1.0 Introduction
Over the past decade, there has been a substantial rise in the use of prescription medications for the management of patients with chronic pain. In addition to opioids, patients are often concurrently prescribed several other analgesic and non-analgesic medications, including antidepressants, anxiolytics/sedatives, anticonvulsants, muscle relaxants, and nonsteroidal anti-inflammatory drugs (NSAIDs) [8,13,18,44]. Despite the potential benefits of each of these medications for the management of patients with pain, it is well-known that the combination of a wide range of medications may lead to a number of adverse side effects, including nausea, dizziness, headaches, constipation, and weakness. These medication side effects are frequently observed in clinical settings and represent a complex pain management issue [8,11,18,21,35,65].
To date, the bulk of research that has been conducted on medication side effects has focused on the determinants of various side effect profiles. Surprisingly, little research has addressed the association between medication side effects and pain-related activity interference (e.g., reductions in daily life activities due to pain). Among patients with chronic pain, medication side effects have been found to be associated with decreased quality of life [2–3] and reduced treatment satisfaction [17,21,34], but the association between patients’ reports of side effects and pain-related activity interference remains largely unexplored. Until now, only one cross-sectional study has found that patients’ reports of side effects were associated with decreased daily functioning [65], while another one found that side effects were associated with decreased work productivity [2].
Another shortcoming of previous studies in this area is the lack of information on the relative (i.e., unique) association between reports of medication side effects and pain-related activity interference. In previous studies, this association has not been examined while controlling for other important variables known to be associated with activity interference, such as patient demographics [15,40,57], pain intensity [30,67,74,76], or negative affect [36,56,66,69,76]. Moreover, previous studies have not examined the variables that might moderate the association between reports of medication side effects and pain-related activity interference. For example, it is possible that medication side effects are associated with pain-related activity interference, but only among certain subgroups of patients, such as men or women, or only among patients experiencing high levels of pain intensity or negative affect. Further research investigating the association between reports of medication side effects and pain-related activity interference is of high clinical relevance, as it has implications for the pharmacological management of patients with pain conditions.
In the present study, 111 patients with chronic musculoskeletal pain were asked to provide, once a month for a period of six months, self-reports of medication use and the presence of any side effects associated with their medications. The primary purpose of the study was to examine the unique (i.e., independent) association between self-reports of medication side effects and pain-related activity interference. It was also of interest to examine whether the association between reports of medication side effects and pain-related activity interference was moderated by patient gender, pain intensity, or negative affect.
2.0 Methods
2.1. Participants
The Human Subjects Committee of Brigham and Women’s Hospital (BWH) approved the study procedures and written informed consent was obtained from every participant. This study was performed in a single, large, urban, university-based pain management center. All patients received a thorough history and underwent a physical examination by a pain physician at BWH. Patients included in the present study (n = 111) were part of a randomized clinical trial (RCT) of a behavioral intervention designed to improve prescription opioid compliance (for methods of the trial, see [29]). Among patients included in the present study, 32 % (n = 35) were part of the experimental treatment arm and 68 % (n = 76) were part of control arms. Given that the pattern of findings obtained in the present study did not differ as a function of treatment groups, data were collapsed and analyses were conducted on the overall sample.
Patients included in the present study met the following inclusion criteria: (1) chronic back or neck pain for more than 6 months, (2) able to speak and understand English, (3) prescribed opioid medication for more than 6 months, and (4) at risk of prescription opioid misuse based on their responses on the SOAPP-R [5] or based on past records of abnormal urine screens. Patients were excluded from participation if they met any of the following criteria: (1) current diagnosis of cancer, (2) acute osteomyelitis or acute bone disease, (3) diagnosis of any psychotic disorder, or (4) current substance abuse or dependence of any kind within the past year (i.e., positive on the Mini International Neuropsychiatric Interview (M.I.N.I. v.5.0; [61]). Patients with an active substance use disorder (SUD) were excluded given current clinical practice guidelines and principles at the BWH Pain Center regarding the management of patients with an active SUD. Patients with an active SUD are generally referred to a local addiction treatment facility before undergoing pain treatment at the Pain Center, and before being eligible for study participation.
2.2. Measures and procedures
2.2.1. Demographic questionnaire
During their first visit, patients were asked to complete a demographic questionnaire, which included information about age, gender, ethnicity, marital status, education level, and employment status.
2.2.2. Screening for substance use disorder
The Mini-International Neuropsychiatric Interview (M.I.N.I. v.5.0; [61]) was used to screen for active opioid addiction and any other active addiction disorder. The M.I.N.I is a brief structured interview for the major Axis I psychiatric disorders included in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). We used section K to assess the presence of a current non-alcohol psychoactive substance use disorder. Section K is designed to identify the use of (1) stimulants, (2) cocaine, (3) non-prescription opioids, (4) hallucinogens, (5) heroin, (6) inhalants, (7) marijuana, (8) non-prescription tranquilizers, and (9) other substances of abuse. The M.I.N.I was administered and scored by a trained research assistant. The M.I.N.I has been shown to be a reliable and valid screening tool for substance use disorders in patients with and without chronic pain conditions [22,29,53,61].
2.2.3. Self-reports of medication use, medication side effects, pain intensity, negative affect, and pain-related activity interference
Using a personal digital assistant (PDA; Hewlett Packard @ IPAQ) running the Pain Electronic Calendar software (New England Research Institute, Watertown, MA, USA), patients were asked to provide, once a month for a period of six months, self-reports of medication use, medication side effects, pain intensity, negative affect, and pain-related activity interference. As will be seen below, all ratings were made on 0 to 10 visual analog scales (VAS). These ratings were then automatically converted and stored on a 0–100 scale.
2.2.3.1. Self-reports of medication use
Using the PDA, patients were asked to report which medications they were currently taking (see Fig. 1). Patients’ reports of medication were verified by a research assistant using the electronic medical record system, and published tables were used to convert opioid doses into morphine equivalents (ME).
Figure 1.
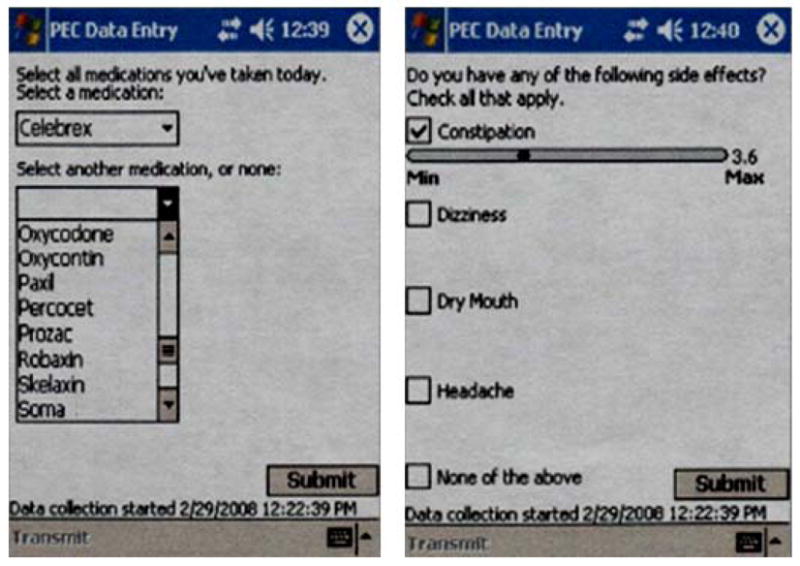
Sample of a personal digital assistant (PDA) screen used to asses medication use and perceived medication side effects
2.2.3.2. Self-reports of medication side effects
Using the PDA, patients were asked to report whether they were currently experiencing any of the following medication side effects: (1) nausea (2) constipation (3) headaches (4) dry mouth (5) itching (6) sneezing (7) sweating (8) weakness (9) dizziness (10) confusion (11) memory problems (12) visual problems. Patients were then asked to rate the intensity of each perceived medication side effect on a VAS that ranged from 0 (minimum) to 10 (maximum). Patients were not asked to provide ratings of medication side effects based on a specific medication, but rather based on all the medications they were taking. Given that chronic pain patients are known to use a wide range of medications concurrently [18–19,33,59], this global medication side effects assessment was assumed to be the most adequate. Self-reports are currently the most commonly used method for assessing side effects among ambulatory patients, whether in the context of observational studies [1,3,77] or randomized controlled trials [21,35,64]. Items used to assess medication side effects in our study are similar to those that have been used in previous work, and the proposed medication side effects rated on the PDA have been found to be among the most frequent side effects associated with opioid and non-opioid analgesic medications [21,24,34,46].
2.2.3.3. Self-reports of pain intensity
Patients were asked to rate their current level of pain on a VAS that ranged from 0 (no pain) to 10 (worst pain possible). This measure is an adaptation of the standard visual analog scale commonly used in the Brief Pain Inventory [68] to assess pain intensity levels among patients with chronic pain.
2.2.3.4. Self-reports of negative affect
Patients were asked to rate their current level of anxiety (“How tense and anxious have you been today?”) and depression (“How depressed and discouraged have you been today?”) on a VAS that ranged from 0 (not much) to 10 (very much). These two measures have been used in our previous studies in order to assess negative affect among patients with chronic pain [27–28,48,50,81].
2.2.3.5. Self-reports of pain-related activity interference
Patients were asked to rate the degree to which pain interfered with their daily routine activities, outdoor activities, and social activities. Ratings were made separately for these three items on a VAS that ranged from 0 (not much) to 10 (very much). Higher scores on these items represent greater pain-related activity interference. These items have been used in our previous studies conducted among patients with chronic pain [28,48–49].
2.3 Data reduction and analysis
Given the high inter-correlations (ranging from .73 to .80; Cronbach’s α = 0.91) among the three items of activity interference, ratings on these items were averaged to create a single index of pain-related activity interference. Consistent with previous research [27,50,79–80], an index of negative affect was also computed by averaging patients’ ratings of anxiety and depression.
The total number of medications used by patients was calculated by summing, for each patient, the different types of medications being used at each of the assessment time points. A medication side effect index (MSE-I) was computed by summing the intensity of each medication side effect being reported. This index was derived in order to simultaneously take into account the number as well as the intensity of medication side effects being reported by patients. Previous studies have highlighted the importance of assessing both the incidence as well as the intensity of medication side effects [24,35,47,54]. This represents a more clinically meaningful medication side effect assessment than assessing the incidence or the intensity of medication side effects independently.
Descriptive data were computed using IBM-SPSS v.21 (Chicago, IL, USA). Descriptive data for continuous variables were presented as means and standard deviations and were analyzed using independent samples t-tests. Descriptive data for categorical variables were presented as percentages and were analyzed using chi-square tests.
Multilevel modeling analyses were conducted using the MIXED command in IBM-SPSS. Multilevel modeling was well-suited to handle the hierarchical nested data structure of the present study, in which repeated monthly assessments (Level 1 units) were nested within participants (Level 2 units). Multilevel modeling was also well-suited to handle the unequal number of data points across participants due to missing data. Given that multilevel modeling can account for unbalanced data set and/or missing data [52,63], all 111 participants could be included in multilevel analyses without using any data imputation procedure.
Across all assessment time points, compliance in our study was high, with an overall completion rate of 87 %. For example, when combining the four main Level 1 variables that were assessed on a monthly basis in this study (i.e., medication side effects, pain-related activity interference, pain intensity, negative affect), there was a total of 2664 possible data points (111 participants * 6 waves/time points * 4 variables). A total of 2338 data points were observed (87%), with varying response rates as a function of waves. Response rates were as follows: Wave 1 (100 %); Wave 2 (99.3 %); Wave 3 (95.5 %); Wave 4 (89.1 %); Wave 5 (75.6 %); Wave 6 (62.8 %). For any given wave, there was a total of 444 possible data points (111 * 4 variables); the lowest/minimum response rate was observed at Wave 6 (279/444; 62.8 %) whereas the highest/maximum response rate was observed at Wave 1 (444/444; 100 %). Analyses indicated that patients with and without missing data did not differ significantly in terms of age, t (109) = −.34, ns, gender, X2 (1) = .01, ns, or in any other demographic variable (all ps > .05). Also, patients with and without missing data did not differ significantly on any of the main study variables, such as pain t (109) = 1.6, ns, negative affect, t (109) = .54, ns, medication side effects, t (109) = .19, ns, or pain-related activity interference, t (109) = .83, ns.
For each independent variable of interest (i.e., perceived medication side effects, pain intensity, negative affect), Level 1 observations (i.e., scores) were centered within participants, a procedure that has been referred to as group-mean centering [16,51]. This was done by computing, for each participant, the mean of Level 1 scores across assessment time points. Mean scores were then subtracted from monthly scores, resulting in a set of “deviation scores” for each independent variable. These scores represent the extent to which a participant, on a given month, deviated from his/her own mean on a specific independent variable [16,39]. Deviation scores allowed us to examine whether elevations on independent variables of interest (e.g., perceived medication side effects) were associated with the study outcome even when controlling for month-to-month changes in relevant covariates. Deviation scores based on this centering procedure also permitted an interpretation of the intercept on the basis of participants’ means on each independent variable [7,16].
Model building was initially guided by examination of variance components for the unconditional (null) models of the main study outcome (i.e., pain-related activity interference). Examination of variance components indicated that the within-person variability in pain-related activity interference was 316.40 (Z = 13.41, p < .001), whereas the between-person variability was 253.94 (Z = 5.47, p < .001). The intraclass correlation (ICC) was 0.45, indicating that approximately 45% and 55% of the total variance in ratings of pain-related activity interference can be explained by between-person variability (Level 2) and within-person variability (Level 1), respectively. Given the significant variance components and results from the ICC, it was thus appropriate to model both between- and within-person variance in pain-related activity interference.
In order to examine the unique (i.e., independent) association between perceived medication side effects and pain-related activity interference, a multilevel model was built using the pain-related activity interference index as the dependent variable. In this model, patient gender (Level 2), pain intensity (Level 1), and negative affect (Level 1) were first added simultaneously to the model as independent variables. The medication side effect index (MSE-I) was then added to the model as a Level 1 independent variable, which permitted examination of the independent association between reports of medication side effects and pain-related activity interference.
In order to examine the potential moderators of the association between medication side effects and pain-related activity interference, three distinct multilevel models were built using the activity interference index as the dependent variable. We were particularly interested in examining whether the association between reports of medication side effects and pain-related activity interference was moderated by patient gender (Level 2), pain intensity (Level 1), or negative affect (Level 1). In these models, two-way interaction terms between the MSE-I and each of the other independent variables (i.e., patient gender, pain intensity, negative affect) were specified. These interaction terms were included in three separate models, after the inclusion of appropriate main effects. Any significant two-way interaction effect would suggest that the association between self-reports of medication side effects and pain-related activity interference is moderated by patient gender, pain intensity, or negative affect.
All the multilevel models that were built in this study followed a sequential procedure [38,58,78], which first involved specifying a random intercept and fixed effects for independent variables. When significant fixed effects emerged, slopes were then treated as random effects, and model fit was re-evaluated using the likelihood ratio test. Random parameters were dropped if they resulted in a significantly worse model fit [60,62–63]. All models were carried out using maximum-likelihood estimation and included a first-order autoregressive variance-covariance matrix (AR1) in order to account for autocorrelations between repeated assessments. As recommended [25,52], effect sizes were estimated by calculating the percentage reduction in unexplained variance at both the between- and within-person level, relative to the unexplained variance of the null model. This measure of explained variance is analogous to the R2 value traditionally reported when conducting linear regression [25–26,52].
3.0 Results
3.1. Descriptive statistics
Descriptive statistics for study measures are presented in Table 1. On average, across assessment time points, men and women did not differ significantly in pain intensity, negative affect, or pain-related activity interference (all p’s > .05). However, men and women differed significantly on the medication side effect index, with self-reports of medication side effects being greater among women than men, t (109) = 2.1, p < .05.
Table 1.
Sample characteristics and descriptive data for main study variables
| Variables | Overall sample | Men (n = 61) | Women (n = 50) | p |
|---|---|---|---|---|
| Age | 48.17 (8.60) | 48.08 (8.43) | 48.28 (8.90) | ns |
| Ethnicity (% white) | 78 % | 77 % | 80 % | ns |
| Marital status (% married/relation) | 50 % | 52 % | 49 % | ns |
| Employment (% unemployed) | 71 % | 71 % | 71 % | ns |
| Education (years) | 12.46 (3.63) | 12.27 (3.77) | 12.68 (3.48) | ns |
| Pain intensity | 59.49 (16.01) | 58.25 (16.72) | 61.01 (15.14) | ns |
| Negative affect | 44.25 (22.68) | 44.03 (20.90) | 44.52 (24.90) | ns |
| Medication side effects (MSE-I) | 124.11 (113..85) | 103.90 (93.78) | 148.76 (131.15) | <.05 |
| Pain-related activity interference | 66.31 (18.51) | 65.80 (18.97) | 66.95 (18.11) | ns |
Note. Values in parentheses are standard deviations; MSE-I, Medication side effect index; ns, nonsignificant.
Table 2 shows the different types of medications that were used by patients included in our study. In addition to prescription opioids (100 %; average daily opioid dose: mean = 141.2; SD = 209.6), the most common medications used by patients over the course of the study were antidepressants (41 %), anticonvulsants (35 %), anxiolytics/sedatives (29 %), muscle relaxants (26 %), and non-steroidal anti-inflammatory drugs (24 %). These different types of medications are representative of medications that are prescribed to patients treated in tertiary pain centers [9,18,43,73]. Analyses indicated that reports of medication side effects were not significantly higher among those who used, in addition to prescription opioids, either antidepressants, t (109) = −.94, ns, anticonvulsants, t (109) = −.32, ns, anxiolytics/sedatives, t (109) = −.28, ns, muscle relaxants, t (109) = −.54, ns, or non-steroidal anti-inflammatory drugs, t (109) = −.88.
Table 2.
Number of patients using the different types of medications
| Opioids | 111 (100 %) |
| Antidepressants | 46 (41 %) |
| Anticonvulsants | 39 (35 %) |
| Muscle relaxants | 29 (26 %) |
| Anxiolytics/sedatives | 32 (29 %) |
| NSAIDs | 27 (24 %) |
Prior to specifying the effects of independent variables on the study outcome in multilevel models, the influence of demographic (i.e., age, ethnicity, education, marital status, employment status) and medication regimen (i.e., total number of medications) variables on pain-related activity interference was examined. Results indicated that employment status was significantly associated with pain-related activity interference, with unemployed patients reporting greater pain-related activity interference scores than employed patients, F (1, 106) = 12.07, p < .001. None of the other variables were significantly associated with pain-related activity interference (all p’s > .05). Employment status was thus the only covariate retained in the multilevel model reported here (see footnote 1).
3.2 Association between self-reports of medication side effects and pain-related activity interference
As can been seen in Table 3, a multilevel model was built using the activity interference index as the dependent variable. Patient gender, pain intensity (Level 1), and negative affect (Level 1) were first added simultaneously to the model as independent variables. Results indicated that the main effect of patient gender on pain-related activity interference was not significant, B = 2.03, SE = 3.38, ns. However, the main effect of pain intensity was significant (B = .47, SE = .05, p < .001), indicating that month-to-month increases in pain were associated with higher ratings of pain-related activity interference. The main effect of negative affect was also significant (B = .13, SE = .04, p < .001), indicating that month-to-month increases in negative affect were associated with higher ratings of pain-related activity interference. Results from a likelihood ratio test indicated that inclusion of pain intensity and negative affect as random effects resulted in a significantly worse model fit, χ2 (2) = 45, p < .001; random parameters were thus dropped from the final model.
Table 3.
Multilevel model examining the unique association between perceived medication side effects and pain-related activity interference
| Fixed effects | β | SE | df | t | p |
|---|---|---|---|---|---|
| Intercept | 56.32 | 3.54 | 107 | 15.9 | <.001 |
| Gender | 1.95 | 3.40 | 114 | .58 | ns |
| Pain intensity | .47 | .05 | 511 | 10.35 | <.001 |
| Negative affect | .12 | .04 | 509 | 3.40 | <.005 |
| Medication side effect index (MSE-I) | .02 | .01 | 499 | 2.07 | <.05 |
| Random effects: Covariance parameters | Subject | β | SE | z | p |
|---|---|---|---|---|---|
| Intercept | ID | 261.09 | 43.73 | 5.97 | <.001 |
| AR (1) | ID | .15 | .06 | 2.68 | <.01 |
| Residuals | ID | 225.49 | 16.24 | 13.88 | <.001 |
Note. Values are from the final model. Independent variables are within-person centered. Employment status was included as a covariate. MSE-I = Medication side effect index; β = unstandardized regression coefficient; SE = Standard error; ns = nonsignificant.
In order to examine the unique (i.e., independent) association between self-reports of medication side effects and pain-related activity interference, the medication side effect index (MSE-I) was subsequently added to the model as a Level 1 independent variable. Results indicated that month-to-month increases in perceived medication side effects were associated with higher ratings of pain-related activity interference, B = .02, SE = .01, p < .05. A likelihood ratio test indicated that inclusion of the MSE-I as a random effect resulted in a significantly worse model fit, χ2 (1) = 62, p < .001; this random parameter was thus dropped from the final model. The within-person pseudo R2 after the inclusion of the MSE-I was .03. Taken together, results from this model indicate that the association between self-reports of medication side effects (MSE-I) and pain-related activity interference was significant even after controlling for the main effects of patient gender, pain intensity, and negative affect.
Given that inspection of variance components from the previous model revealed substantial between-person variability in mean pain-related activity interference scores (i.e., intercept) (B = 261.09, p < .001), a follow-up model was built in order to examine whether the association between self-reports of medication side effects and pain-related activity interference remained significant even after controlling for all potential between-person variables (i.e., “observed” or “unobserved”) that could have been responsible for this association. While the previous analysis controlled for month-to-month changes in pain and negative affect when examining the association between perceived side effects and pain-related activity interference, a wide range of pre-existing (i.e., time-invariant) variables could have also been responsible for this association. We thus retained all the parameters from the previous model, but we controlled for the influence between-person variables by adding each participant (i.e., ID) as a fixed effect in the model (see Footnote 2). This model building strategy allowed us to control for the influence of any “observed” or “unobserved” between-person variables on the study outcome [4,42,55]. Results from this model indicated that the main effect of medication side effects (MSE-I) on pain-related activity interference remained significant even after controlling for the fixed effects of participants (B = .02, SE = .01, p < .01). Taken together, results from this model suggest that the association between perceived medication side effects and pain-related activity interference cannot be entirely accounted for by any potential “observed” or “unobserved” between-person variables.
3.3. Moderators of the association between patients’ reports of medication side effects and pain-related activity interference
Three distinct multilevel models were built in order to examine whether patient gender, pain intensity (Level 1), or negative affect (Level 1) moderated the association between self-reports of medication side effects and pain-related activity interference. As can be seen in Table 4, models were built using the activity interference index as the dependent variable. In all these models, two-way interaction terms between the MSE-I and each of the other independent variables (i.e., patient gender, pain intensity, negative affect) were specified and were preceded by appropriate main effects. Results indicated that two-way interaction effects between the MSE-I and patient gender (B = .001, SE = .020, ns), pain intensity (B = .000, SE = .001, ns), and negative affect (B = .001, SE = .001, ns) were not significant. Taken together, results from these models indicated that the association between reports of medication side effects and pain-related activity interference was not moderated by any of these variables.
Table 4.
Multilevel model examining the potential moderators of the association between perceived medication side effects and pain-related activity interference
| Fixed effects | β | SE | df | t | p |
|---|---|---|---|---|---|
| Model 4a | |||||
| MSE-I x gender | .001 | .020 | 523 | .05 | ns |
| Model 4b | |||||
| MSE-I x pain intensity | .000 | .001 | 551 | .38 | ns |
| Model 4c | |||||
| MSE-I x negative affect | .001 | .001 | 562 | 1.91 | ns |
Note. Values are from the final multilevel model. Level 1 independent variables are within-person centered. In all these models, appropriate main effects preceded the modeling of each interaction effect. MSE-I = Medication side effect index;
β = unstandardized regression coefficient; SE = Standard error; ns = nonsignificant.
4.0 Discussion
The primary purpose of the present study was to examine the unique (i.e., independent) association between reports of medication side effects and pain-related activity interference in a sample of patients with chronic pain. It was also of interest to examine the potential role of patients’ gender, pain intensity, and negative affect as moderators of the association between self-reports of medication side effects and pain-related activity interference.
In our study, we examined the unique association between reports of medication side effects and pain-related activity interference by controlling for a number of pain-relevant variables that have previously been found to be associated with activity interference in patients with chronic pain. For example, in line with many previous studies, we found that month-to-month increases in pain intensity were associated with higher ratings of pain-related activity interference [30,67,74–75]. We also found that increases in negative affect were associated with higher ratings of pain-related activity interference, which is consistent with the findings of previous studies that have examined the psychological determinants of pain-related disability [36,41,56,66,76].
The main finding that emerged from our study is that increases in perceived medication side effects were associated with higher ratings of pain-related activity interference even after controlling for month-to-month changes in patients’ levels of pain and negative affect. This “unique” association between perceived medication side effects and pain-related activity interference suggests that medication side effects have the potential to interfere with activities independent of patients’ pain and affective states. We found that pain intensity, negative affect, and perceived medication side effects all emerged as having significant unique influences on pain-related activity interference, suggesting that these variables might represent additive risk factors for poor functional outcomes in patients with chronic pain.
The results of a subsequent analysis indicated that the unique association between reports of medication side effects and pain-related activity interference could not be attributable to differences across patients in any baseline or pre-existing characteristics. While it was important to control for month-to-month changes in pain and negative affect when examining the association between perceived side effects and pain-related activity interference, a wide range of pre-existing (i.e., time-invariant) variables could have also been responsible for this association. In our study, we ruled out this possibility by including each participant as a fixed effect in the multilevel model. Although stringent, this model building strategy allowed us to control for the influence of any “observed” or “unobserved” between-person variables on the study outcome. Importantly, this strategy allowed for a pure estimate of the within-person (Level 1) association between perceived medication side effects and pain-related activity interference, without the influence of between-person variables. In our study, the magnitude of the association between self-reports of side effects and pain-related activity interference might possibly have been influenced by some unobserved variables (e.g., patients’ tendency to over-report symptoms), but results indicated that these variables could not entirely account for the association that was found between side effects and pain-related activity interference.
In the present study, we were also interested in examining some of the variables that might moderate the association between reports of medication side effects and pain-related activity interference. Consistent with previous studies [6,54,70], we found that women experienced significantly greater medication side effects than men; however, the magnitude of the association between reports of side effects and pain-related activity interference did not differ significantly as a function of patient gender. Moreover, neither pain intensity nor negative affect moderated the association between reports of side effects and pain-related activity interference. The latter results suggest that the association between medication side effects and pain-related activity interference was not particularly more pronounced when pain or negative affect increased from month to month.
The findings of the present study have implications for clinicians who are involved in the pharmacologic management of patients with chronic pain. To date, most pain management guidelines encourage clinicians to identify and monitor the occurrence of various medication side effects. Prescribing clinicians have also repeatedly been encouraged to find a ‘satisfactory balance’ between pain relief and the adverse effects of medication [10–11,13,32,45–46,65]. To the extent that one of the primary goals of chronic pain management is to enhance activity engagement or function [12,23,31,37,72], our findings suggest that greater efforts should be placed on the assessment and treatment of medication side effects. The assessment of patients’ side effects and pain-related activity interference, both prior and after the initiation of any new medication, would be consistent with guidelines for the pharmacological management of patients with pain [11,13,20], and could facilitate clinicians’ prescribing decisions. Importantly, if side effects do emerge as a result of medication use, they should be targeted directly and treated using specific interventions in order to prevent their potentially deleterious impact on patients’ activity engagement.
A number of limitations must be considered when interpreting the findings of the present study. First, the effect size observed in our study for the association between reports of side effects and pain-related activity interference was relatively small. However, small effect sizes are typical of most longitudinal studies that involve modest within-person changes in variables of interest. Second, our study was based on self-report measures of pain-related activity interference. While self-report instruments are the most frequently used methods for assessing activity interference [14,31,71,74], future studies should include more objective measures. Similarly, our medication side effect assessment was based on patients’ subjective perceptions of side effects associated with their medications. As with any other self-reported measures, self-reports of medication side effects are subject to potential response bias, and limits in patients’ ability to accurately report their side effects must be considered when interpreting our findings. Moreover, even though patients were questioned about potential symptoms or “side effects” associated with their medications, it remains unclear whether these symptoms were specifically due to their medication. Given that chronic pain itself may lead to symptoms such as headaches or weakness, the possibility that patients have erroneously attributed some of their symptoms to their medication must be considered. Third, patients included in the present study were at risk for prescription opioid misuse due to their inclusion in the broader parent study. While this has no direct implications for the nature of findings observed in the present study, this sample characteristic places limits on the generalizability of our findings. Finally, given that most patients were taking more than one medication, our findings on medication side effects are not specific to analgesic medications, and non-analgesic medications might also have contributed to patients’ reports of side effects. It is well-known, however, that medication regimens of most patients being treated in tertiary care pain management settings, like in the present study, involve more than one medication. The inclusion of polymedication users in our study sample should thus be seen as strength rather than a methodological limitation.
In summary, the findings of our study provide preliminary evidence that reports of medication side effects are associated with heightened pain-related activity interference in patients with chronic pain. The key finding of our study is that perceived medication side effects were associated with heightened pain-related activity interference even after controlling for patient demographics, pain intensity, and negative affect. Additional studies will need to be conducted using a more frequent (e.g., day-to-day) data collection schedule in order to better capture the dynamic inter-relationships between side effects and pain-related activity interference. Further research should also further examine the variables that might moderate the impact of medication side effects on pain-related activity interference in patients with pain. Advances in this domain might pave the way for the development of more effective pharmacologic interventions, and might ultimately lead to improved pain management outcomes in patients with pain-related conditions.
Acknowledgments
This study was supported in part by Grants (R21 DA024298, Jamison, PI; K23 DA020682, Wasan, PI) from the National Institute on Drug Abuse (NIDA), and by a postdoctoral fellowship from the Canadian Institutes of Health Research (CIHR) awarded to Marc O. Martel.
Abbreviations and Acronyms
- PDA
Personal digital assistant
- SUD
Substance use disorder
- VAS
Visual analogue scale
- MSE-I
Medication side effect index
Footnotes
Additional multilevel analyses were conducted in order to explore any potential systematic time effect on the main study outcome. In these analyses, the variable “time” was treated as a continuous Level 1 independent variable, and the activity interference index was used as the dependent variable. Results indicated that the main effect of time was not significant, B = .65, SE = .50, ns. A follow-up multilevel analysis was then conducted to examine whether “time” interacted with medication side effects in predicting pain-related activity interference, but this interaction effect was not significant, B = .02, SE = .01, ns. The variable “time” was thus not included in any further models.
Modeling each participant (ID) as a fixed effect was done by creating 110 dummy coded variables, which were then entered simultaneously in the multilevel model (see Refs. 5, 41, 53). This resulted in a model with 110 new parameters. The random effect for the intercept was set to zero, indicating that the between-person variance in the intercept was entirely accounted for by the inclusion of all participants (IDs) as fixed effects to the model.
Conflicts of Interest
The authors have no financial interests in the results of this research and no conflicts of interest. All of the listed authors have read and approved the manuscript.
References
- 1.Baldini A, Von Korff M, Lin EH. A Review of Potential Adverse Effects of Long-Term Opioid Therapy: A Practitioner’s Guide. Prim Care Companion CNS Disord. 2012:14. doi: 10.4088/PCC.11m01326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell T, Annunziata K, Leslie JB. Opioid-induced constipation negatively impacts pain management, productivity, and health-related quality of life: findings from the National Health and Wellness Survey. J Opioid Manag. 2009;5:137–144. doi: 10.5055/jom.2009.0014. [DOI] [PubMed] [Google Scholar]
- 3.Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Opioid complications and side effects. Pain Physician. 2008;11:S105–120. [PubMed] [Google Scholar]
- 4.Beteille T, Kalogrides D, Loeb S. Stepping stones: Principal career paths and school outcomes. Social Science Research. 2012;41:904–919. doi: 10.1016/j.ssresearch.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Butler SF, Fernandez K, Benoit C, Budman SH, Jamison RN. Validation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPP-R) J Pain. 2008;9:360–372. doi: 10.1016/j.jpain.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cepeda MS, Farrar JT, Baumgarten M, Boston R, Carr DB, Strom BL. Side effects of opioids during short-term administration: effect of age, gender, and race. Clin Pharmacol Ther. 2003;74:102–112. doi: 10.1016/S0009-9236(03)00152-8. [DOI] [PubMed] [Google Scholar]
- 7.Cepeda MS, Fife D, Chow W, Mastrogiovanni G, Henderson SC. Opioid shopping behavior: how often, how soon, which drugs, and what payment method. J Clin Pharmacol. 2013;53:112–117. doi: 10.1177/0091270012436561. [DOI] [PubMed] [Google Scholar]
- 8.Chapman CR, Lipschitz DL, Angst MS, Chou R, Denisco RC, Donaldson GW, Fine PG, Foley KM, Gallagher RM, Gilson AM, Haddox JD, Horn SD, Inturrisi CE, Jick SS, Lipman AG, Loeser JD, Noble M, Porter L, Rowbotham MC, Schoelles KM, Turk DC, Volinn E, Von Korff MR, Webster LR, Weisner CM. Opioid pharmacotherapy for chronic non-cancer pain in the United States: a research guideline for developing an evidence-base. J Pain. 2010;11:807–829. doi: 10.1016/j.jpain.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Chou R. Pharmacological management of low back pain. Drugs. 2010;70:387–402. doi: 10.2165/11318690-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Chou R, Ballantyne JC, Fanciullo GJ, Fine PG, Miaskowski C. Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:147–159. doi: 10.1016/j.jpain.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, Foley KM, Fudin J, Gilson AM, Kelter A, Mauskop A, O’Connor PG, Passik SD, Pasternak GW, Portenoy RK, Rich BA, Roberts RG, Todd KH, Miaskowski C. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Main CJMS, Watson PJ. Pain Management: Practical applications of the biopsychosocial perspective in clinical and occupational settings. Edinburgh: Churchill Livingstone; 2008. [Google Scholar]
- 13.Dworkin RH, O’Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, Kalso EA, Loeser JD, Miaskowski C, Nurmikko TJ, Portenoy RK, Rice AS, Stacey BR, Treede RD, Turk DC, Wallace MS. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237–251. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 14.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Edwards RR. Age differences in the correlates of physical functioning in patients with chronic pain. J Aging Health. 2006;18:56–69. doi: 10.1177/0898264305280976. [DOI] [PubMed] [Google Scholar]
- 16.Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: a new look at an old issue. Psychol Methods. 2007;12:121–138. doi: 10.1037/1082-989X.12.2.121. [DOI] [PubMed] [Google Scholar]
- 17.Evans CJ, Trudeau E, Mertzanis P, Marquis P, Pena BM, Wong J, Mayne T. Development and validation of the Pain Treatment Satisfaction Scale (PTSS): a patient satisfaction questionnaire for use in patients with chronic or acute pain. Pain. 2004;112:254–266. doi: 10.1016/j.pain.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Fishman SM, Teichera D. Challenges and choices in drug therapy for chronic pain. Cleve Clin J Med. 2003;70:119–121. 125–117, 131–112. doi: 10.3949/ccjm.70.2.119. passim. [DOI] [PubMed] [Google Scholar]
- 19.Fleming MF, Balousek SL, Klessig CL, Mundt MP, Brown DD. Substance use disorders in a primary care sample receiving daily opioid therapy. J Pain. 2007;8:573–582. doi: 10.1016/j.jpain.2007.02.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furlan AD, Reardon R, Weppler C. Opioids for chronic noncancer pain: a new Canadian practice guideline. CMAJ. 2010;182:923–930. doi: 10.1503/cmaj.100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E. Opioids for chronic noncancer pain: a meta-analysis of effectiveness and side effects. Cmaj. 2006;174:1589–1594. doi: 10.1503/cmaj.051528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao K, Kemp DE, Conroy C, Ganocy SJ, Findling RL, Calabrese JR. Comorbid anxiety and substance use disorders associated with a lower use of mood stabilisers in patients with rapid cycling bipolar disorder: a descriptive analysis of the cross-sectional data of 566 patients. Int J Clin Pract. 2010;64:336–344. doi: 10.1111/j.1742-1241.2009.02284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133:581–624. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- 24.Gregorian RS, Jr, Gasik A, Kwong WJ, Voeller S, Kavanagh S. Importance of side effects in opioid treatment: a trade-off analysis with patients and physicians. The journal of pain: official journal of the American Pain Society. 2010;11:1095–1108. doi: 10.1016/j.jpain.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Hayes AF. A Primer on Multilevel Modeling. Human communication research. 2007;32:385–410. [Google Scholar]
- 26.Heck RH, Thomas SL, Tabata LN. Multilevel and Longitudinal Modeling with IBM SPSS. New York: Taylor & Francis; 2014. [Google Scholar]
- 27.Jamison RN, Edwards RR, Liu X, Ross EL, Michna E, Warnick M, Wasan AD. Relationship of negative affect and outcome of an opioid therapy trial among low back pain patients. Pain Pract. 2013;13:173–181. doi: 10.1111/j.1533-2500.2012.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jamison RN, Link CL, Marceau LD. Do pain patients at high risk for substance misuse experience more pain? A longitudinal outcomes study. Pain Med. 2009;10:1084–1094. doi: 10.1111/j.1526-4637.2009.00679.x. [DOI] [PubMed] [Google Scholar]
- 29.Jamison RN, Ross EL, Michna E, Chen LQ, Holcomb C, Wasan AD. Substance misuse treatment for high-risk chronic pain patients on opioid therapy: a randomized trial. Pain. 2010;150:390–400. doi: 10.1016/j.pain.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen MP, Dworkin RH, Gammaitoni AR, Olaleye DO, Oleka N, Galer BS. Do pain qualities and spatial characteristics make independent contributions to interference with physical and emotional functioning? J Pain. 2006;7:644–653. doi: 10.1016/j.jpain.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 31.Jensen MP, Turner JA, Romano JM. Changes after multidisciplinary pain treatment in patient pain beliefs and coping are associated with concurrent changes in patient functioning. Pain. 2007;131:38–47. doi: 10.1016/j.pain.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalso E. Opioids for persistent non-cancer pain. Bmj. 2005;330:156–157. doi: 10.1136/bmj.330.7484.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalso E. The Vicious Circle in chronic pain management: balancing efficacy and adverse effects. Curr Med Res Opin. 2011;27:2069–2071. doi: 10.1185/03007995.2011.619436. [DOI] [PubMed] [Google Scholar]
- 34.Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004;112:372–380. doi: 10.1016/j.pain.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 35.Katz NP. The measurement of symptoms and side effects in clinical trials of chronic pain. Contemp Clin Trials. 2012;33:903–911. doi: 10.1016/j.cct.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Kerns RD, Haythornthwaite JA. Depression among chronic pain patients: cognitive-behavioral analysis and effect on rehabilitation outcome. J Consult Clin Psychol. 1988;56:870–876. doi: 10.1037//0022-006x.56.6.870. [DOI] [PubMed] [Google Scholar]
- 37.Kerns RD, Sellinger J, Goodin BR. Psychological treatment of chronic pain. Annu Rev Clin Psychol. 2011;7:411–434. doi: 10.1146/annurev-clinpsy-090310-120430. [DOI] [PubMed] [Google Scholar]
- 38.Kopala-Sibley DC, Zuroff DC, Russell JJ, Moskowitz DS, Paris J. Understanding heterogeneity in borderline personality disorder: differences in affective reactivity explained by the traits of dependency and self-criticism. J Abnorm Psychol. 2012;121:680–691. doi: 10.1037/a0028513. [DOI] [PubMed] [Google Scholar]
- 39.Kreft I, De Leeuw J. Introducing multilevel modeling. New York: Sage; 1998. [Google Scholar]
- 40.Leresche L. Defining gender disparities in pain management. Clin Orthop Relat Res. 2011;469:1871–1877. doi: 10.1007/s11999-010-1759-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linton SJ. A review of psychological risk factors in back and neck pain. Spine (Phila Pa 1976) 2000;25:1148–1156. doi: 10.1097/00007632-200005010-00017. [DOI] [PubMed] [Google Scholar]
- 42.Loeb S, Kalogrides D, Beteille T. Effective schools: Teacher hiring, assignment, development, and retention. Education Finance and Policy. 2012;7:269–304. [Google Scholar]
- 43.Lynch ME. The pharmacotherapy of chronic pain. Rheum Dis Clin North Am. 2008;34:369–385. doi: 10.1016/j.rdc.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Lynch ME, Watson CP. The pharmacotherapy of chronic pain: a review. Pain Res Manag. 2006;11:11–38. doi: 10.1155/2006/642568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manchikanti L, Abdi S, Atluri S, Balog CC, Benyamin RM, Boswell MV, Brown KR, Bruel BM, Bryce DA, Burks PA, Burton AW, Calodney AK, Caraway DL, Cash KA, Christo PJ, Damron KS, Datta S, Deer TR, Diwan S, Eriator I, Falco FJ, Fellows B, Geffert S, Gharibo CG, Glaser SE, Grider JS, Hameed H, Hameed M, Hansen H, Harned ME, Hayek SM, Helm S, 2nd, Hirsch JA, Janata JW, Kaye AD, Kaye AM, Kloth DS, Koyyalagunta D, Lee M, Malla Y, Manchikanti KN, McManus CD, Pampati V, Parr AT, Pasupuleti R, Patel VB, Sehgal N, Silverman SM, Singh V, Smith HS, Snook LT, Solanki DR, Tracy DH, Vallejo R, Wargo BW. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part 2--guidance. Pain Physician. 2012;15:S67–116. [PubMed] [Google Scholar]
- 46.Manchikanti L, Abdi S, Atluri S, Balog CC, Benyamin RM, Boswell MV, Brown KR, Bruel BM, Bryce DA, Burks PA, Burton AW, Calodney AK, Caraway DL, Cash KA, Christo PJ, Damron KS, Datta S, Deer TR, Diwan S, Eriator I, Falco FJ, Fellows B, Geffert S, Gharibo CG, Glaser SE, Grider JS, Hameed H, Hameed M, Hansen H, Harned ME, Hayek SM, Helm S, 2nd, Hirsch JA, Janata JW, Kaye AD, Kaye AM, Kloth DS, Koyyalagunta D, Lee M, Malla Y, Manchikanti KN, McManus CD, Pampati V, Parr AT, Pasupuleti R, Patel VB, Sehgal N, Silverman SM, Singh V, Smith HS, Snook LT, Solanki DR, Tracy DH, Vallejo R, Wargo BW. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part I--evidence assessment. Pain Physician. 2012;15:S1–65. [PubMed] [Google Scholar]
- 47.Manchikanti L, Manchikanti KN, Pampati V, Cash KA. Prevalence of side effects of prolonged low or moderate dose opioid therapy with concomitant benzodiazepine and/or antidepressant therapy in chronic non-cancer pain. Pain Physician. 2009;12:259–267. [PubMed] [Google Scholar]
- 48.Marceau LD, Link C, Jamison RN, Carolan S. Electronic diaries as a tool to improve pain management: is there any evidence? Pain Med. 2007;8 (Suppl 3):S101–109. doi: 10.1111/j.1526-4637.2007.00374.x. [DOI] [PubMed] [Google Scholar]
- 49.Marceau LD, Link CL, Smith LD, Carolan SJ, Jamison RN. In-clinic use of electronic pain diaries: barriers of implementation among pain physicians. J Pain Symptom Manage. 2010;40:391–404. doi: 10.1016/j.jpainsymman.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martel MO, Dolman AJ, Edwards RR, Jamison RN, Wasan AD. The association between negative affect and prescription opioid misuse in patients with chronic pain: the mediating role of opioid craving. J Pain. 2014;15:90–100. doi: 10.1016/j.jpain.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nezlek J. Multilevel random coefficient analyses of event and interval contingent data in social and personality psychology research. Psychological Bulletin. 2001;27:771–785. [Google Scholar]
- 52.Peugh JL. A practical guide to multilevel modeling. J Sch Psychol. 2010;48:85–112. doi: 10.1016/j.jsp.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Reme SE, Tangen T, Moe T, Eriksen HR. Prevalence of psychiatric disorders in sick listed chronic low back pain patients. Eur J Pain. 2011;15:1075–1080. doi: 10.1016/j.ejpain.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Riley JL, 3rd, Hastie BA, Glover TL, Fillingim RB, Staud R, Campbell CM. Cognitive-affective and somatic side effects of morphine and pentazocine: side-effect profiles in healthy adults. Pain Med. 2010;11:195–206. doi: 10.1111/j.1526-4637.2009.00680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rothstein J. Student sorting and bias in value-added estimation: selection on observables and unobservables. Education Finance and Policy. 2009;4:537–571. [Google Scholar]
- 56.Rudy TE, Lieber SJ, Boston JR, Gourley LM, Baysal E. Psychosocial predictors of physical performance in disabled individuals with chronic pain. Clin J Pain. 2003;19:18–30. doi: 10.1097/00002508-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Rudy TE, Weiner DK, Lieber SJ, Slaboda J, Boston JR. The impact of chronic low back pain on older adults: a comparative study of patients and controls. Pain. 2007;131:293–301. doi: 10.1016/j.pain.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Russell JJ, Moskowitz DS, Zuroff DC, Bleau P, Pinard G, Young SN. Anxiety, emotional security and the interpersonal behavior of individuals with social anxiety disorder. Psychol Med. 2011;41:545–554. doi: 10.1017/S0033291710000863. [DOI] [PubMed] [Google Scholar]
- 59.Saunders KW, Von Korff M, Campbell CI, Banta-Green CJ, Sullivan MD, Merrill JO, Weisner C. Concurrent use of alcohol and sedatives among persons prescribed chronic opioid therapy: prevalence and risk factors. J Pain. 2012;13:266–275. doi: 10.1016/j.jpain.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwartz JE, Stone AA. Strategies for analyzing ecological momentary assessment data. Health Psychol. 1998;17:6–16. doi: 10.1037//0278-6133.17.1.6. [DOI] [PubMed] [Google Scholar]
- 61.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry. 1998;59 (Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 62.Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. Journal of Educational and Behavioral Statistics. 1998;23:323–325. [Google Scholar]
- 63.Singer JD, Willett JB. Applied longitudinal data analysis: modeling change and event occurrence. Oxford; New York: Oxford University Press; 2003. [Google Scholar]
- 64.Smith SM, Wang AT, Katz NP, McDermott MP, Burke LB, Coplan P, Gilron I, Hertz SH, Lin AH, Rappaport BA, Rowbotham MC, Sampaio C, Sweeney M, Turk DC, Dworkin RH. Adverse event assessment, analysis, and reporting in recent published analgesic clinical trials: ACTTION systematic review and recommendations. Pain. 2013;154:997–1008. doi: 10.1016/j.pain.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 65.Sullivan MD, Von Korff M, Banta-Green C, Merrill JO, Saunders K. Problems and concerns of patients receiving chronic opioid therapy for chronic non-cancer pain. Pain. 2010;149:345–353. doi: 10.1016/j.pain.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sullivan MJ, Stanish W, Sullivan ME, Tripp D. Differential predictors of pain and disability in patients with whiplash injuries. Pain research & management: the journal of the Canadian Pain Society = journal de la societe canadienne pour le traitement de la douleur. 2002;7:68–74. doi: 10.1155/2002/176378. [DOI] [PubMed] [Google Scholar]
- 67.Sullivan MJ, Stanish W, Waite H, Sullivan M, Tripp DA. Catastrophizing, pain, and disability in patients with soft-tissue injuries. Pain. 1998;77:253–260. doi: 10.1016/S0304-3959(98)00097-9. [DOI] [PubMed] [Google Scholar]
- 68.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5:133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 69.Tan GJM, Thomby J, Sloan PA. Negative emotions, pain, and functioning. Psychol Serv. 2008;5:26–35. [Google Scholar]
- 70.Trafton JA, Cucciare MA, Lewis E, Oser M. Somatization is associated with non-adherence to opioid prescriptions. J Pain. 2011;12:573–580. doi: 10.1016/j.jpain.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 71.Turk DC, Dworkin RH, Revicki D, Harding G, Burke LB, Cella D, Cleeland CS, Cowan P, Farrar JT, Hertz S, Max MB, Rappaport BA. Identifying important outcome domains for chronic pain clinical trials: an IMMPACT survey of people with pain. Pain. 2008;137:276–285. doi: 10.1016/j.pain.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 72.Turk DC, Swanson KS, Tunks ER. Psychological approaches in the treatment of chronic pain patients--when pills, scalpels, and needles are not enough. Can J Psychiatry. 2008;53:213–223. doi: 10.1177/070674370805300402. [DOI] [PubMed] [Google Scholar]
- 73.Turk DC, Wilson HD, Cahana A. Treatment of chronic non-cancer pain. Lancet. 2011;377:2226–2235. doi: 10.1016/S0140-6736(11)60402-9. [DOI] [PubMed] [Google Scholar]
- 74.Turner JA, Franklin G, Heagerty PJ, Wu R, Egan K, Fulton-Kehoe D, Gluck JV, Wickizer TM. The association between pain and disability. Pain. 2004;112:307–314. doi: 10.1016/j.pain.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 75.Turner JA, Franklin G, Turk DC. Predictors of chronic disability in injured workers: a systematic literature synthesis. Am J Ind Med. 2000;38:707–722. doi: 10.1002/1097-0274(200012)38:6<707::aid-ajim10>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 76.Turner JA, Jensen MP, Romano JM. Do beliefs, coping, and catastrophizing independently predict functioning in patients with chronic pain? Pain. 2000;85:115–125. doi: 10.1016/s0304-3959(99)00259-6. [DOI] [PubMed] [Google Scholar]
- 77.Turner JA, Sears JM, Loeser JD. Programmable intrathecal opioid delivery systems for chronic noncancer pain: a systematic review of effectiveness and complications. Clin J Pain. 2007;23:180–195. doi: 10.1097/01.ajp.0000210955.93878.44. [DOI] [PubMed] [Google Scholar]
- 78.Wallace D, Green BS. Analysis of repeated measures designs with linear mixed models. In: Moscovitz DM, Hershberger SL, editors. Modeling intraindividual variability with repeated measures data. Mahwah, NJ: Lawrence Erlbaum; 2002. [Google Scholar]
- 79.Wasan AD, Davar G, Jamison R. The association between negative affect and opioid analgesia in patients with discogenic low back pain. Pain. 2005;117:450–461. doi: 10.1016/j.pain.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 80.Wasan AD, Jamison RN, Pham L, Tipirneni N, Nedeljkovic SS, Katz JN. Psychopathology predicts the outcome of medial branch blocks with corticosteroid for chronic axial low back or cervical pain: a prospective cohort study. BMC Musculoskelet Disord. 2009;10:22. doi: 10.1186/1471-2474-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wasan AD, Ross EL, Michna E, Chibnik L, Greenfield SF, Weiss RD, Jamison RN. Craving of prescription opioids in patients with chronic pain: a longitudinal outcomes trial. J Pain. 2012;13:146–154. doi: 10.1016/j.jpain.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


