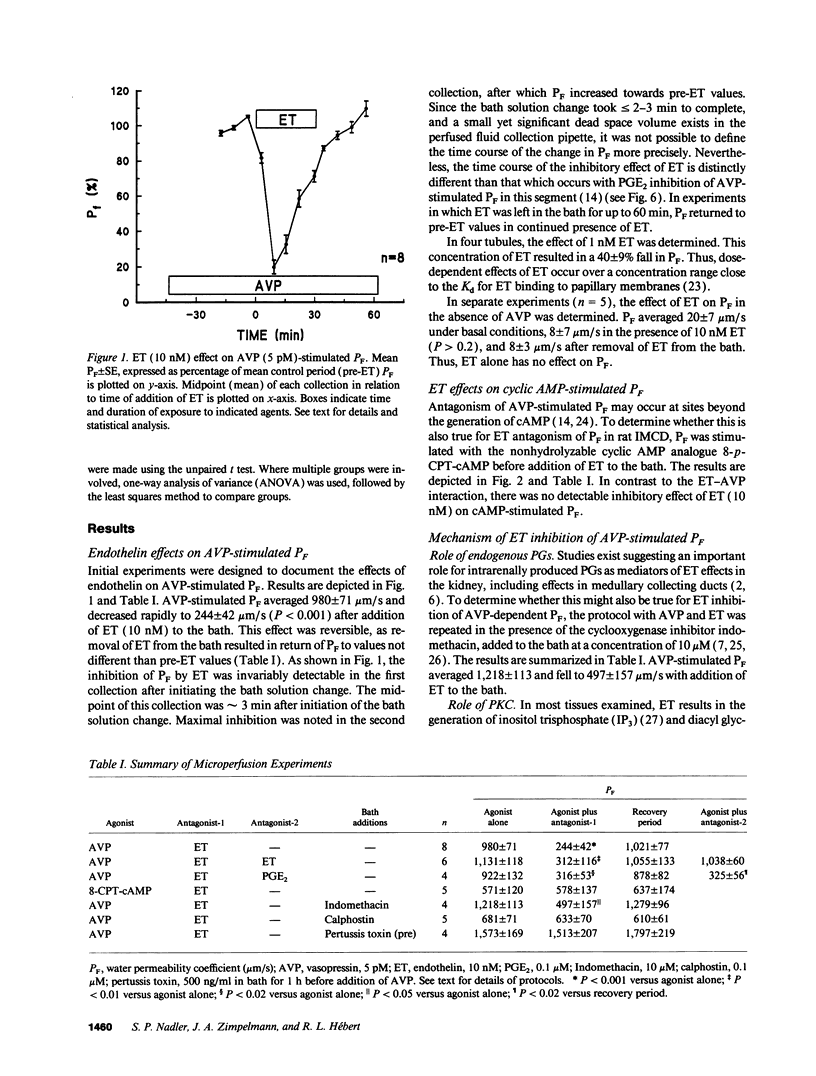
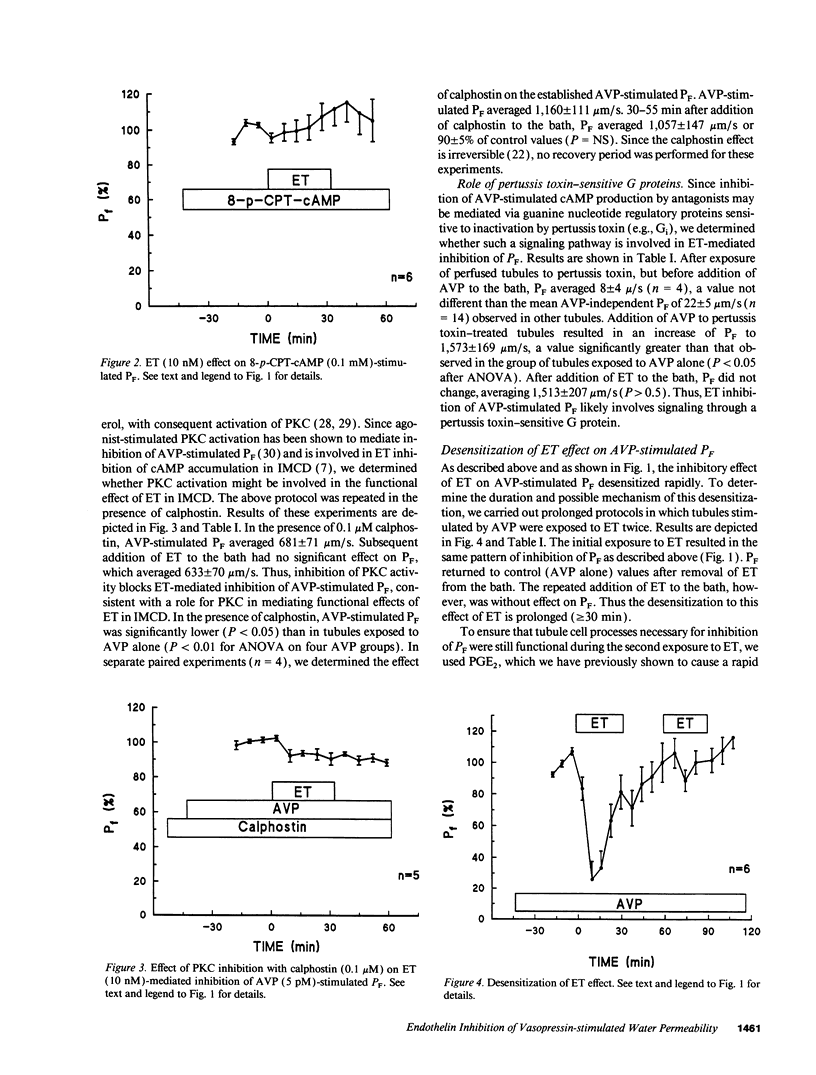
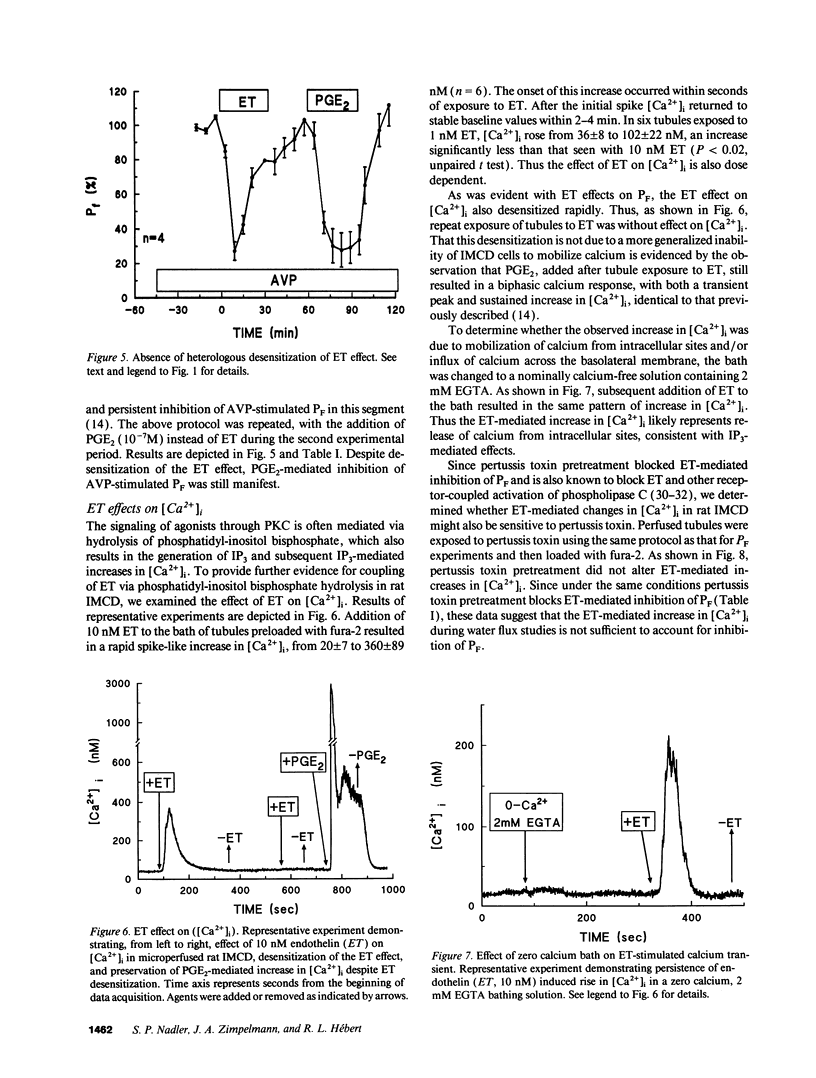
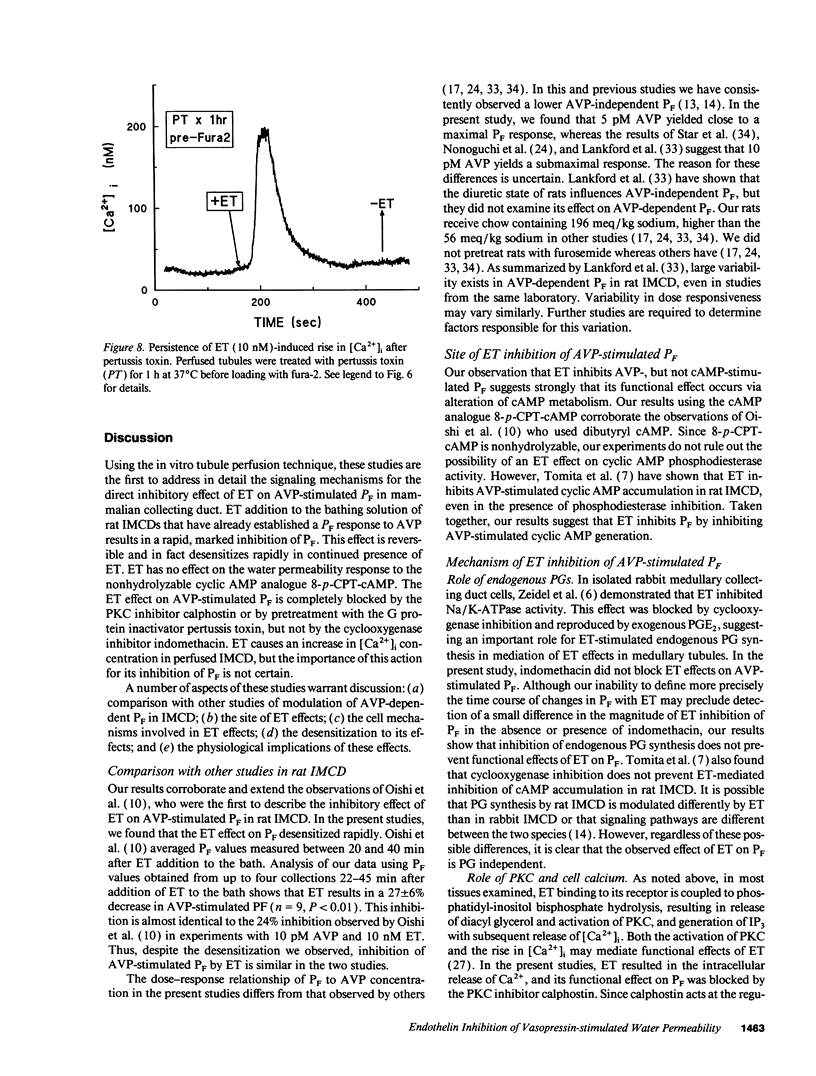
Abstract
Renal tubule solute and water transport is subject to regulation by numerous factors. To characterize direct effects of the recently discovered peptide endothelin (ET) on renal tubule transport, we determined signaling mechanisms for ET effects on vasopressin (AVP)-stimulated water permeability (PF) in rat terminal inner medullary collecting duct (IMCD) perfused in vitro. ET caused a rapid, dose-dependent, and reversible fall in AVP- but not cyclic AMP-stimulated PF, suggesting that its effect on PF is by inhibition of cyclic AMP accumulation. Indomethacin did not block ET actions, ruling out a role for prostaglandins in its effect. The protein kinase C (PKC) inhibitor calphostin, or pretreatment of perfused tubules with pertussis toxin, blocked ET-mediated inhibition of AVP-stimulated PF. ET caused a transient increase in intracellular calcium ([Ca2+]i) in perfused tubules, an effect unchanged in zero calcium bath or by PT pretreatment. ET effects on PF and [Ca2+]i desensitized rapidly. Inhibition of PF was transient and largely abolished by 20 min ET preexposure, and repeat exposure to ET did not alter [Ca2+]i. In contrast, PGE2-mediated inhibition of AVP-stimulated PF and increase of [Ca2+]i were sustained and unaltered by prior exposure of IMCD to ET. Thus desensitization to ET is homologous. We conclude that ET is a potent inhibitor of AVP-stimulated water permeability in rat terminal IMCD. Signaling pathways for its effects involve both an inhibitory guanine nucleotide-binding protein and phospholipase-mediated activation of PKC. Since ET is synthesized by IMCD cells, this peptide may be an important autocrine modulator of renal epithelial transport.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badr K. F., Murray J. J., Breyer M. D., Takahashi K., Inagami T., Harris R. C. Mesangial cell, glomerular and renal vascular responses to endothelin in the rat kidney. Elucidation of signal transduction pathways. J Clin Invest. 1989 Jan;83(1):336–342. doi: 10.1172/JCI113880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L., Brown A. M. G proteins and the mechanism of action of hormones, neurotransmitters, and autocrine and paracrine regulatory factors. Am Rev Respir Dis. 1990 Mar;141(3 Pt 2):S106–S114. doi: 10.1164/ajrccm/141.3_Pt_2.S106. [DOI] [PubMed] [Google Scholar]
- Breyer M. D., Jacobson H. R., Hebert R. L. Cellular mechanisms of prostaglandin E2 and vasopressin interactions in the collecting duct. Kidney Int. 1990 Oct;38(4):618–624. doi: 10.1038/ki.1990.251. [DOI] [PubMed] [Google Scholar]
- Bruns R. F., Miller F. D., Merriman R. L., Howbert J. J., Heath W. F., Kobayashi E., Takahashi I., Tamaoki T., Nakano H. Inhibition of protein kinase C by calphostin C is light-dependent. Biochem Biophys Res Commun. 1991 Apr 15;176(1):288–293. doi: 10.1016/0006-291x(91)90922-t. [DOI] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Clozel M., Fischli W., Guilly C. Specific binding of endothelin on human vascular smooth muscle cells in culture. J Clin Invest. 1989 May;83(5):1758–1761. doi: 10.1172/JCI114078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Effects of extracellular sodium on cytosolic calcium, PGE2 and cAMP in papillary collecting tubule cells. Kidney Int. 1991 Apr;39(4):591–597. doi: 10.1038/ki.1991.69. [DOI] [PubMed] [Google Scholar]
- Du Bois R., Vernoiry A., Abramow M. Computation of the osmotic water permeability of perfused tubule segments. Kidney Int. 1976 Dec;10(6):478–479. doi: 10.1038/ki.1976.135. [DOI] [PubMed] [Google Scholar]
- Flores A. G., Sharp G. W. Endogenous prostaglandins and osmotic water flow in the toad bladder. Am J Physiol. 1972 Dec;223(6):1392–1397. doi: 10.1152/ajplegacy.1972.223.6.1392. [DOI] [PubMed] [Google Scholar]
- Griendling K. K., Tsuda T., Alexander R. W. Endothelin stimulates diacylglycerol accumulation and activates protein kinase C in cultured vascular smooth muscle cells. J Biol Chem. 1989 May 15;264(14):8237–8240. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hirata Y., Yoshimi H., Takaichi S., Yanagisawa M., Masaki T. Binding and receptor down-regulation of a novel vasoconstrictor endothelin in cultured rat vascular smooth muscle cells. FEBS Lett. 1988 Oct 24;239(1):13–17. doi: 10.1016/0014-5793(88)80536-2. [DOI] [PubMed] [Google Scholar]
- Hébert R. L., Jacobson H. R., Breyer M. D. PGE2 inhibits AVP-induced water flow in cortical collecting ducts by protein kinase C activation. Am J Physiol. 1990 Aug;259(2 Pt 2):F318–F325. doi: 10.1152/ajprenal.1990.259.2.F318. [DOI] [PubMed] [Google Scholar]
- Hébert R. L., Jacobson H. R., Breyer M. D. Prostaglandin E2 inhibits sodium transport in rabbit cortical collecting duct by increasing intracellular calcium. J Clin Invest. 1991 Jun;87(6):1992–1998. doi: 10.1172/JCI115227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. J., Brenner B. M., Anderson S. Endothelin: a potent renal and systemic vasoconstrictor peptide. Am J Physiol. 1989 Jun;256(6 Pt 2):F1051–F1058. doi: 10.1152/ajprenal.1989.256.6.F1051. [DOI] [PubMed] [Google Scholar]
- Kohan D. E., Fiedorek F. T., Jr Endothelin synthesis by rat inner medullary collecting duct cells. J Am Soc Nephrol. 1991 Aug;2(2):150–155. doi: 10.1681/ASN.V22150. [DOI] [PubMed] [Google Scholar]
- Kon V., Badr K. F. Biological actions and pathophysiologic significance of endothelin in the kidney. Kidney Int. 1991 Jul;40(1):1–12. doi: 10.1038/ki.1991.172. [DOI] [PubMed] [Google Scholar]
- Kudo L. H., van Baak A. A., Rocha A. S. Effect of vasopressin on sodium transport across inner medullary collecting duct. Am J Physiol. 1990 May;258(5 Pt 2):F1438–F1447. doi: 10.1152/ajprenal.1990.258.5.F1438. [DOI] [PubMed] [Google Scholar]
- Lankford S. P., Chou C. L., Terada Y., Wall S. M., Wade J. B., Knepper M. A. Regulation of collecting duct water permeability independent of cAMP-mediated AVP response. Am J Physiol. 1991 Sep;261(3 Pt 2):F554–F566. doi: 10.1152/ajprenal.1991.261.3.F554. [DOI] [PubMed] [Google Scholar]
- Lee T. S., Chao T., Hu K. Q., King G. L. Endothelin stimulates a sustained 1,2-diacylglycerol increase and protein kinase C activation in bovine aortic smooth muscle cells. Biochem Biophys Res Commun. 1989 Jul 14;162(1):381–386. doi: 10.1016/0006-291x(89)92008-1. [DOI] [PubMed] [Google Scholar]
- Lee T. S., Chao T., Hu K. Q., King G. L. Endothelin stimulates a sustained 1,2-diacylglycerol increase and protein kinase C activation in bovine aortic smooth muscle cells. Biochem Biophys Res Commun. 1989 Jul 14;162(1):381–386. doi: 10.1016/0006-291x(89)92008-1. [DOI] [PubMed] [Google Scholar]
- Lin W. W., Lee C. Y., Chuang D. M. Endothelin- and sarafotoxin-induced phosphoinositide hydrolysis in cultured cerebellar granule cells: biochemical and pharmacological characterization. J Pharmacol Exp Ther. 1991 Jun;257(3):1053–1061. [PubMed] [Google Scholar]
- Martin E. R., Marsden P. A., Brenner B. M., Ballermann B. J. Identification and characterization of endothelin binding sites in rat renal papillary and glomerular membranes. Biochem Biophys Res Commun. 1989 Jul 14;162(1):130–137. doi: 10.1016/0006-291x(89)91972-4. [DOI] [PubMed] [Google Scholar]
- Nadler S. P., Hebert S. C., Brenner B. M. PGE2, forskolin, and cholera toxin interactions in rabbit cortical collecting tubule. Am J Physiol. 1986 Jan;250(1 Pt 2):F127–F135. doi: 10.1152/ajprenal.1986.250.1.F127. [DOI] [PubMed] [Google Scholar]
- Nadler S. P., Zimpelmann J. A., Hébert R. L. PGE2 inhibits water permeability at a post-cAMP site in rat terminal inner medullary collecting duct. Am J Physiol. 1992 Feb;262(2 Pt 2):F229–F235. doi: 10.1152/ajprenal.1992.262.2.F229. [DOI] [PubMed] [Google Scholar]
- Nonoguchi H., Sands J. M., Knepper M. A. Atrial natriuretic factor inhibits vasopressin-stimulated osmotic water permeability in rat inner medullary collecting duct. J Clin Invest. 1988 Oct;82(4):1383–1390. doi: 10.1172/JCI113742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi R., Nonoguchi H., Tomita K., Marumo F. Endothelin-1 inhibits AVP-stimulated osmotic water permeability in rat inner medullary collecting duct. Am J Physiol. 1991 Dec;261(6 Pt 2):F951–F956. doi: 10.1152/ajprenal.1991.261.6.F951. [DOI] [PubMed] [Google Scholar]
- Rüegg U. T., Burgess G. M. Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol Sci. 1989 Jun;10(6):218–220. doi: 10.1016/0165-6147(89)90263-0. [DOI] [PubMed] [Google Scholar]
- Sands J. M., Nonoguchi H., Knepper M. A. Hormone effects on NaCl permeability of rat inner medullary collecting duct. Am J Physiol. 1988 Sep;255(3 Pt 2):F421–F428. doi: 10.1152/ajprenal.1988.255.3.F421. [DOI] [PubMed] [Google Scholar]
- Sands J. M., Nonoguchi H., Knepper M. A. Vasopressin effects on urea and H2O transport in inner medullary collecting duct subsegments. Am J Physiol. 1987 Nov;253(5 Pt 2):F823–F832. doi: 10.1152/ajprenal.1987.253.5.F823. [DOI] [PubMed] [Google Scholar]
- Sato M., Dunn M. J. Osmolality, vasopressin-stimulated cAMP, and PGE2 synthesis in rat collecting tubule cells. Am J Physiol. 1986 May;250(5 Pt 2):F802–F810. doi: 10.1152/ajprenal.1986.250.5.F802. [DOI] [PubMed] [Google Scholar]
- Simonson M. S., Dunn M. J. Ca2+ signaling by distinct endothelin peptides in glomerular mesangial cells. Exp Cell Res. 1991 Jan;192(1):148–156. doi: 10.1016/0014-4827(91)90169-u. [DOI] [PubMed] [Google Scholar]
- Simonson M. S., Dunn M. J. Cellular signaling by peptides of the endothelin gene family. FASEB J. 1990 Sep;4(12):2989–3000. doi: 10.1096/fasebj.4.12.2168326. [DOI] [PubMed] [Google Scholar]
- Star R. A., Nonoguchi H., Balaban R., Knepper M. A. Calcium and cyclic adenosine monophosphate as second messengers for vasopressin in the rat inner medullary collecting duct. J Clin Invest. 1988 Jun;81(6):1879–1888. doi: 10.1172/JCI113534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuwa Y., Kasuya Y., Takuwa N., Kudo M., Yanagisawa M., Goto K., Masaki T., Yamashita K. Endothelin receptor is coupled to phospholipase C via a pertussis toxin-insensitive guanine nucleotide-binding regulatory protein in vascular smooth muscle cells. J Clin Invest. 1990 Mar;85(3):653–658. doi: 10.1172/JCI114488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki T., Takahashi I., Kobayashi E., Nakano H., Akinaga S., Suzuki K. Calphostin (UCN1028) and calphostin related compounds, a new class of specific and potent inhibitors of protein kinase C. Adv Second Messenger Phosphoprotein Res. 1990;24:497–501. [PubMed] [Google Scholar]
- Thomas C. P., Kester M., Dunn M. J. A pertussis toxin-sensitive GTP-binding protein couples endothelin to phospholipase C in rat mesangial cells. Am J Physiol. 1991 Mar;260(3 Pt 2):F347–F352. doi: 10.1152/ajprenal.1991.260.3.F347. [DOI] [PubMed] [Google Scholar]
- Tomita K., Nonoguchi H., Marumo F. Effects of endothelin on peptide-dependent cyclic adenosine monophosphate accumulation along the nephron segments of the rat. J Clin Invest. 1990 Jun;85(6):2014–2018. doi: 10.1172/JCI114667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]



