Abstract
Objective
To assess the incidence of giant cell arteritis (GCA) in the current era (2000-2009).
Methods
We extended the previously identified population-based cohort of Olmsted County, MN residents who fulfill 1990 ACR criteria for GCA (1950-1999) for another decade.
Results
In 2000-2009, 74 cases of GCA were identified (mean age: 78.1 years; 80% women; 79% temporal artery biopsy positive; 7 included based on radiological criteria). The incidence of GCA was 19.8 per 100,000 population.
Conclusions
The GCA incidence rates have remained steady since 1970, and the age at incidence, which was progressively increasing, appears to have reached a plateau.
Keywords: Giant cell arteritis, incidence
Introduction
Giant cell arteritis (GCA) is a primary vasculitis of uncertain etiology, usually involving granulomatous inflammation of the aorta and its major branches with a predilection for extra cranial branches of the carotid artery.(1, 2) It primarily affects women over 50 years of age and is most common in populations of northern European descent. The incidence increases with age with the highest incidence reported in those individuals in their seventh decade of life.
The incidence of GCA has varied widely across the world depending on the characteristics of the population, from 1.7/100,000 in Japan to 22 per 100,000 in Gothenburg, Sweden(3, 4) The incidence of GCA in Olmsted County, Minnesota has been previously reported for a fifty year period from 1950 until 1999 as 18.8 per 100,000 population.(5) With this present study we aimed to update the annual incidence rates for 2000 until 2009 and to compare and analyze the time trends over a sixty year time period spanning from 1950 until 2009.
Materials and Methods
The population of Olmsted County (mixed rural/urban) is well suited for studying the epidemiology of GCA since the county's population is overwhelmingly of northern European origin, the population among whom the disease is most common. Our comprehensive record linkage system (The Rochester Epidemiology Project) and its importance in the field of population-based studies has been widely credited in other major studies.(6)
Following the methodology of our previous study capturing all cases diagnosed from January 1, 1950 to December 31, 1999, additional cases were ascertained which were diagnosed between January 1, 2000 and December 31, 2009. We reviewed all patient records that showed a surgical index entry of temporal or occipital artery biopsy or a medical diagnosis of GCA between January 1, 2000 and December 31, 2009. The information about clinical manifestation, disease course and laboratory findings were collected and if the diagnosis was questionable, the records were reviewed by two rheumatologists and a consensus was reached.
The diagnosis was based on 1990 American College of Rheumatology criteria for classification of GCA.(7) Patients greater than 50 years of age with elevation of erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP) and computed tomography/angiography (CTA), magnetic resonance arteriography (MRA) or positron emission tomography (PET) evidence of large vessel vasculitis involving the ascending aorta and/or its branches were also included. Asymptomatic patients with incidental finding of aortitis on histopathologic examination of specimens obtained at aortic aneurysm repair or aortic valve replacement were not included. The medical records of all patients from the previous study were also reviewed and updated.(5)
Statistical Methods
Descriptive statistics (means, percentages, etc.) were used to summarize the data. Age- and sex-specific incidence rates were calculated using the number of incident cases as the numerator and population estimates based on decennial census counts as the denominator; linear interpolation was used to estimate population size for intercensal years. Overall rates were age- and sex-adjusted to the 2010 United States white population. Ninety-five percent confidence intervals (95% CIs) were computed for incidence rates assuming the incident cases follow a Poisson distribution. Annual incidence rates were illustrated using a 3-year centered moving average.
Results
A total of 74 patients were newly diagnosed with GCA from 2000 to 2009. Of these, a majority, 59 (80%), were women and 15 (20%) were men. Of 71 patients undergoing biopsy, the temporal artery biopsy was positive in 56 (79%), and negative in 15(21%). Among the 18 patients with missing or negative temporal artery biopsy, 7 patients were included based on positive radiologic criteria. The average time from symptom onset to diagnosis was 1.6 (SD 2.6) months.
The annual incidence of GCA in persons over the age of 50 was 19.8 (95% CI 15.2-24.3) per 100,000 population in 2000-2009, age and sex adjusted to 2010 US population (Table 1). As expected, the overall annual incidence rate was higher in women than in men, 27.0 (95% CI 20.0 to 33.9) versus 10.1 (95% CI 5.0 – 15.3). The annual incidence increased with advancing age, from 0.6/100,000 population in the 50-59 age groups to 73.9/100,000 in the over 80 age group.
Table 1. Annual incidence of giant cell arteritis among residents of Olmsted County, Minnesota, 2000-2009 per 100,000 population by sex and age group.
| Men | Women | Total | ||||
|---|---|---|---|---|---|---|
| Age Group | N | Rate (95% CI) | N | Rate (95% CI) | N | Rate (95% CI) |
| 50-59 | 0 | 0 | 1 | 1.2 | 1 | 0.6 |
| 60-69 | 3 | 6.3 | 8 | 15.3 | 11 | 11.0 |
| 70-79 | 6 | 20.5 | 22 | 61.4 | 28 | 43.1 |
| 80+ | 6 | 38.3 | 28 | 92.3 | 34 | 73.9 |
| Total | 15 | 10.1* (5.0, 15.3) |
59 | 27.0* (20.0, 33.9) |
74 | 19.8** (15.2, 24.3) |
Incidence per 100,000 population, age adjusted to the 2010 US white population aged ≥ 50 years.
Incidence per 100,000 population, age- and sex-adjusted to the 2010 US white population aged ≥ 50 years.
Comparison of annual incidence rates of GCA per 100,000 population (age and sex adjusted to the 2010 US Caucasian population age ≥50 years from 1950 to 2009) are illustrated in table 2 and figure 1. The overall annual incidence rates and incidence rates increased progressively from 5.0 (95% CI 0.8 to 9.2) in the decade 1950-1959 to 19.8 (95% CI 13.3 to 26.3) in 1970-1979. Around 1980, the incidence rates leveled off and have since remained steady with minimal fluctuations through 2009. These overall trends were similar in both men and women.
Table 2. Annual incidence of giant cell arteritis among residents of Olmsted County, Minnesota, 1950-2009 per 100,000 population by sex and decade.
| Men | Women | Total | ||||
|---|---|---|---|---|---|---|
| Time period | N | Rate* (95% CI) |
N | Rate* (95% CI) |
N | Rate* (95% CI) |
| 1950-59 | 1 | 1.7 (0.0, 5.0) | 5 | 7.5 (0.7, 14.3) | 6 | 5.0 (0.8, 9.2) |
| 1960-69 | 3 | 4.2 (0.0, 9.0) | 17 | 19.1 (9.9, 28.2) | 20 | 12.4 (6.9, 17.9) |
| 1970-79 | 10 | 15.3 (5.5, 25.1) | 27 | 23.7 (14.7, 32.8) | 37 | 19.8 (13.3, 26.3) |
| 1980-89 | 7 | 8.3 (2.0, 14.5) | 48 | 33.5 (24.0, 43.1) | 55 | 22.9 (16.8, 29.0) |
| 1990-99 | 16 | 15.6 (7.8, 23.4) | 40 | 24.1 (16.5, 31.7) | 56 | 20.0 (14.7, 25.3) |
| 2000-09 | 15 | 10.1 (5.0, 15.3) | 59 | 27.0 (20.0, 33.9) | 74 | 19.8 (15.2, 24.3) |
Incidence per 100,000 population, age adjusted to the 2010 US white population aged ≥ 50 years.
Incidence per 100,000 population, age- and sex-adjusted to the 2010 US white population aged ≥ 50 years.
Figure 1.
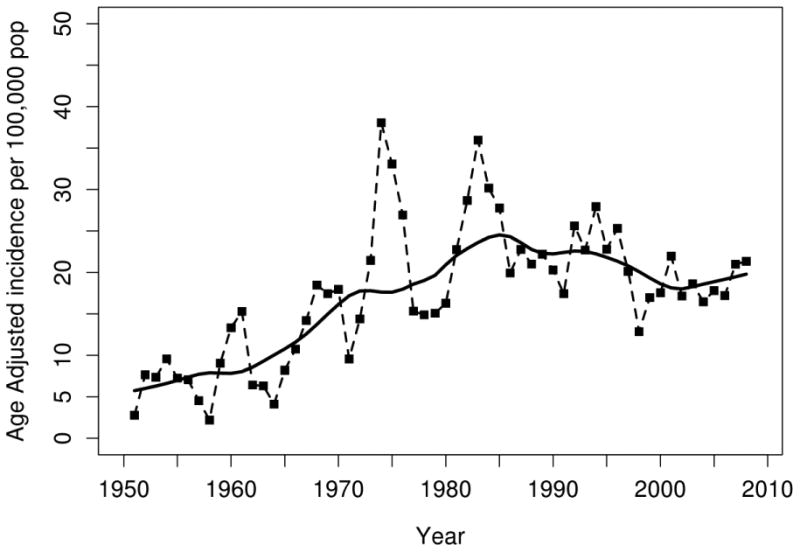
Age- and sex- adjusted (to US white 2010 population) incidence rates for GCA in Olmsted County, Minnesota, 1950-2009, per 100,000 persons age ≥ 50 years. Points plotted represent 3 year moving averages. Solid line estimated using smoothing techniques.
There was a significant cyclic pattern noticed until 1999 with peaks appearing every 7 to 10 years. The cases were clustered over six peak periods (1953-55, 1959-61, 1967-69, 1974-76, 1982-84 and 1992-95). No peak has emerged in the last decade of our study (2000 - 2009).
The trends in the age at the incidence of GCA between 1950 and 2009 were also analyzed. The average age at diagnosis has increased from 74.7 years in 1950-59 to 78.1 in 2000-09 (79.3 in 2000-04 and 77.1 in 2005-09). The average age at diagnosis has been progressively increasing from 1950 through 2009 but the trend seems to have levelled off after 2000.
Discussion
The incidence of GCA in the Olmsted County population was 19.8 per 100,000 population. This was almost identical to the annual incidence rate recorded from 1970 until 2000 which ranged from 19.8 to 22.9 per 100,000 population.(5) The annual incidence rates had been progressively increasing prior to 1980 and have remained relatively stable after that. Even among women the same trend of progression was noted with the incidence range remaining constant after 1979.
Even with the use of modern imaging diagnostic technologies such as MRA, PET and CTA, the incidence rates have remained almost unchanged in recent decades. This might be due to the fact that most patients suspected of having GCA present with cranial disease and/or polymyalgic symptoms, and occult large vessel disease is not often suspected in patients without these more typical cranial disease symptoms. In the current study, only 5% of case diagnoses were based on large vessel imaging alone.
A recent study from southern Sweden reported a decreasing trend in the annual incidence rates to 14.1 per 100, 000 population from 16 per 100, 000 population in 1991.(8) This difference may in part be because that study was based on positive temporal artery biopsies, the rate of which decreased significantly during the study period.(8) We did not see this trend toward decreasing incidence rates in our population from Olmsted County, Minnesota, which is also predominantly northern European in origin. The incidence rate in our current study has remained almost the same as in our previous studies and also the same as reported from some of the other studies from northern Europe.(4, 9-12) The most recent study from Gothenburg reports their incidence at 22 per 100,000 population in 1993.(4) Our incidence rates are much higher than those more recently reported in another population from Otago, New Zealand as 12.7 per 100,000 population in 2005.(13)
Our cohort is the population-based cohort of patients with GCA followed continuously for the longest period of time. Incident cases have been added to the cohort since 1950. Case ascertainment in this cohort is complete including both biopsy positive GCA as well as biopsy negative GCA, and our unique record linkage system with easy accessibility to all the health providers of Olmsted County minimizes the risk of under ascertainment of GCA cases as opposed to other studies using administrative data bases.(14)
It is possible that more frequent use of advanced imaging including PET, CTA and MRA may result in increased detection of GCA, and therefore increase the incidence rates for this disease in coming years. The role of advanced imaging in screening for large vessel inflammation in patients with GCA, or suspected of having GCA is not certain, nor is as yet the usefulness of advanced imaging as a criterion for the classification of GCA.
The age at onset of diagnosis of GCA has been steadily increasing from 1950 until 2004, even after adjusting for the increase in age of the general population.(15) There was a slight decrease from 79.3 years of age seen in 2000-04 to 77.1 in 2005-09. It is possible that the progressively increasing age trend at diagnosis has reached a plateau. The previously noted seasonal cyclic variation in the diagnosis has not been noticed in the last decade. The significance of this, particularly in terms of putative environmental influences on disease expression, is uncertain and is a topic for future research.
Incidence rates for GCA have remained fairly stable in the past three decades and the seasonal cyclic variation which suggested an environmental cause to a probable etiology for GCA was not noticed in the last 10 year period. The age at onset of the disease which was increasing seems to have leveled off.
Acknowledgments
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Funding: Research reported in this publication was made possible by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Salvarani C, Gabriel SE, O'Fallon WM, Hunder GG. The incidence of giant cell arteritis in Olmsted County, Minnesota: apparent fluctuations in a cyclic pattern. Ann Intern Med. 1995;123:192–4. doi: 10.7326/0003-4819-123-3-199508010-00006. [DOI] [PubMed] [Google Scholar]
- 2.Bas-Lando M, Breuer GS, Berkun Y, Mates M, Sonnenblick M, Nesher G. The incidence of giant cell arteritis in Jerusalem over a 25-year period: annual and seasonal fluctuations. Clin Exp Rheumatol. 2007;25(Suppl 44):15–7. [PubMed] [Google Scholar]
- 3.Kobayashi S, Yano T, Matsumoto Y, Numano F, Nakajima N, Yasuda K, et al. Clinical and epidemiologic analysis of giant cell (temporal) arteritis from a nationwide survey in 1998 in Japan: the first government-supported nationwide survey. Arthritis Rheum. 2003;49:594–8. doi: 10.1002/art.11195. [DOI] [PubMed] [Google Scholar]
- 4.Petursdottir V, Johansson H, Nordborg E, Nordborg C. The epidemiology of biopsy-positive giant cell arteritis: special reference to cyclic fluctuations. Rheumatology (Oxford) 1999;38:1208–12. doi: 10.1093/rheumatology/38.12.1208. [DOI] [PubMed] [Google Scholar]
- 5.Salvarani C, Crowson CS, O'Fallon WM, Hunder GG, Gabriel SE. Reappraisal of the epidemiology of giant cell arteritis in Olmsted County, Minnesota, over a fifty-year period. Arthritis Rheum. 2004;51:264–8. doi: 10.1002/art.20227. [DOI] [PubMed] [Google Scholar]
- 6.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173:1059–68. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122–8. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- 8.Mohammad AJ, Nilsson JA, Jacobsson LT, Merkel PA, Turesson C. Incidence and mortality rates of biopsy-proven giant cell arteritis in southern Sweden. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204652. [DOI] [PubMed] [Google Scholar]
- 9.Boesen P, Sorensen SF. Giant cell arteritis, temporal arteritis, and polymyalgia rheumatica in a Danish county. A prospective investigation, 1982-1985. Arthritis Rheum. 1987;30:294–9. doi: 10.1002/art.1780300308. [DOI] [PubMed] [Google Scholar]
- 10.Franzen P, Sutinen S, von Knorring J. Giant cell arteritis and polymyalgia rheumatica in a region of Finland: an epidemiologic, clinical and pathologic study, 1984-1988. J Rheumatol. 1992;19(2):273–6. [PubMed] [Google Scholar]
- 11.Noltorp S, Svensson B. High incidence of polymyalgia rheumatica and giant cell arteritis in a Swedish community. Clin Exp Rheumatol. 1991;9:351–5. [PubMed] [Google Scholar]
- 12.Baldursson Ó, Steinsson K, Björnsson J, Lie JT. Giant cell arteritis in Iceland. Arthritis & Rheumatism. 1994;37:1007–12. doi: 10.1002/art.1780370705. [DOI] [PubMed] [Google Scholar]
- 13.Abdul-Rahman AM, Molteno AC, Bevin TH. The epidemiology of giant cell arteritis in Otago, New Zealand: a 9-year analysis. N Z Med J. 2011;124:44–52. [PubMed] [Google Scholar]
- 14.Dunstan E, Lester SL, Rischmueller M, Dodd T, Black R, Ahern M, et al. Epidemiology of biopsy-proven giant cell arteritis in South Australia. Intern Med J. 2014;44:32–9. doi: 10.1111/imj.12293. [DOI] [PubMed] [Google Scholar]
- 15.Kermani TA, Schafer VS, Crowson CS, Hunder GG, Gabriel SE, Matteson EL, et al. Increase in age at onset of giant cell arteritis: a population-based study. Ann Rheum Dis. 2010;69:780–1. doi: 10.1136/ard.2009.111005. [DOI] [PubMed] [Google Scholar]


