Abstract
Obstructive sleep apnoea is a common disease that is now more widely recognised because of the rise in prevalence and the increasingly compelling data that shows major neurocognitive and cardiovascular sequelae. At the same time, the clinical practice of sleep medicine is changing rapidly, with novel diagnostics and treatments that have established a home-based (rather than laboratory-based) management approach. We review the most recent insights and discoveries in obstructive sleep apnoea, with a focus on diagnostics and therapeutics. As will be discussed, management of obstructive sleep apnoea could soon transition from a so-called one size fits all approach to an individualised approach.
Definition
The apnoea-hypopnoea index (AHI) is the number of cessations in breathing (apnoea) and the number of reductions (hypopnoea) in breathing (tidal volume) per hour of sleep. Although this definition seems straightforward, much debate is ongoing with regards to the definition of hypopnoea. Liberal definitions of hypopnoea could increase the likelihood that a patient might qualify for positive airway pressure (PAP) therapy on the assumption that the patient would benefit. Conversely, stricter definitions of hypopnoea have been used, particularly by US Medicare and others who have relied on pulse oximetry for a diagnosis of obstructive sleep apnoea. The AHI is regarded as the gold standard for diagnosis and determining severity of obstructive sleep apnoea; however, results from recent published work show that the predictive value of AHI for complications is low. Crucial to this debate on the validity of the AHI is the recognition that different definitions are relevant for the various outcomes and complications of obstructive sleep apnoea. For example, emerging data show that 4% desaturation is predictive of hypertension, 2% de saturation is predictive of insulin resistance, increased arousal frequency is predictive of impaired memory consolidation, percent time saturation lower than 90% is predictive of platelet aggregation, and none of these variables are predictive of motor vehicle accidents.1–6 In view of the myriad of effects associated with obstructive sleep apnoea, a single definition is unlikely to be predictive of all known complications. The optimum definition of sleep apnoea for an individual patient will take into account the outcome of interest, as well as sleep study parameters such as AHI.7
We review the most important recent developments in obstructive sleep apnoea in relation to epidemiology, pathogenesis, diagnosis, comorbidities, outcomes, and treatments. The salient points are summarised in the table.
Table.
Recent developments in obstructive sleep apnoea
| Where next? | |
|---|---|
| Epidemiology | |
| New data suggest that moderate-to-severe OSA is highly prevalent if rigorous methods are used in diagnostic approaches | OSA might represent a range of disease rather than a definable cutoff; public health measures might be needed to increase awareness and to tackle the burden of disease |
| Pathogenesis | |
| Non-anatomical traits are important in some patients | Personalised treatment |
| Diagnostics | |
| Shift from laboratory-based testing to home sleep testing Ability to measure some physiological traits from clinical polysomnograms |
Patient initiated testing (ie, smartphone applications) Ability to measure traits from home studies |
| Outcomes | |
| Recognition that different sequelae of OSA are important for different outcomes—eg, arousal from sleep affects memory consolidation and 4% oxygen desaturation predicts hypertension Treatment of OSA in various patient populations—eg, elderly patients have subjective benefit from PAP |
Personalised risk profile Continued understanding of OSA treatment in different patient groups, and specific strategies to improve adherence |
| Treatments | |
| Hypoglossal nerve stimulation PAP is superior to oxygen therapy for blood pressure reduction Substantial reductions in blood pressure with medical weight loss |
Define the role of novel devices Comparative efficacy research Optimise strategies for medical and surgical weight loss and weight maintenance |
OSA=obstructive sleep apnoea. PAP=positive airway pressure.
Epidemiology
The results of a study by Young and colleagues8 in 1993 showed that 4% of men and 2% of women in the USA had clinically important obstructive sleep apnoea. The prevalence of obstructive sleep apnoea has since increased worldwide, and this increase is thought to be associated with the obesity pandemic, improvements in diagnostic technology, and ageing of the population. 20 years after the study by Young and colleagues,8 Peppard and colleagues9 reported that 13% of men and 6% of women in the USA had clinically important obstructive sleep apnoea (AHI >15). These prevalence figures might have been higher if Peppard and colleagues9 had reported hypopnoea frequency using contemporary methods10 and if lower thresholds had been used to define the obstructive sleep apnoea. Indeed, Heinzer and colleagues,11 using sensitive methods, reported a prevalence of moderate-to-severe obstructive sleep apnoea (AHI ≥15) of 23·4% (95% CI 20·9–26·0) in women and 49·7% (46·6–52·8) in men. Ultimately, obstructive sleep apnoea could be regarded as a spectrum of disease rather than a diagnosis with a rigid cutoff point because traditional definitions of obstructive sleep apnoea severity do not necessarily portray the risk of adverse outcomes.12 Treatment approaches to obstructive sleep apnoea could then be applied along a continuum, in the same way that serum cholesterol or bodyweight is treated, whereby more aggressive treatments could be used depending on the extent of the disease and the effectiveness of available interventions.
Data have been published on the risk of obstructive sleep apnoea in specific subsets of patients. For example, Patil and colleagues13 have shown high prevalence of obstructive sleep apnoea (>70%) in people with HIV. The mechanisms underlying this high prevalence have been debated, but the finding is probably indicative of the increased survival rates of individuals with HIV, the weight gain associated with antiretroviral therapy, and the side-effects associated with specific drugs used to treat HIV.14
Key messages.
The rise in prevalence of obstructive sleep apnoea is due to an increase in rates of obesity, among other factors, and more cases coming to the attention of physicians because of the wide availability of diagnostic equipment
The apnoea-hypopnoea index is an imperfect metric for the definition of obstructive sleep apnoea with respect to symptoms and outcomes
A diverse range of people have obstructive sleep apnoea for various reasons, which leads to the concept of personalised medicine and the need for individualised therapy
Pathogenesis
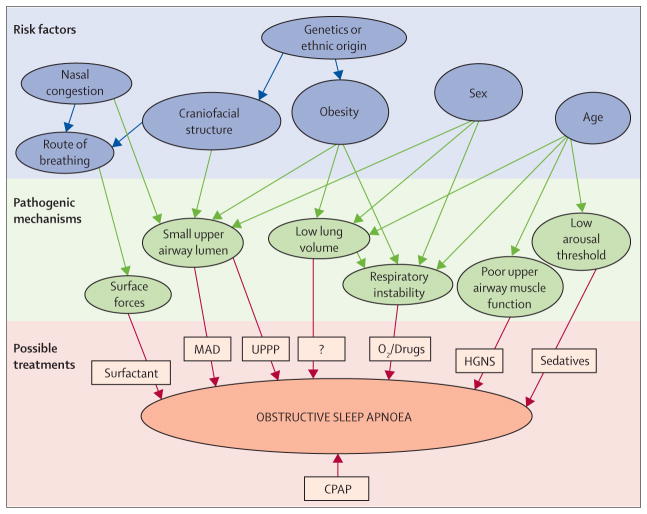
People with obstructive sleep apnoea have compromised pharyngeal anatomy but increased activation of the pharyngeal dilator muscle by protective reflexes during wakefulness that maintain pharyngeal patency until the onset of sleep, when these protective reflexes fail and the pharyngeal airway is susceptible to collapse. Obesity contributes to pharyngeal collapse especially in patients without robust responsiveness of the upper airway dilator muscle.15 Traditionally, obstructive sleep apnoea has been regarded as a disease of anatomical compromise coupled with dysfunction in pharyngeal dilator muscles during sleep.16 However, recent evidence has suggested that the mechanisms underlying apnoea are broad ranging and highly variable, such that different patients have varying degrees of abnormality in various pathophysiological factors.17–19 While in some patients the primary cause may be anatomical compromise of the pharyngeal airway, in other patients dysfunction in pharyngeal dilator muscles, unstable ventilatory control (elevated loop gain), or a low threshold for arousal from sleep might be important contributors towards the development of apnoea (figure 1).20,21
Figure 1. Risk factors, pathogenic mechanisms, and treatments for obstructive sleep apnoea.
Risk factors for obstructive sleep apnoea have long been recognised, but novel pathogenic mechanisms have now been detected in patients with the disorder. Although CPAP is the current treatment of choice irrespective of underlying cause, treatments based on tackling individual pathogenic mechanisms might prove a successful alternative approach in the future. CPAP=continuous positive airway pressure. MAD=mandibular advancement device. UPPP=uvulopalatopharyngoplasty. HGNS=hypoglossal nerve stimulation. Figure adapted from Jordan and colleagues,16 by permission of Elsevier.
Interventions targeting underlying abnormality are likely to be beneficial if individualised on the basis of the underlying mechanism. For example, uvulopalato-pharyngoplasty (UPPP) is beneficial for a subset of patients with anatomical compromise of the velopharynx.16,17 Similarly, efforts to increase hypoglossal nerve output, either by drug treatment or electrical stimulation, could be beneficial for those patients with pharyngeal dilator muscle dysfunction,17 and efforts to stabilise breathing control (with oxygen or aceta zolamide) will benefit patients with unstable ventilatory control as a major predisposing factor.22 Patients with a low arousal threshold wake up prematurely, have inadequate time for the accumulation of respiratory stimuli to activate pharyngeal dilator muscles, and have repetitive apnoea as a result.23,24 Agents that increase the arousal threshold improve obstructive sleep apnoea by allowing time for activation of pharyngeal dilator muscle.25,26 Some patients might have several abnormalities and require combination therapy to eliminate apnoea. Thus, a concept of personalised medicine is now emerging such that targeted therapy can be provided to people with obstructive sleep apnoea in an individualised manner on the basis of the underlying mechanism.17
One limitation of the personalised medicine approach has been the need for complex, invasive overnight testing to identify the mechanism underlying apnoea in each patient. Recent progress has been made such that clinicians now have readily available information to assess some of the pathophysiological traits. Terrill and colleagues27 used quantitative methods to assess control of breathing in clinical sleep recordings to establish the extent of the breathing abnormality. Instability in ventilatory control (loop gain) can be established by the response to a ventilatory disturbance, such as apnoea, which occurs spontaneously during obstructive sleep apnoea. Sleep studies can therefore be used to quantify the loop gain of a particular individual, which could enable clinicians to target loop gain without the need for complicated experiments. Such approaches are being assessed in clinical trials.27
Edwards and colleagues28 reported that more than 60% of the variance in arousal threshold can be determined by use of standard polysomnographic data. By combining the AHI, degree of oxygen desaturation, and number of hypopnoeas compared with apnoeas, Edwards and colleagues28 established which patients might respond to pharmacological manipulation of arousal threshold. Recent studies25,26 have shown substantial improvements in obstructive sleep apnoea by using a pharmacological approach to raise the arousal threshold in selected patients. Thus, a treating practitioner could stratify clinically the patients that are likely to respond to hypnotic therapy, although multicentre clinical trials will be needed to assess hard clinical outcomes before recommendations can be made.
Diagnosis
The diagnostic approach for obstructive sleep apnoea is transitioning from gold standard polysomnography in the sleep laboratory to home sleep testing (HST).29 This shift in diagnostic approach is driven in the USA by economic pressures, but many other countries such as the UK had already adopted an HST-focused approach. Data are increasingly compelling that diagnosis and treatment of patients can be well managed in the home. At present, HST is the diagnostic procedure of choice for most patients with suspected obstructive sleep apnoea. However, there are some caveats with the use of HST because most commonly used testing devices do not record sleep time or body position.
Sleep duration is not recorded during HST; practitioners are therefore reliant on total recording time rather than total sleep time to calculate time-derived indices such as AHI. In patients who sleep poorly or have comorbid insomnia, the AHI might be underestimated with HST because if the patient is awake, they are not likely to experience respiratory events. Thus, patients who have a high pre-test probability of obstructive sleep apnoea might benefit from either repeat HST or in-laboratory testing.
The relevance of respiratory events that occur during rapid eye movement (REM) sleep is controversial. Chami and colleagues30 showed that respiratory events that occur during REM sleep might be normal physiological variations and might not have clinical consequences. However, Mokhlesi and colleagues31 showed that REM-predominant obstructive sleep apnoea is associated with a risk of hypertension. These findings are clinically relevant because treatment of obstructive sleep apnoea is often limited to the first half of the sleep period leaving most of REM sleep untreated. Recent findings32 provide reassurance that HST can be used to guide PAP therapy regardless of the sleep stage in which the events are occurring.
Most HST devices do not reliably track body position, therefore, diagnosis of supine predominant obstructive sleep apnoea is less common with HST than with in-laboratory monitoring. As a result, positional therapy, which might be useful for some patients, is more difficult to apply. Positional therapy has traditionally been difficult to implement because of an inability to track postural manipulations in the home, particularly in long-term follow-up. Nonetheless, some patients do well with avoidance of supine posture either as primary therapy or as adjunctive therapy with oral devices or in patients who experience partial responses to surgical procedures.33
Most, but not all, HST devices have no reliable means to assess arousal from sleep. Arousal frequency might be the best predictor of impaired sleep-dependent memory consolidation in obstructive sleep apnoea.5 Recent findings have also suggested that fragmentation of sleep might have a role in glucose dysregulation and accelerated tumour growth. Indeed, Hakim and colleagues34,35 added to the growing body of published work that links sleep disturbance with tumour growth. Previous epidemiological data showed an important association between obstructive sleep apnoea and cancer risk,36 and more recent findings support a mechanistic role of sleep fragmentation in the absence of hypoxaemia on cancer growth. The clinical relevance of these findings, and sleep fragmentation in general, will require further study, but the absence of information on sleep arousal with HST is notable.37,38
The benefits of HST for patients with obstructive sleep apnoea are predicated on the use of auto-titration PAP (APAP) therapy for disease management. Adherence is similar for APAP and continuous positive airway pressure (CPAP) but auto-titration equipment is more expensive.32 Worse clinical outcomes have been reported for patients treated with APAP as compared to CPAP, on the basis of either sleep fragmentation or haemodynamic effects of variable intra-thoracic pressure. More data are needed to establish whether outcomes from APAP and CPAP are similar. At present, once the therapeutic pressure has been established, fixed pressure PAP is the treatment used for most patients.39
Much of the research done to date to understand the pathogenesis of obstructive sleep apnoea has relied on in-laboratory sleep recordings. The use of HST to derive mechanisms underlying obstructive sleep apnoea requires further development and study for clinical applicability.
Comorbidities
Obstructive sleep apnoea is very common in patients with other medical disorders such as chronic obstructive pulmonary disease (COPD), diabetes mellitus, atrial fibrillation, and congestive heart failure.40,41 New insights into many of these comorbid disorders have come to light, but we will focus on COPD in this Review.
The prevalence of clinically important obstructive sleep apnoea in people with severe airflow obstruction is much higher than previously suggested. Soler and colleagues42 reported that 57% of people with severe COPD referred for pulmonary rehabilitation had clinically important obstructive sleep apnoea. This finding is striking because overlap syndrome (obstructive sleep apnoea with COPD) has been associated with increased risk of death due to cardiovascular causes compared with COPD alone.40 Furthermore, the most common cause of death in COPD is probably cardiovascular-related disease, although the mechanisms underlying this association are unclear.42 New research has shown that patients with overlap syndrome have increased right ventricular mass compared with that of people with COPD only of matched severity.43 Dose-dependent use of nasal CPAP has been associated with decreased mortality;40 however, randomised clinical trials are needed to draw definitive conclusions. People with COPD frequently complain of sleep disturbances of various kinds and thus a comprehensive management strategy of these patients must take into account sleep problems given the prevalence and therapeutic options for these complaints.44
New research has also suggested a major role for nocturnal ventilation, using high levels of inspiratory pressure support or fully controlled modes, in patients with hypercapnia and COPD. Results from a large scale trial by Kohnlein and colleagues45 showed a mortality benefit with bilevel PAP (ie, differing inspiratory and expiratory pressures, rather than continuous pressure) compared with usual care for patients with hypercapnia and COPD in Germany and Austria. Despite many deaths of study participants during the trial period,45 hospital admissions among all participants were rare, which raises questions regarding the generalisability of these findings to a clinical practice setting. Regardless, these findings encourage the provision of bilevel therapy for patients with hypercapnic COPD, although the optimum therapy for people with overlap syndrome is unclear.
Clinical outcomes
Results from clinical trials show that nasal CPAP is the treatment of choice for obstructive sleep apnoea.46,47 CPAP can be transformative for some patients who experience substantial symptomatic improvement and reduced cardiometabolic risk. However, controversy remains with regards to the optimum therapy for CPAP-intolerant patients and identification of patients for whom CPAP therapy is indicated.
Elderly people with obstructive sleep apnoea are a unique group with a distinct physiological phenotype.48 The causes and consequences of obstructive sleep apnoea in this subset of patients have been debated.49 Data from observational studies and subgroup analyses have yielded equivocal results. Some data suggest that obstructive sleep apnoea has an adverse effect on elderly people,50 whereas others have noted no major adverse effects,51 or even a protective role with respect to mortality for obstructive sleep apnoea in octogenarians.52 McMillan and colleagues50 did a multicentre clinical trial examining the role of CPAP for the treatment of obstructive sleep apnoea in elderly people (aged 65 years and older). Results from the study showed a significant improvement in clinical outcome on the basis of the coprimary outcome measure of Epworth sleepiness score. Although the study was unblinded and used subjective outcomes, the results are compelling in view of the magnitude and consistency of the observed benefits. More data are needed, but on the basis of these new findings, nasal CPAP should be made available as a treatment choice for elderly people with obstructive sleep apnoea.
The association between obstructive sleep apnoea and hypertension has been studied extensively with various techniques. The effect of CPAP therapy on blood pressure compared with usual care and oxygen therapy was assessed in a clinical trial by Gottlieb et al.53 Results from the trial showed a slight reduction in blood pressure with CPAP therapy, which is consistent with results from previous studies and meta-analyses.37 Blood pressure was not affected in patients receiving oxygen therapy despite correction of hypoxaemia.53
Patients with refractory hypertension are thought to be particularly amenable to CPAP therapy. However, blood pressure reduction with CPAP in patients with obstructive sleep apnoea with refractory hypertension is similar to that seen in other populations with milder hypertension.54 Conversely, a decrease in blood pressure was noted with CPAP in an individual patient meta-analysis,55 even in patients with mild obstructive sleep apnoea. Predictors of blood pressure reduction with CPAP therapy in obstructive sleep apnoea in study-level meta-analyses include adherence with PAP therapy, severity of obstructive sleep apnoea, blood pressure elevation at baseline, young age, and daytime sleepiness.51,55,56 Since reduction of blood pressure is better with pharmacotherapy than with CPAP therapy,57 reasons other than daytime blood pressure must motivate treatment of obstructive sleep apnoea. For example, nocturnal surges in blood pressure are not typically captured in blood pressure trials but probably improved greatly with CPAP therapy in people with obstructive sleep apnoea.51,56 Furthermore, CPAP therapy also improves other symptoms of obstructive sleep apnoea, and might have additional benefits when compared with pharmacological treatment of blood pressure.58
Chirinos and colleagues59 examined weight loss as an adjunct to CPAP therapy to assess the role of C-reactive protein as a clinically important biomarker. The data showed major benefits with weight loss compared with CPAP therapy, although a combination of weight loss and CPAP treatment might be an optimum treatment approach for some patients. Medical and surgical weight loss strategies have been compared but the optimum weight loss strategy for patients with obstructive sleep apnoea is undefined.
Application of CPAP without the consideration of bodyweight is ill-advised, particularly since data show substantial weight gain in some patients after CPAP application.60 The mechanism underlying weight gain during CPAP treatment is unclear, but is probably multifactorial. Reduced work of breathing during CPAP treatment might contribute to weight gain owing to the associated reduction in metabolic expenditure.61 Hormonal changes following CPAP initiation have been reported, which might promote weight gain via changes in leptin, growth hormone, and other mediators. Furthermore, social behaviours often change after CPAP treatment; for example, patients who experience substantial benefits with treatment will often resume previous social activities, such as eating dinner with their spouse or going out for drinks with friends, which can contribute to weight gain. Regardless of the reason, diet and exercise advice should be provided to all patients with obstructive sleep apnoea. Respiratory physicians can no longer ignore management of bodyweight in these patients.
Treatment
CPAP is the gold standard treatment for patients with symptomatic obstructive sleep apnoea. CPAP has few major side-effects, and for most patients an initial trial with CPAP is recommended. Some patients have transformative benefits from CPAP,62,63 but new therapies or improvements in existing therapies for obstructive sleep apnoea are needed in view of the large number of patients who are intolerant of CPAP or avoid a diagnosis of obstructive sleep apnoea because of their concerns about therapy.63,64
Recent studies of hypoglossal nerve stimulation (HGNS) have rejuvenated enthusiasm for this approach to the treatment of obstructive sleep apnoea.65,66 The hypo glossal nerve innervates upper airway dilator muscles and electrical stimulation is thought to promote pharyngeal patency in patients with the disorder (figure 2). Results from an open-label clinical trial65 showed significant improvements in the AHI in patients with obstructive sleep apnoea who were selected to participate in the trial.65 Although the study was uncontrolled, results from a randomised withdrawal group of the study provided reassurance that the observed benefits were real.67 Questions remain about the proportion and type of patients that might respond to HGNS, whether the approach affects long-term hard (quantitative) clinical outcomes, and whether HGNS is cost effective, but recent approval of this treatment for obstructive sleep apnoea by the US Food and Drug Administration will drive use of this treatment.
Figure 2. Polysomnography during hypoglossal nerve stimulation.
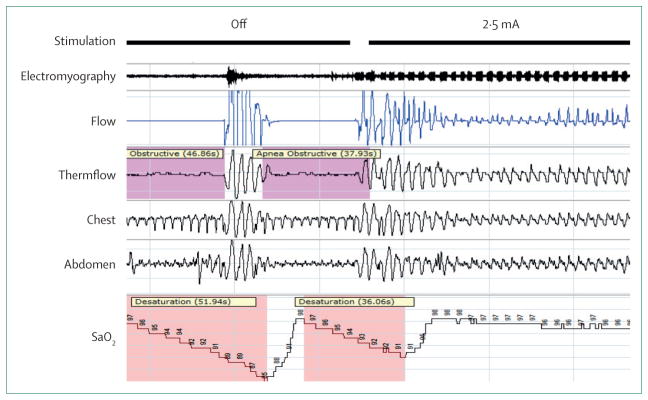
Initially, hypoglossal nerve stimulation is off, with periods of obstructive apnoea accompanied by oxygen desaturation and arousal. Stimulation is then turned on at a level above the capture threshold, in this example at 2·5 mA. Regular impulses can be seen in the electromyography. Improvement is noted in flow characteristics such that desaturation and arousal are no longer present, however, flow limitation remains. Variable response to hypoglossal nerve stimulation between patients emphasises the importance of refining patient selection. By permission of Inspire Medical Systems.
Recent clinical data have shown improvements in obstructive sleep apnoea with oral pressure therapy.68 Oral suction is used to pull the tongue and soft palate anteriorly to prevent posterior displacement of these structures during sleep. More studies to establish the proportion of patients responsive to therapy, improved methods to observe benefits, and careful patient selection are needed. Oral devices are an important second-line option for patients who are intolerant of positive airway pressure therapy.69 New data suggest that inexpensive non-adjustable mandibular splint devices can provide acceptable and cost-effective results.70
Where next?
An outdated assessment of obstructive sleep apnoea would be that obstructive sleep apnoea is a disease of obesity, the major goal of treatment is reduction in the AHI, and CPAP is the treatment of choice. In this Review, we have described how our understanding of obstructive sleep apnoea has progressed substantially in recent years as a result of improved diagnostic technologies, improved therapeutic approaches, and readily available clinical outcome data. Today, pathogenesis of obstructive sleep apnoea is recognised as multifactorial, improvement in AHI might not be the only indication for treatment and measure of success, and many alternative treatments have been proposed or developed.
Search strategy and selection criteria.
We reviewed articles published in peer-reviewed journals from Jan 1, 2014, to Dec 31, 2014, that related to obstructive sleep apnoea. Selection for inclusion was based on our expertise and our perception of the relevance and impact on the field of sleep medicine. We included older articles to provide background information and context.
Future work will focus on the causes of obstructive sleep apnoea at the individual patient level and therapy will be tailored accordingly. Sites of upper airway collapse will be localised for targeted therapy using either upper airway surgery or devices. Patients with non-anatomical traits, such as arousal threshold or loop gain, will be assessed with the use of clinically available data rather than data from specialised research studies and given appropriate treatment. When the underlying causes of obstructive sleep apnoea in an individual are established, an individualised treatment plan will need to be created. To do this, pharmacotherapy for non-anatomical traits will need to move from proof-of-principle to clinical trials. Similarly, the role of novel devices will need to be better defined. For example, how do devices manipulate the upper airway, what is the magnitude of the effect, and which patients best respond to such therapies?
Patient-centred outcomes will also increasingly have a role in treatment decisions. Reduction in the AHI and cardiovascular risk has been the primary goal of therapies so far. However, patients might be more concerned with the prevention of other comorbidities associated with obstructive sleep apnoea, such as diabetes or neuro-cognitive outcomes. Similarly, in obese patients with obstructive sleep apnoea, a comprehensive treatment plan will include focus on weight reduction.
Footnotes
Contributors
All authors contributed equally to the research, writing, editing, and preparation of the report, table, and figures.
Declaration of interests
AM and JEO declare no competing interests. RLO reports personal fees from Philips Respironics, outside of the submitted work.
References
- 1.Punjabi NM, Newman AB, Young TB, Resnick HE, Sanders MH. Sleep-disordered breathing and cardiovascular disease: an outcome-based definition of hypopneas. Am J Respir Crit Care Med. 2008;177:1150–55. doi: 10.1164/rccm.200712-1884OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahangdale S, Yeh SY, Novack V, et al. The influence of intermittent hypoxemia on platelet activation in obese patients with obstructive sleep apnoea. J Clin Sleep Med. 2011;7:172–78. [PMC free article] [PubMed] [Google Scholar]
- 3.Djonlagic I, Guo M, Matteis P, Carusona A, Stickgold R, Malhotra A. Untreated sleep-disordered breathing: links to aging-related decline in sleep-dependent memory consolidation. PLoS One. 2014;9:e85918. doi: 10.1371/journal.pone.0085918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Djonlagic I, Saboisky J, Carusona A, Stickgold R, Malhotra A. Increased sleep fragmentation leads to impaired off-line consolidation of motor memories in humans. PLoS One. 2012;7:e34106. doi: 10.1371/journal.pone.0034106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Punjabi NM, Beamer BA. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med. 2009;179:235–40. doi: 10.1164/rccm.200809-1392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strohl KP, Brown DB, Collop N, et al. An official American Thoracic Society Clinical Practice Guideline: sleep apnoea, sleepiness, and driving risk in noncommercial drivers. An update of a 1994 Statement. Am J Respir Crit Care Med. 2013;187:1259–66. doi: 10.1164/rccm.201304-0726ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward NR, Roldao V, Cowie MR, et al. The effect of respiratory scoring on the diagnosis and classification of sleep disordered breathing in chronic heart failure. Sleep. 2013;36:1341–48. doi: 10.5665/sleep.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;32:1230–35. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 9.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosselet JJ, Norman RG, Ayappa I, Rapoport DM. Detection of flow limitation with a nasal cannula/pressure transducer. Am J Respir Crit Care Med. 1998;157:1461–67. doi: 10.1164/ajrccm.157.5.9708008. [DOI] [PubMed] [Google Scholar]
- 11.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3:310–18. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 13.Patil SP, Brown TT, Jacobson LP, et al. Sleep disordered breathing, fatigue, and sleepiness in HIV-infected and -uninfected men. PLoS One. 2014;9:e99258. doi: 10.1371/journal.pone.0099258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darquenne C, Hicks CB, Malhotra A. The ongoing need for good physiological investigation: obstructive sleep apnoea in HIV patients as a paradigm. J Appl Physiol. 2015;118:244–46. doi: 10.1152/japplphysiol.00656.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sands SA, Eckert DJ, Jordan AS, et al. Enhanced upper-airway muscle responsiveness is a distinct feature of overweight/obese individuals without sleep apnoea. Am J Respir Crit Care Med. 2014;190:930–37. doi: 10.1164/rccm.201404-0783OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383:736–47. doi: 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malhotra A. Hypoglossal-nerve stimulation for obstructive sleep apnoea. N Engl J Med. 2014;370:170–71. doi: 10.1056/NEJMe1314084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnoea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owens RL, Edwards BA, Eckert DJ, et al. An integrative model of physiological traits can be used to predict obstructive sleep apnea and response to non positive airway pressure therapy. Sleep. 2014 doi: 10.5665/sleep.4750. published online Nov 9. pii: sp-00478-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnoea. Am J Respir Crit Care Med. 2001;163:1181–90. doi: 10.1164/ajrccm.163.5.2007013. [DOI] [PubMed] [Google Scholar]
- 21.Khoo M. Determinants of ventilatory instability and variability. Respir Physiol. 2000;122:167–82. doi: 10.1016/s0034-5687(00)00157-2. [DOI] [PubMed] [Google Scholar]
- 22.Horner RL, Hughes SW, Malhotra A. State-dependent and reflex drives to the upper airway: basic physiology with clinical implications. J Appl Physiol. 2014;116:325–36. doi: 10.1152/japplphysiol.00531.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanchina M, Malhotra A, Fogel RB, et al. Genioglossus muscle responsiveness to chemical and mechanical loading during NREM sleep. Am J Respir Crit Care Med. 2002;165:945–49. doi: 10.1164/ajrccm.165.7.2108076. [DOI] [PubMed] [Google Scholar]
- 24.Johnson KD, Patel SR, Baur DM, et al. Association of sleep habits with accidents and near misses in United States transportation operators. J Occup Environ Med. 2014;56:510–15. doi: 10.1097/JOM.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinzer RC, White DP, Jordan AS, et al. Trazodone increases arousal threshold in obstructive sleep apnoea. Eur Respir J. 2008;31:1308–12. doi: 10.1183/09031936.00067607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckert DJ, Owens RL, Kehlmann GB, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci. 2011;120:505–14. doi: 10.1042/CS20100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terrill PI, Edwards BA, Nemati S, et al. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur Respir J. 2014 doi: 10.1183/09031936.00062914. published online Oct 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edwards BA, Eckert DJ, McSharry DG, et al. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnoea. Am J Respir Crit Care Med. 2014;190:1293–300. doi: 10.1164/rccm.201404-0718OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuna ST, Gurubhagavatula I, Maislin G, et al. Noninferiority of functional outcome in ambulatory management of obstructive sleep apnoea. Am J Respir Crit Care Med. 2011;183:1238–44. doi: 10.1164/rccm.201011-1770OC. [DOI] [PubMed] [Google Scholar]
- 30.Chami HA, Baldwin CM, Silverman A, et al. Sleepiness, quality of life, and sleep maintenance in REM versus non-REM sleep-disordered breathing. Am J Respir Crit Care Med. 2010;181:997–1002. doi: 10.1164/rccm.200908-1304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mokhlesi B, Finn LA, Hagen EW, et al. Obstructive sleep apnoea during REM sleep and hypertension: results of the Wisconsin Sleep Cohort. Am J Respir Crit Care Med. 2014;190:1158–67. doi: 10.1164/rccm.201406-1136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powell ED, Gay PC, Ojile JM, Litinski M, Malhotra A. A pilot study assessing adherence to auto-bilevel following a poor initial encounter with CPAP. J Clin Sleep Med. 2012;8:43–47. doi: 10.5664/jcsm.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oksenberg A, Silverberg DS, Arons E, Radwan H. Positional vs nonpositional obstructive sleep apnoea patients: anthropomorphic, nocturnal polysomnographic, and multiple sleep latency test data. Chest. 1997;112:629–39. doi: 10.1378/chest.112.3.629. [DOI] [PubMed] [Google Scholar]
- 34.Hakim F, Wang Y, Zhang SX, et al. Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and TLR4 signaling. Cancer Res. 2014;74:1329–37. doi: 10.1158/0008-5472.CAN-13-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Almendros I, Wang Y, Becker L, et al. Intermittent hypoxia-induced changes in tumor-associated macrophages and tumor malignancy in a mouse model of sleep apnoea. Am J Respir Crit Care Med. 2014;189:593–601. doi: 10.1164/rccm.201310-1830OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farre R. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2012;186:190–94. doi: 10.1164/rccm.201201-0130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bakker JP, Montesi SB, Malhotra A. Obstructive sleep apnoea: new associations and approaches. Lancet Respir Med. 2013;1:e15–16. doi: 10.1016/S2213-2600(12)70059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kohli P, Sarmiento K, Malhotra A. Update in sleep medicine 2012. Am J Respir Crit Care Med. 2013;187:1056–60. doi: 10.1164/rccm.201302-0315UP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bakker JP, Marshall NS. Flexible pressure delivery modification of continuous positive airway pressure for obstructive sleep apnoea does not improve compliance with therapy: systematic review and meta-analysis. Chest. 2011;139:1322–30. doi: 10.1378/chest.10-2379. [DOI] [PubMed] [Google Scholar]
- 40.Stanchina ML, Welicky LM, Donat W, Lee D, Corrao W, Malhotra A. Impact of CPAP use and age on mortality in patients with combined COPD and obstructive sleep apnoea: the overlap syndrome. J Clin Sleep Med. 2013;9:767–72. doi: 10.5664/jcsm.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnoea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc. 2013;2:e000421. doi: 10.1161/JAHA.113.000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soler X, Gaio E, DeYoung P, et al. High prevalence of obstructive sleep apnea in patients with moderate to severe COPD. D39 connecting the dots: drawing lines between copd and comorbid conditions. American Thoracic Society. 2014:A5844-A (abstr). [Google Scholar]
- 43.Sharma B, Neilan TG, Kwong RY, et al. Evaluation of right ventricular remodeling using cardiac magnetic resonance imaging in co-existent chronic obstructive pulmonary disease and obstructive sleep apnoea. COPD. 2013;10:4–10. doi: 10.3109/15412555.2012.719050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soler X, Diaz-Piedra C, Ries AL. Pulmonary rehabilitation improves sleep quality in chronic lung disease. COPD. 2013;10:156–63. doi: 10.3109/15412555.2012.729622. [DOI] [PubMed] [Google Scholar]
- 45.Köhnlein T, Windisch W, Köhler D, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med. 2014;2:698–705. doi: 10.1016/S2213-2600(14)70153-5. [DOI] [PubMed] [Google Scholar]
- 46.Pepperell J, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359:204–10. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 47.Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet. 1999;353:2100–05. doi: 10.1016/S0140-6736(98)10532-9. [DOI] [PubMed] [Google Scholar]
- 48.Edwards BA, Wellman A, Sands SA, et al. Obstructive sleep apnoea in older adults is a distinctly different physiological phenotype. Sleep. 2014;37:1227–36. doi: 10.5665/sleep.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carlisle T, Carthy ER, Glasser M, et al. Upper airway factors that protect against obstructive sleep apnoea in healthy older males. Eur Respir J. 2014;44:685–93. doi: 10.1183/09031936.00177213. [DOI] [PubMed] [Google Scholar]
- 50.McMillan A, Bratton DJ, Faria R, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med. 2014;2:804–12. doi: 10.1016/S2213-2600(14)70172-9. [DOI] [PubMed] [Google Scholar]
- 51.Montesi SB, Edwards BA, Malhotra A, Bakker JP. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med. 2012;8:587–96. doi: 10.5664/jcsm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lavie P, Herer P, Peled R, et al. Mortality in sleep apnoea patients: a multivariate analysis of risk factors. Sleep. 1995;18:149–57. doi: 10.1093/sleep/18.3.149. [DOI] [PubMed] [Google Scholar]
- 53.Gottlieb DJ, Punjabi NM, Mehra R, et al. CPAP versus oxygen in obstructive sleep apnoea. N Engl J Med. 2014;370:2276–85. doi: 10.1056/NEJMoa1306766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnoea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310:2407–15. doi: 10.1001/jama.2013.281250. [DOI] [PubMed] [Google Scholar]
- 55.Bakker JP, Edwards BA, Gautam SP, et al. Blood pressure improvement with continuous positive airway pressure is independent of obstructive sleep apnoea severity. J Clin Sleep Med. 2014;10:365–69. doi: 10.5664/jcsm.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montesi SB, Bajwa EK, Malhotra A. Biomarkers of sleep apnoea. Chest. 2012;142:239–45. doi: 10.1378/chest.11-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pepin JL, Tamisier R, Barone-Rochette G, Launois SH, Levy P, Baguet JP. Comparison of continuous positive airway pressure and valsartan in hypertensive patients with sleep apnoea. Am J Respir Crit Care Med. 2010;182:954–60. doi: 10.1164/rccm.200912-1803OC. [DOI] [PubMed] [Google Scholar]
- 58.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnoea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chirinos JA, Gurubhagavatula I, Teff K, et al. CPAP, weight loss, or both for obstructive sleep apnoea. N Engl J Med. 2014;370:2265–75. doi: 10.1056/NEJMoa1306187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quan SF, Budhiraja R, Clarke DP, et al. Impact of treatment with continuous positive airway pressure (CPAP) on weight in obstructive sleep apnoea. J Clin Sleep Med. 2013;9:989–93. doi: 10.5664/jcsm.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stenlof K, Grunstein R, Hedner J, Sjostrom L. Energy expenditure in obstructive sleep apnoea: effects of treatment with continuous positive airway pressure. Am J Physiol. 1996;271:E1036–43. doi: 10.1152/ajpendo.1996.271.6.E1036. [DOI] [PubMed] [Google Scholar]
- 62.Weaver TE, Mancini C, Maislin G, et al. Continuous positive airway pressure treatment of sleepy patients with milder OSA: results of the CPAP Apnea Trial North American Program (CATNAP) randomized clinical trial. Am J Respir Crit Care Med. 2012;186:677–83. doi: 10.1164/rccm.201202-0200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwab RJ, Badr SM, Epstein LJ, et al. An official American Thoracic Society statement: continuous positive airway pressure adherence tracking systems. The optimal monitoring strategies and outcome measures in adults. Am J Respir Crit Care Med. 2013;188:613–20. doi: 10.1164/rccm.201307-1282ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin HS, Zuliani G, Amjad EH, et al. Treatment compliance in patients lost to follow-up after polysomnography. Otolaryngol Head Neck Surg. 2007;136:236–40. doi: 10.1016/j.otohns.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 65.Strollo PJ, Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnoea. N Engl J Med. 2014;370:139–49. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 66.Schwartz AR, Barnes M, Hillman D, et al. Acute upper airway responses to hypoglossal nerve stimulation during sleep in obstructive sleep apnoea. Am J Respir Crit Care Med. 2012;185:420–26. doi: 10.1164/rccm.201109-1614OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woodson BT, Gillespie MB, Soose RJ, et al. Randomized Controlled Withdrawal Study of Upper Airway Stimulation on OSA: Short- and Long-term Effect. Otolaryngol Head Neck Surg. 2014;151:880–87. doi: 10.1177/0194599814544445. [DOI] [PubMed] [Google Scholar]
- 68.Colrain IM, Black J, Siegel LC, et al. A multicenter evaluation of oral pressure therapy for the treatment of obstructive sleep apnoea. Sleep medicine. 2013;14:830–37. doi: 10.1016/j.sleep.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnoea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187:879–87. doi: 10.1164/rccm.201212-2223OC. [DOI] [PubMed] [Google Scholar]
- 70.Quinnell TG, Bennett M, Jordan J, et al. A crossover randomised controlled trial of oral mandibular advancement devices for obstructive sleep apnoea-hypopnoea (TOMADO) Thorax. 2014;69:938–45. doi: 10.1136/thoraxjnl-2014-205464. [DOI] [PubMed] [Google Scholar]