on HDL cholesterol or truncal fat percentage, controlling for overall body fat percentage. See Table 2.
Sleep Duration and Metabolic Risk Factors
Partial correlations between sleep duration as measured by sleep diary and actigraphy and the metabolic risk factors were conducted, first in the full sample and then separately in the PTSD and control groups. In the full sample, there were negative correlations between diary-measured sleep duration and triglycerides, cholesterol, VLDL cholesterol, and truncal fat percentage. In the PTSD group, there were negative correlations between diary-measured sleep duration and triglycerides and diary-measured sleep duration and VLDL cholesterol. The five non-significant correlations were all in the predicted direction (see Table 3). The same partial correlation analysis was then conducted in the control group. There was a significant correlation between truncal fat percentage and diary-measured sleep duration, indicating that less sleep was associated with more truncal fat. No other correlations were significant and two of the six non-significant associations were in the predicted direction.
Table 3.
Partial Correlations between Sleep Duration and Metabolic Risk Factors
| Sleep diary-measuredtotal sleep time | Actigraphy-measured total sleep time | |||
|---|---|---|---|---|
|
| ||||
| r | P | r | p | |
| All participants | ||||
| Triglycerides | −.35 | .002 | −.30 | .009 |
| Cholesterol | −.28 | .017 | −.20 | .09 |
| VLDL cholesterol | −.36 | .002 | −.31 | .007 |
| LDL cholesterol | −.18 | .12 | −.23 | .049 |
| HDL cholesterol | −.02 | .88 | .27 | .022 |
| HDL: cholesterol ratio | −.17 | .14 | −.37 | .001 |
| Truncal fat percentage | −.31 | .007 | −.44 | <.001 |
| PTSD group | ||||
| Triglycerides | −.40 | .011 | −.41 | .007 |
| Cholesterol | −.17 | .28 | −.17 | .30 |
| VLDL cholesterol | −.40 | .010 | −.42 | .006 |
| LDL cholesterol | −.04 | .79 | −.19 | .23 |
| HDL cholesterol | .02 | .91 | .40 | .009 |
| HDL: cholesterol ratio | −.09 | .60 | −.40 | .009 |
| Truncal fat percentage | −.24 | .13 | −.42 | .006 |
| Control group | ||||
| Triglycerides | .06 | .74 | .05 | .77 |
| Cholesterol | −.14 | .43 | −.13 | .48 |
| VLDL cholesterol | .05 | .76 | .05 | .78 |
| LDL cholesterol | −.21 | .24 | −.23 | .19 |
| HDL cholesterol | .04 | .85 | .11 | .53 |
| HDL: cholesterol ratio | −.14 | .42 | −.22 | .22 |
| Truncal fat percentage | −.44 | .009 | −.48 | .004 |
Note. Partial correlations controlled for body fat percentage. VLDL = very low density lipoprotein. LDL = low density lipoprotein. HDL = high density lipoprotein.
Next, the partial correlations between actigraphy-measured sleep duration and metabolic risk factors were examined in the full sample and then in the PTSD group andcontrol group. In the full sample, there were negative correlations between actigraphy-measured sleep duration and triglycerides, cholesterol, VLDL cholesterol, LDL cholesterol, cholesterol: HDL ration, and trunkal fat percentage, and a positive correlation between actigraphy-measured sleep duration and HDL cholesterol. In the PTSD group, five associations were significant. There were negative correlations between actigraphy-measured sleep duration and triglycerides, VLDL cholesterol, cholesterol: HDL ratio, and truncal fat percentage. There was a positive correlation between actigraphy-measured sleep duration and HDL cholesterol. These significant correlations were all in the predicted direction and suggest that less sleep duration is associated with higher metabolic risk. The two non-significant correlations were also in the predicted direction. Interestingly, there were more significant sleep-metabolic risk factor correlations based on actigraphy than on diary. Finally, the same partial correlations between actigraphy-measured sleep duration and metabolic risk factors were examined in the control group. Only one significant correlation emerged: sleep duration and truncal fat percentage, such that less sleep was associated with more truncal fat. Four of the six non-significant correlations were in the predicted direction.
Finally, on an exploratory basis, sleep duration (both diary and actigraphy-measured) was examined as a mediator of the effects of PTSD on metabolic risk factors using path models. Results indicated that diary TST partially mediated the effects of PTSD on triglycerides: The standardized mediated effect was .24 (p < .001), while the standardized direct effect was .40 (p = .003). These results indicate that there was a PTSD vs. control group difference of .24 standard deviation units in triglycerides attributable to differences in sleep duration, plus an additional .40 standard deviation units difference in triglycerides not attributable to sleep duration. Moreover, diary TST completely mediated the effect of PTSD on truncal fat percent (standardized mediated effect = .29, p < .001) compared to a standardized direct effect = −.10, p = .660). Diary TST did not mediate any of the cholesterol measures. We note that diary TST mediated the effect on truncal fat even though there was no total effect of PTSD on truncal fat. This result is due to the effect of group on TST and the effect of TST on truncal fat, contributing to a significant indirect pathway from PTST to TST to truncal fat (for more on mediation effects in the absence of direct effects, see 38). Actigraphy-based TST did not mediate any relationships.
Discussion
We examined metabolic risk factors and habitual sleep duration in individuals with PTSD compared to a healthy control group. In support of our hypothesis, we observed that individuals with PTSD demonstrated a worse metabolic profile, including higher levels of triglycerides, total cholesterol, LDL cholesterol, VLDL cholesterol, and cholesterol: HDL ratio. We did not observe increased truncal fat or reduced HDL cholesterol in PTSD. Overall, our findings are consistent with the accruing evidence suggesting higher metabolic risks in PTSD (1, 2, 4–7, 9, 11, 12, 39). However, the differences we observed are particularly notable given the relatively young age of the sample and the lack of co-occurring medical issues and medications. For example, many previous studies have utilized older samples which may present a confound since metabolic syndrome increases with age (e.g., 40). Our data suggest that the poor health risks and outcomes associated with PTSD are present by early adulthood and as such should be assessed early in clinical settings.
Our second hypothesis was that sleep duration would account for the group differences in metabolic risks between individuals with PTSD and healthy control participants. To test this hypothesis, we first examined sleep duration in the sample. We observed that individuals with PTSD reported less total sleep time compared to the healthy control participants based on sleep diary, consistent with substantial research indicating sleep disturbance in PTSD (e.g., 15, 16, 17). Interestingly, we did not observe worse sleep in PTSD as measured by actigraphy, though the means were in the predicted direction. The r = 0.49 correlation between the two measures in this study was not surprising. It is common for discrepancies to emerge between objective and subjective measures of sleep, particularly in individuals with psychiatric disorders, e.g., (41, 42). While both measures are useful for longitudinal use as in the current study and present a low participant burden, sleep diaries rely on morning estimates of the previous night’s sleep. Evidence suggests that individuals may have difficulty with such estimates (e.g., 43) and particularly that individuals with insomnia may overestimate their sleep disturbance (for a review see 44). On the other hand, actigraphs, which estimate sleep from wrist accelerometry, may present issues such as incorrectly considering physical inactivity in the bed as sleep or restlessness/movement in sleep was wake time. Finally, age and gender may affect concordance between diary- and actigraphy-measured sleep (45).
As the second step in testing the hypothesis that sleep would account for group differences in metabolic risk factors, we conducted correlation analyses. These analyses demonstrated relationships between sleep duration and metabolic risk factors, such that shorter sleep duration was associated with higher metabolic risk. We then examined the diagnostic groups separately to see if one group drove these differences. In the PTSD group, there were numerous associations between sleep duration and metabolic risk factors, while the same analyses in the healthy control group yielded few associations. It is possible that the habitually longer sleep duration in the control group served as a protective factor. Overall, the data suggest that sleep may contribute to the metabolic risk profile observed in PTSD, although sleep does not fully explain the heightened risk. Treating sleep disturbance in PTSD continues to be important because initial evidence suggests that improving sleep may improve overall functioning (46) and increasing sleep duration could decrease metabolic risks. At the same time, treatment for sleep disturbance is unlikely to serve as a panacea and other interventions that focus on management of chronic stress, diet, and exercise may also be important, given the likelihood that other mechanisms also contribute to these metabolic health risks.
We also note that exploratory mediation analyses demonstrated that diary-measured total sleep time partially mediated the effect of PTSD on triglycerides and completely mediated the effect of PTSD on truncal fat, consistent with previous research, while actigraphy-measured sleep duration did not. These results are consistent with previous research which demonstrated a link between subjectively-measured, but not objectively-measured, sleep and urinary cortisol (another metabolism variable)(47).
Considering other possible contributors to metabolic health risks—beyond sleep—allostatic load may comprise one possible mechanism. Allostatic load refers to the physiologic damage as a result of repeated activation of the body’s stress response system (48). For example, chronic glucocorticoid secretion can contribute to abdominal fat deposition directly (49, 50) as well as indirectly via increased intake of so-called “comfort foods” (i.e., foods high in fat and/or sugar; e.g., 51, 52), resulting in visceral obesity. In another example, increased activation of the sympathetic nervous system increases production of lipoprotein lipase, which leads to increased cholesterol and triglycerides (53). Additionally, stress may act through the neuropeptide Y system (54), particularly in the context of a high caloric, fat and sugar diet, which may be more common in PTSD (34, 54). Future research should examine multiple contributory mechanisms to the heightened metabolic risk in PTSD including both sleep disturbance and allostatic load in order to begin delineate causal pathways.
Considering the seven specific metabolic risk factors examined in the present study, no factor in particular emerged as the one most likely to demonstrate heightened risk in this young, healthy sample. All seven of the metabolic risk factors examined demonstrated either an elevated mean in PTSD compared to the healthy control group or an association with sleep in the PTSD group (and most factors demonstrated both of these). As such, in samples such as this one in which full metabolic syndrome is unlikely to be present in the majority of individuals, it may be important to continue to examine and treat individual risk factors.
We do note, however, that the sleep-truncal fat correlations were the only associations in this sample that were significant in both the individuals with PTSD (according to actigraphy, and marginally significant according to sleep diary) and the healthy control group (significant according to both actigraphy and diary). While we cannot assume directionality from a correlation, the data raise the possibility that sleep duration and truncal fat may be closely connected. The correlations in the PTSD and healthy control groups across both sleep diary and actigraphy with truncal fat ranged from r = .3 to r = .5, which is noteworthy given that the group means for habitual sleep duration were not extremely low (i.e., the sleep diary and actigraphy means ranged from 6.6–7.0 hours in the PTSD group and 7.3–7.4 hours in the healthy control group). Consistent with our findings, one recent study has observed an association between short sleep and visceral obesity in a community sample of middle-aged Korean adults.(23) The topic has otherwise been understudied but it is an important direction for future research, particularly to establish whether abdominal obesity is an early result of habitually low sleep.
Some limitations of the present study are important to consider. First, the relatively small sample size may have limited the statistical power of the study and therefore reduced the ability to detect group differences. For example, there were some nonsignificant correlations in the PTSD and control groups between sleep and metabolic risk factors. Given the small sample size, it is not clear whether there were truly no relationships in these instances or whether the study was underpowered to detect potential relationships. Future studies should employ larger sample sizes. Second, we used multiple statistical tests to examine the metabolic risk factors, and as such there is an increased possibility of type I errors. We note, however, that all hypotheses were a priori and all significant correlations were in the predicted direction. Also, metabolic risk factors are correlated, and therefore the multiple tests do not represent independent chances for type II error. Third, given the cross-sectional design of the present study, it is not possible to determine causal relationships among PTSD, sleep, and metabolic risk factors. Future research should include longitudinal assessments. Finally, the study used a convenience rather than a representative sample, thus introducing the possibility of bias. Nonetheless, the initial data suggest that PTSD is associated with higher metabolic risks even in a young to middle-aged, medically-healthy, and medication-free sample. Additionally, sleep duration is associated with these metabolic risks in individuals with PTSD but does not fully account for them. Future longitudinal research should assess whether sleep disturbance in PTSD contributes to heightened metabolic risk as well as explore other potential contributory mechanisms.
Acknowledgments
This research was supported by grants from the National Institute for Mental Health (TCN: 5R01MH073978-04, 5R34MH077667-03) and the Mental Illness Research and Education Clinical Center of the U.S. Veterans Health Administration. This project was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Sources of funding: This research was supported by grants to TCN from the National Institute for Mental Health (TCN: 5R01MH073978-04, 5R34MH077667-03) and the Mental Illness Research and Education Clinical Center of the U.S. Veterans Health Administration. This project was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131 to TCN. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Acronyms
- CAPS
Clinician-Administered PTSD Scale
- CNS
central nervous system
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- DEXA
dual-energy X-ray absorptiometry
- HDL
high density lipoprotein
- LDL
low density lipoprotein
- MANCOVA
multivariate analysis of covariance
- PTSD
posttraumatic stress disorder
- TST
total sleep time
- VA
Veterans Affairs
- VLDL
very low density lipoprotein
Footnotes
Conflicts of interest: Dr. Cohen reported that her spouse is employed by Gilead Sciences, Inc. Dr. Neylan reported receiving study medication from Actelion for a study funded by the Department of Defense and receiving study medication from Glaxo Smith Kline for a study funded by the Department of Veterans Affairs. For the remaining authors none were declared.
References
- 1.Kagan BL, Leskin G, Haas B, Wilkins J, Foy D. Elevated lipid levels in Vietnam veterans with chronic posttraumatic stress disorder. Biol Psychiatry. 1999;45:374–7. doi: 10.1016/s0006-3223(98)00059-6. [DOI] [PubMed] [Google Scholar]
- 2.Karlovic D, Buljan D, Martinac M, Marcinko D. Serum lipid concentrations in Croatian veterans with post-traumatic stress disorder, post-traumatic stress disorder comorbid with major depressive disorder, or major depressive disorder. J Korean Med Sci. 2004;19:431–6. doi: 10.3346/jkms.2004.19.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karlovic D, Martinac M, Buljan D, Zoricic Z. Relationship between serum lipid concentrations and posttraumatic stress disorder symptoms in soldiers with combat experiences. Acta Med Okayama. 2004;58:23–7. doi: 10.18926/AMO/32118. [DOI] [PubMed] [Google Scholar]
- 4.Solter V, Thaller V, Karlovic D, Cmkovic D. Elevated serum lipids in veterans with combat-related chronic posttraumatic stress disorder. Croat Med J. 2002;43:685–9. [PubMed] [Google Scholar]
- 5.Maia DB, Marmar CR, Mendlowicz MV, Metzler T, Nobrega A, Peres MC, Coutinho ES, Volchan E, Figueira I. Abnormal serum lipid profile in Brazilian police officers with post-traumatic stress disorder. J Affect Disord. 2008;107:259–63. doi: 10.1016/j.jad.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen BE, Marmar C, Ren L, Bertenthal D, Seal KH. Association of cardiovascular risk factors with mental health diagnoses in Iraq and Afghanistan war veterans using VA health care. JAMA. 2009;302:489–92. doi: 10.1001/jama.2009.1084. [DOI] [PubMed] [Google Scholar]
- 7.Bartoli F, Carra G, Crocamo C, Carretta D, Clerici M. Metabolic syndrome in people suffering from posttraumatic stress disorder: a systematic review and meta-analysis. Metab Syndr Relat D. 2013;11:301–8. doi: 10.1089/met.2013.0010. [DOI] [PubMed] [Google Scholar]
- 8.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SCJ, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive Summary. Cardiol Rev. 2005;13:322–27. [PubMed] [Google Scholar]
- 9.Violanti JM, Andrew M, Burchfiel CM, Hartley TA, Charles LE, Miller DB. Post-traumatic stress symptoms and cortisol patterns among police officers. Policing: an Intl J Police Strategies & Mgmt. 2007;30:189–202. [Google Scholar]
- 10.Jin H, Lanouette NM, Mudaliar S, Henry R, Folsom DP, Khandrika S, Glorioso DK, Jeste DV. Association of posttraumatic stress disorder with increased prevalence of metabolic syndrome. Journal of clinical Psychopharmacology. 2009;29:210–5. doi: 10.1097/JCP.0b013e3181a45ed0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heppner PS, Crawford EF, Haji UA, Afari N, Hauger RL, Dashevsky BA, Horn PS, Nunnink SE, Baker DG. The association of posttraumatic stress disorder and metabolic syndrome: a study of increased health risk in veterans. BMC Medicine. 2009;7:1. doi: 10.1186/1741-7015-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heppner PS, Lohr JB, Kash TB, Jin H, Wang H, Baker DG. Metabolic syndrome: relative risk associated with post-traumatic stress disorder (PTSD) severity and antipsychotic medication use. Psychosomatics. 2012;53:550–8. doi: 10.1016/j.psym.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Weiss T, Skelton K, Phifer J, Javanovic T, Gillespie CF, Smith A, Umpierrez G, Bradley B, Ressler KJ. Posttraumatic stress disorder is a risk factor for metabolic syndrome in an impoverished urban population. Gen Hosp Psychiat. 2011;33:135–42. doi: 10.1016/j.genhosppsych.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tochigi M, Umekage T, Otani T, Kato T, Iwanami A, Asukai N, Sasaki T, Kato N. Serum cholesterol, uric acid and cholinesterase in victims of the Tokyo subway sarin poisoning: a relation with post-traumatic stress disorder. Neurosci Res. 2002;44:267–72. doi: 10.1016/s0168-0102(02)00146-3. [DOI] [PubMed] [Google Scholar]
- 15.Roszell DK, McFall ME, Malas KL. Frequency of symptoms and concurrent psychiatric disorder in Vietnam veterans with chronic PTSD. Hosp Community Psych. 1991;42:293–6. doi: 10.1176/ps.42.3.293. [DOI] [PubMed] [Google Scholar]
- 16.Kilpatrick DG, Resnick HS, Freedy JR, Pelcovitz D, Resick PA, Roth S, van der Kolk B. Posttraumatic stress disorder field trial: Evaluation of the PTSD construct–Criteria A through E. In: Widiger TA, Frances AJ, Pincus HA, Ross R, First MB, Davis W, editors. DSM-IV sourcebook. 4. Washington, DC: American Psychiatric Association; 1998. pp. 803–44. [Google Scholar]
- 17.Neylan TC, Marmar CR, Metzler TJ, Weiss DS, Zatzick DF, Delucchi KL, Wu RM, Schoenfeld FB. Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam veterans. Am J Psychiatry. 1998;155:929–33. doi: 10.1176/ajp.155.7.929. [DOI] [PubMed] [Google Scholar]
- 18.Morselli LL, Guyon A, Spiegel K. Sleep and metabolic function. Pflugers Arch. 2012;463:139–60. doi: 10.1007/s00424-011-1053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xi B, He D, Zhang M, Xue J, Zhou D. Short sleep duration predicts risk of metabolic syndrome: A systematic review and meta-analysis. Sleep Med Rev. 2013 doi: 10.1016/j.smrv.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Jennings JR, Muldoon MF, Hall M, Buysse DJ, Manuck SB. Self-reported sleep quality is associated with the metabolic syndrome. SLEEP. 2007;30:219–23. doi: 10.1093/sleep/30.2.219. [DOI] [PubMed] [Google Scholar]
- 21.Troxel WM, Buysse DJ, Matthews KA, Kip KE, Strollo PJ, Hall M, Drumheller O, Reis SE. Sleep symptoms predict the development of the metabolic syndrome. SLEEP. 2010;33:1633–40. doi: 10.1093/sleep/33.12.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaput JP, McNeil J, Despres JP, Bouchard C, Tremblay A. Seven to eight hours of sleep a night is associated with a lower prevalence of the metabolic syndrome and reduced overall cardiometabolic risk in adults. PLoS. 2013;8 doi: 10.1371/journal.pone.0072832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim NH, Lee SK, Eun CR, Seo JA, Kim SG, Choi KM, Baik SH, Choi DS, Yun CH, Kim NH, Shin C. Short sleep duration combined with obstructive sleep apnea is associated with visceral obesity in Korean adults. SLEEP. 2013;36:723–9. doi: 10.5665/sleep.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, D.C.: APA; 2000. text revision ed. [Google Scholar]
- 25.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 26.Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD Scale: a review of the first ten years of research. Depress Anxiety. 2001;13:132–56. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- 27.First M, Spitzer R, Williams J, Gibbon M. Structured Clincal Interview for the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) 4. New York: Biomedics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 28.Williams JB, Gibbon M, First MB, Spitzer RL, Davies M, Borus J, Howes MJ, Kane J, Pope HG, Jr, Rounsaville B, Wittchen HU. The Structured Clinical Interview for DSM-III-R (SCID): Multisite test-retest reliability. Arch Gen Psychiatry. 1992;49:630–6. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- 29.Morin CM, Espie CA. Insomnia: a clinical guide to assessment and treatment. New York: Kluwer Academic/Plenum Publishers; 2003. [Google Scholar]
- 30.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. SLEEP. 2006;29:1155–73. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 31.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollack CP. The role of actigraphy in the study of sleep and circadian rhythms. SLEEP. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 32.O’Donovan A, Epel E, Lin J, Wolkowitz O, Cohen B, Maguen S, Metzler T, Lenoci M, Blackburn E, Neylan TC. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry. 2011;70:465–71. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richards A, Metzler TJ, RUoff LM, Inslicht SS, Rao M, Talbot LS, Neylan TC. Sex differences in objective measures of sleep in post-traumatic stress disorder and healthy control subjects. J Sleep Res. 2013 doi: 10.1111/jsr.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talbot LS, Maguen S, Epel ES, Metzler TJ, Neylan TC. Posttraumatic stress disorder is associated with emotional eating. J Traum Stress. 2013;26:521–5. doi: 10.1002/jts.21824. [DOI] [PubMed] [Google Scholar]
- 35.Brown H, Prescott R. Applied Mixed Models in Medicine. Second. England: John Wiley & Sons Ltd; 2006. [Google Scholar]
- 36.Hall MH, Muldoon MF, Jennings JR, Buysse DJ, Flory JD, Manuck SB. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. SLEEP. 2008;31:635–43. doi: 10.1093/sleep/31.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ju SY, Choi WS. Sleep duration and metabolic syndrome in adult populations: a meta-analysis of observational studies. Nutr Diabetes. 2013 doi: 10.1038/nutd.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kenny DA, Judd CM. Power anomalies in testing mediation. Psychological Science. 2014;25:334–9. doi: 10.1177/0956797613502676. [DOI] [PubMed] [Google Scholar]
- 39.Weiss T, Skelton K, Phifer J, Jovanovic T, Gillespie CF, Smith A, Umpierrez G, Bradley B, Ressler KJ. Posttraumatic stress disorder is a risk factor for metabolic syndrome in an impoverished urban population. Gen Hosp Psychiatr. 2011;33:135–42. doi: 10.1016/j.genhosppsych.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexander CM, Landsman PB, Teutsch SM, Haffner SM. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210–4. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- 41.Dagan Y, Zinger Y, Lavie P. Actigraphic sleep monitoring in posttraumatic stress disorder (PTSD) patients. J Psychosom Res. 1997;42:577–81. doi: 10.1016/s0022-3999(97)00013-5. [DOI] [PubMed] [Google Scholar]
- 42.Harvey AG, Schmidt DA, Scarna A, Semler CN, Goodwin GM. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. Am J Psychiatry. 2005;162:50–7. doi: 10.1176/appi.ajp.162.1.50. [DOI] [PubMed] [Google Scholar]
- 43.Carskadon MA, Dement WC, Mitler MM, Guilleminault C, Zarcone VP, Spiegel R. Self-reports versus sleep laboratory findings in 122 drug-free subjects with complaints of chronic insomnia. Am J Psychiatry. 1976;133:1382–8. doi: 10.1176/ajp.133.12.1382. [DOI] [PubMed] [Google Scholar]
- 44.Harvey AG, Tang NKY. (Mis)perception of sleep in insomnia: a puzzle and a resolution. Psych Bull. 2012;138:77–101. doi: 10.1037/a0025730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reyner A, Horne JA. Gender- and age-related differences in sleep determined by home-recorded sleep logs and actimetry from 400 adults. SLEEP. 1995;18:127–34. [PubMed] [Google Scholar]
- 46.Talbot LS, Maguen S, Metzler TJ, Schmitz M, McCaslin SE, Richards A, Perlis ML, Posner DA, Weiss B, Ruoff L, Varbel J, Neylan TC. Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: a randomized controlled trial. SLEEP. 2014;37:327–341. doi: 10.5665/sleep.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rao MN, Blackwell T, Redline S, Punjabi NM, Barrett-Connor E, Neylan TC, Stone KL. Association between sleep duration and 24-hour urine free cortisol in the MrOS Sleep Study. PLOS One. 2013;8 doi: 10.1371/journal.pone.0075205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 49.Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19:275–80. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Bjorntorp P, Rosmond R. Neuroendocrine abnormalities in visceral obesity. Int J Obes RelaT Metab Disord. 2000;24:S80–5. doi: 10.1038/sj.ijo.0801285. [DOI] [PubMed] [Google Scholar]
- 51.Zellner DA, Loaiza S, Gonzalez Z, Pita J, Morales J, Pecora D, Wolf A. Food selection changes under stress. Physiology & Behavior. 2006;87:789–93. doi: 10.1016/j.physbeh.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 52.Gibson EL. Emotional influences on food choice: sensory, physiological and psychological pathways. Physiology & Behavior. 2006;89:51–61. doi: 10.1016/j.physbeh.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 53.Rosmond R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrino. 2005;30:1–10. doi: 10.1016/j.psyneuen.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 54.Rasmusson AM, Schnurr PP, Zukowska Z, Scioli E, Forman DE. Adaptation to extreme stress: post-traumatic stress disorder, neuropeptide Y and metabolic syndrome. Exp Biol Med (Maywood) 2010;235:1150–62. doi: 10.1258/ebm.2010.009334. [DOI] [PubMed] [Google Scholar]


