Abstract
PURPOSE/OBJECTIVE(S)
To identify prognostic factors and patterns of relapse for patients with Ewing sarcoma (ES) who underwent chemotherapy and R0 resection without radiation therapy (RT).
METHODS AND MATERIALS
We reviewed the medical records of patients who underwent surgical resection at our institution between 2000 and 2013 for an initial diagnosis of ES. The associations of demographic and clinical factors with local control (LC) and patient outcome were determined by Cox regression. Time to events was measured from the time of surgery. Survival curves were estimated by the Kaplan-Meier method and compared by the log rank test.
RESULTS
A total of 66 patients (median age 19 years, range: 4–55) met the study criteria. The median follow-up was 5.6 years for living patients. In 43 patients (65%) for whom imaging studies were available, the median tumor volume reduction was 73% and at least partial response (PR) by RECIST was achieved in 17 patients (40%). At 5 years, LC was 78%, progression-free survival (PFS) was 59%, and overall survival (OS) was 65%. Poor histologic response (necrosis ≤ 95%) was an independent predictor of LC (HR=6.8, P=0.004), PFS (HR=5.2, P=0.008), and OS (HR=5.0, P=0.008). Metastasis on presentation was also an independent predictor of LC (HR=6.3, P=0.011), PFS (HR=6.8, P=0.002) and OS (HR=6.7, P=0.002). Radiologic PR was a predictor of PFS (HR=0.26, P=0.012) and post-chemotherapy tumor volume was associated with OS (HR=1.06, P=0.015). All deaths were preceded by distant relapse. Of the 8 initial local-only relapses, 5 (63%) were soon followed by distant relapse. Predictors of poor post-recurrence survival were time to recurrence <1 year (HR=11.5, P=0.002) and simultaneous local and distant relapse (HR=16.8, P=0.001).
CONCLUSIONS
Histologic and radiologic response to chemotherapy were independent predictors of outcome. Additional study is needed to determine the role of adjuvant RT for patients who have poor histologic response after R0 resection.
Introduction
The Ewing sarcoma (ES) family of tumors commonly bearing a pathognomonic EWS-FL1 translocation is the second most common bone malignancy in children and adolescents, with an annual incidence of 200–500 cases in the United States[1,2]. With advances in multidisciplinary care, 5-year survival rates for pediatric ES patients have improved from 50% during the period from 1983–1990 to 70% during the period from 2004–2010 [3,4]. The current standard of care combines a 5-drug chemotherapy regimen VDC/IE (vincristine, doxorubicin, and cyclophosphamide alternating with ifosfamide and etoposide) and local control comprising surgery and/or radiation therapy (RT)[5,6]. In the United States, surgery is generally favored for initial local therapy[7], with adjuvant RT typically reserved for cases with positive microscopic margins (R1 resection) and excluded if clear margins are obtained (R0 resection)[8].
Prior studies have shown that overt metastases at presentation and poor histologic response to induction chemotherapy are indicators of poor prognosis in terms of disease progression and overall survival (OS)[9–11]. The goal of this study was to identify prognostic factors for disease outcomes and patterns of relapse in patients with ES who underwent neoadjuvant chemotherapy and successful R0 resection without adjuvant RT. The results of this study would then serve as hypothesis generating for future investigations of appropriate timepoints for delivering RT.
Materials and Methods
Patient selection
The study was approved by the local Institutional Review Board, and the informed consent requirement was waived. Medical records were retrospectively reviewed for all ES patients entered into the institutional cancer registry for the years 2000–2013. Patients were eligible for the study if they underwent an R0 surgical resection at our institution for an initial diagnosis of ES and had pathology material available for review. R0 resection was defined as no viable or necrotic tumor on inked margins. The data collected included patient age and sex, tumor location (extremity versus central), presence or absence of metastasis at diagnosis, neoadjuvant and adjuvant chemotherapy regimens received and their durations, pre-chemotherapy and pre-surgical imaging studies, surgical pathology reports, follow-up imaging studies, and patient outcome. Patients were excluded if they had positive surgical margins, presented with recurrent disease, or received neoadjuvant, definitive, or adjuvant RT. Institutional practice generally reserved adjuvant RT for patients with positive surgical margins.
Chemotherapy
The neoadjuvant chemotherapy regimens were grouped into three categories: high-dose VDC (vincristine, doxorubicin, and cyclophosphamide); VIDE (vincristine, ifosfamide, doxorubicin, and etoposide); and alternating VDC/IE. Postoperative chemotherapy was given at the discretion of the medical oncologist, and was most often a continuation of the induction chemotherapy regimen (47%) or high-dose ifosfamide with or without etoposide (29%).
Radiologic imaging
Magnetic resonance images (MRI) or computed tomography scans (CT) of the primary tumor taken after induction chemotherapy and before surgery were reviewed by a staff radiologist (AM). A one-dimensional tumor size was determined by the single longest of the superior-inferior (SI), anterior-posterior (AP), and transverse (LR) measurements. A three-dimensional volume was calculated by approximating the tumor as an ellipsoid, measured as: . Patients whose images were analog, consisted only of outside imaging reports without corresponding digital imaging files, or were imported from an outside institution and incorrectly calibrated were excluded from the analysis of imaging studies. Radiologic response was assessed by both relative three-dimensional volumetric reduction and at least partial response (PR) by Response Evaluation Criteria in Solid Tumors (RECIST), defined as at least a 30% decrease in one-dimensional tumor size[12].
Pathology review
The pathology reports and available slides were reviewed by one pathologist (WLW) to confirm the histologic diagnosis and extent of tumor necrosis. Molecular confirmation of ES diagnosis by fluorescence in situ hybridization (FISH) or reverse-transcriptase polymerase chain reaction (RT-PCR) was noted. Tumor necrosis was determined by mapping the specimen onto a grid and averaging the percentage of necrotic cells in each section. Cases for which the diagnosis was not certain by histology and that were without molecular confirmation were excluded. Histologic response was defined as good if tumor necrosis was >95% and poor if tumor necrosis was ≤95%. Only patients with both clear soft tissue and bone margins were included.
Statistical analysis
Correlation between radiologic and histologic response was assessed by Spearman correlation. Other predictors of good histologic response were assessed by binary logistic regression. Proportions were compared with the Fisher exact test. A two-sided P-value of <0.05 was deemed significant. Statistical analysis was performed using Spotfire S+ software v8.2 (TIBCO, Boston, MA).
Post-relapse survival (PRS) was measured from the time of initial recurrence. As the study cohort was defined by the event of an R0 surgical resection, the time to all other events was measured from the time of post-chemotherapy surgical resection. Patients who underwent amputation were excluded from local control (LC) analysis. Clinic notes and the social security death index were used to determine OS, and progression-free survival (PFS) was defined as the absence of local relapse (LR) and distant relapse (DR). LC, PFS, OS, and PRS were estimated by Kaplan-Meier analysis, and 95% confidence intervals (CIs) were calculated. Only the initial relapse was included in Kaplan-Meier analysis; an initial simultaneous LR and DR was scored for both events. Subsequent relapse events were recorded to describe patterns of failure. Factors associated with event outcomes were determined by Cox regression, and Kaplan-Meier curves were compared by the log rank test. The influence of LC on OS was evaluated as a time-varying covariate in Cox regression. Continuous variables considered in Cox regression that had significant outliers as identified by boxplot were analyzed as categorical variables compared to the median value. A multivariate analysis (MVA) was performed to identify variables associated with outcomes while adjusting for confounding by other study factors. Due to the number of variables compared to the dataset size, covariates for the MVA were screened based on a P<0.20 on univariate analysis (UVA). All patients who died had either LR or DR, so competing risks analysis was not performed and PFS was the same as event-free survival (EFS).
Results
Patient characteristics
A total of 66 patients met the study inclusion criteria, with patient and disease characteristics shown in Table 1. Of the remaining 249 patients in the ES registry, the most common reasons for exclusion were presentation for recurrent disease (32%), neoadjuvant, definitive, or adjuvant RT to the primary site (28%), or management of the primary site at an outside hospital prior to presentation at our cancer center (24%). Additional exclusions included 7 patients based on pathology review and 6 patients due to positive surgical margins. Median follow-up duration was 3.6 years (range: 0.3–13.7 years) for all patients and 5.6 years (range: 0.5–13.7 years) for living patients.
Table 1.
Baseline patient, tumor, and treatment characteristics (N=66)
| Characteristic | No. | (Range)/% |
|---|---|---|
| Median age, years | 19 | (4–55) |
| Sex | ||
| Male | 49 | 74% |
| Female | 17 | 26% |
| Tumor type | ||
| Bone | 51 | 77% |
| Extraosseous | 4 | 6% |
| Chest wall | 11 | 17% |
| Tumor location | ||
| Central | 33 | 50% |
| Extremity | 33 | 50% |
| Metastasis at presentation | 10 | 15% |
| Pre-chemotherapy imaging (N=43) | 65% | |
| MRI | 37 | 56% |
| CT | 6 | 9% |
| Median longest dimension, cm | 10 | (5.1–17.3) |
| Median volume, cm3 | 144 | (11–1149) |
| Neoadjuvant chemotherapy | ||
| Number of cycles | 6 | (2–17) |
| VDC | 13 | 20% |
| VIDE | 30 | 45% |
| VDC/IE | 23 | 35% |
| Post-chemotherapy imaging (N=51) | 77% | |
| MRI | 45 | 68% |
| CT scan | 6 | 9% |
| Median longest dimension, cm | 6.6 | (2.2–16.3) |
| Median volume, cm3 | 32 | (1–768) |
| Radiologic response (N=43) | ||
| Partial response by RECIST | 17 | 40% |
| Median volume reduction, % | 73% | (−139–99%) |
| Pathology | ||
| Molecular confirmation | 41 | 62% |
| Median necrosis extent | 98% | (10–100%) |
VDC: vincristine, doxorubicin, cyclophosphamide; VIDE: doxorubicin and ifosfamide with or without vincristine and etoposide; VDC/IE: VDC alternating with ifosfamide with or without etoposide; MRI: magnetic resonance imaging; CT: computed tomography; RECIST: Response Evaluation Criteria in Solid Tumors.
Radiologic and histologic responses to chemotherapy
Radiologic and histologic responses are shown in Table 1. Pre-chemotherapy imaging was available for review for 43 patients (65%). Seventeen patients (40%) had PR by RECIST and good histologic response was seen in 39 patients (58%). The 25 surgical specimens that were not molecularly confirmed were included as morphologically and immunohistochemically compatible with ES. No correlation was found between the volumetric and histologic responses to chemotherapy (r=0.02, 95% CI: −0.28 to 0.32, P=0.91). None of the analyzed factors was associated significantly with histologic response, including sex, age, tumor location, metastasis, initial tumor size and volume, chemotherapy regimen and duration, pre-operative tumor size and volume, and volumetric response to chemotherapy.
Outcomes analysis
Kaplan-Meier survival curves for the entire study population are shown in Supplemental Figure 1. The 2- and 5-year LC rates were both 78% (95% CI: 66–89%). The 2- and 5-year OS rates were 79% (95% CI: 69–90%) and 65% (95% CI: 52–77%), respectively. The 2- and 5-year PFS rates were both 58% (95% CI: 46–71%).
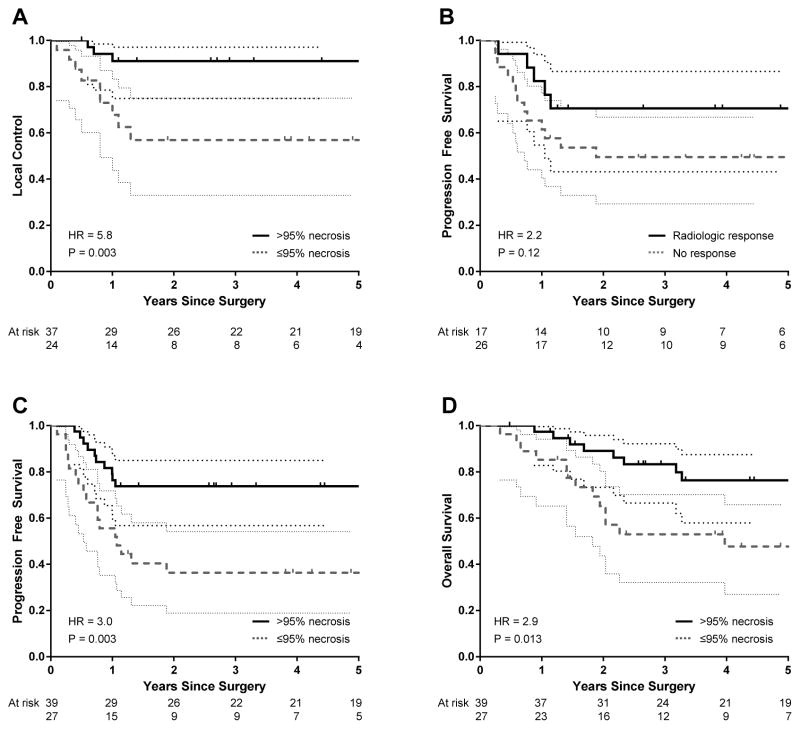
Detailed results of the UVA and MVA performed to identify factors associated with disease outcomes are listed in Table 2 and Table 3. Five patients (8%) underwent amputation and were excluded from LC analysis. On UVA, good histologic response, metastasis on presentation, and initial tumor volume were predictive of LC. On MVA, good histologic response and metastasis on presentation remained independent predictors. The 2-year LC rates were 91% (95% CI: 81–100%) for patients with good histologic response and 56% (95% CI: 32–78%) for those with poor histologic response (P=0.003) (Figure 1A). For the 53 patients with non-metastatic disease, an initial LR was seen in 1 of 32 patients (3%) with good histologic response and 8 of 21 patients (38%) with poor histologic response (P=0.001).
Table 2.
Univariate analysis by Cox regression of factors associated with disease outcomes in patients with ES treated with neoadjuvant chemotherapy and R0 resection
| Covariate | Local control (12 events) | Progression-free survival (28 events) | Overall survival (21 events) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Age, per 1 year | 1.02 | 0.97–1.08 | 0.497 | 1.04 | 1.01–1.08 | 0.022 | 1.06 | 1.02–1.10 | 0.002 |
| Male sex | 0.95 | 0.26–3.52 | 0.942 | 1.07 | 0.47–2.44 | 0.865 | 0.91 | 0.33–2.47 | 0.905 |
| Metastasis at presentation | 4.55 | 1.18–17.6 | 0.028 | 4.31 | 1.83–10.1 | 0.001 | 6.02 | 2.43–14.9 | <0.001 |
| Extremity location | 0.39 | 0.11–1.43 | 0.154 | 1.39 | 0.66–2.95 | 0.385 | 1.20 | 0.51–2.85 | 0.680 |
| No. chemotherapy cycles | 1.05 | 0.81–1.35 | 0.730 | 1.06 | 0.90–1.25 | 0.497 | 0.96 | 0.76–1.23 | 0.772 |
| Initial tumor size, per cm | 1.02 | 0.99–1.04 | 0.169 | 1.01 | 1.00–1.02 | 0.132 | 1.01 | 1.00–1.03 | 0.110 |
| Post-chemotherapy tumor size, per cm | 1.00 | 0.99–1.02 | 0.705 | 1.01 | 1.00–1.02 | 0.085 | 1.01 | 1.00–1.02 | 0.155 |
| PR by RECIST | 1.26 | 0.32–5.05 | 0.743 | 0.46 | 0.16–1.26 | 0.131 | 0.59 | 0.18–1.92 | 0.381 |
| Initial tumor volume, per 10 cm3 | 1.02 | 1.00–1.04 | 0.045 | 1.01 | 0.99–1.02 | 0.380 | 1.01 | 0.99–1.03 | 0.224 |
| Post-chemotherapy tumor volume, per 10 cm3 | 1.00 | 0.93–1.07 | 0.988 | 1.03 | 1.00–1.07 | 0.064 | 1.04 | 1.00–1.08 | 0.059 |
| Volume reduction > 75% | 1.49 | 0.36–6.25 | 0.583 | 0.54 | 0.21–1.37 | 0.196 | 0.81 | 0.27–2.41 | 0.701 |
| Necrosis ≤ 95% | 5.92 | 1.59–21.7 | 0.008 | 3.04 | 1.42–6.54 | 0.004 | 2.90 | 1.20–6.99 | 0.018 |
HR: hazard ratio; CI: confidence interval; PR: partial response; RECIST: Response Evaluation Criteria in Solid Tumors
Table 3.
Multivariate analysis by Cox regression of factors associated with disease outcomes in patients with ES treated with neoadjuvant chemotherapy and R0 resection
| Covariate | HR | 95% CI | P |
|---|---|---|---|
| Local control (12 events) | |||
| Necrosis ≤ 95% | 6.85 | 1.82–25.6 | 0.004 |
| Metastasis at presentation | 6.34 | 1.52–26.4 | 0.011 |
| Distant control (20 events) | |||
| Metastasis at presentation | 9.52 | 2.64–34.4 | 0.001 |
| Necrosis ≤ 95% | 5.24 | 1.54–17.9 | 0.008 |
| PR by RECIST | 0.21 | 0.05–0.80 | 0.022 |
| Progression-free survival (28 events) | |||
| Necrosis ≤ 95% | 5.92 | 2.07–16.9 | 0.001 |
| Metastasis at presentation | 6.86 | 2.08–22.7 | 0.002 |
| PR by RECIST | 0.26 | 0.09–0.74 | 0.012 |
| Post-relapse survival (21 events) | |||
| Simultaneous local and distant failure | 16.8 | 3.41–82.9 | 0.001 |
| Recurrence <1 year after surgery | 11.5 | 2.52–52.2 | 0.002 |
| Overall survival (21 events) | |||
| Metastasis at presentation | 6.75 | 2.05–22.3 | 0.002 |
| Necrosis ≤ 95% | 5.05 | 1.54–16.7 | 0.008 |
| Post-chemotherapy tumor volume, per 10 cm3 | 1.06 | 1.01–1.10 | 0.015 |
HR: hazard ratio; CI: confidence interval; PR: Partial response; RECIST: Response Evaluation Criteria in Solid Tumors
Figure 1.
Kaplan-Meier curves showing the prognostic effect on local control of tumor necrosis ≤95% (A); on progression-free survival by partial response by Response Evaluation Criteria in Solid tumors (B) and tumor necrosis (C); and on overall survival by necrosis (D). Thin lines denote 95% confidence intervals.
Factors associated with poorer PFS on UVA included older age, metastasis at presentation, and poor histologic response to induction chemotherapy. The 2-year PFS of 71% (95% CI: 48–93%) for patients with at least PR by RECIST was not significantly different than that of the no-response group (50%; 95% CI: 30–70%) by the log rank test (P=0.12) (Figure 1B). However, radiologic response by RECIST became an independent predictor when controlled for metastasis at presentation and histologic response on MVA. The 2-year PFS rates for good vs poor histologic response were 74% (95% CI: 60–88%) vs 36% (95% CI: 18–55%) (P=0.003, Figure 1C) and for localized vs metastatic disease were 66% (95% CI: 53–79%) vs 11% (95% CI: 0–33%) (P<0.001).
Independent predictors of OS were histologic response, metastasis at presentation, and post-chemotherapy tumor volume. Increased patient age was associated with poor prognosis on UVA but was not statistically significant on MVA. Rates of OS at 5 years was 76% (95% CI: 62–91%) vs 48% (95% CI: 27–68%) (P=0.013, Figure 1D) based on histologic response and 74% (95% CI: 62–87%) vs 11% (95% CI: 0–32%) (P<0.001) based on metastasis at presentation.
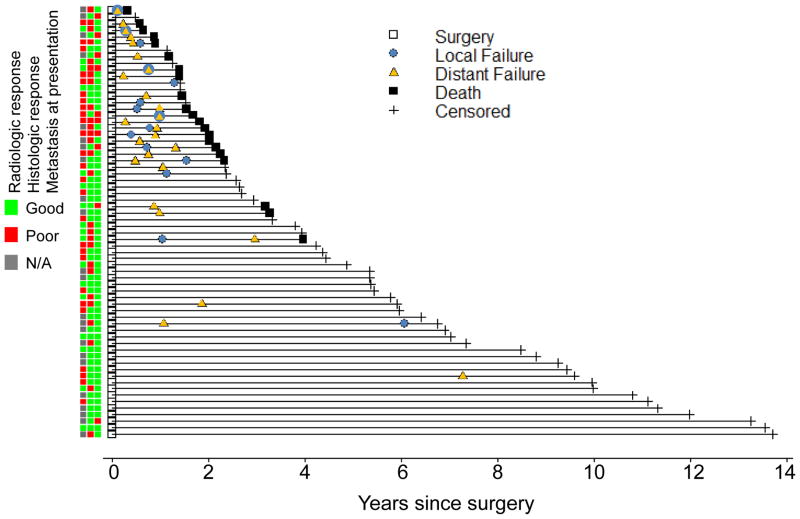
Patterns of relapse
All events were plotted on a single chart (Figure 2). The median time to initial relapse was 0.7 years (range: 0.1–7.3) after surgery. All but one initial recurrence event (96%) occurred within 2 years of surgery; that DR occurred at 7.3 years. All but one of the LR events (93%) occurred within 2 years of surgery; that LR occurred at 6.1 years after an initial DR at 1.1 years. All recorded death events were within 4 years of surgery. The hazard ratio (HR) for LR on OS was 12.7 (95% CI: 5.2–31.1, P<0.001) on UVA and 9.2 (95% CI: 3.6–23.2, P<0.001) after adjustment for metastasis and histologic response. As all deceased patients had DR prior to death, an HR was not computable, implicating DR as the primary driver of OS.
Figure 2.
A plot of disease outcome events in 66 patients with Ewing sarcoma treated with chemotherapy and R0 resection with accompanying heat map showing corresponding prognostic factor status.
The patterns of relapse and local salvage therapies are listed in Table 4. Of the 28 patients with recurrent disease, the initial recurrence was most often distant only (57%), followed by local only (29%) and both distant and local (14%). A total of 12 initial LR events were observed (8 LR-only and 4 simultaneous LR and DR). Of the 8 patients with initial LR-only, 5 (63%) subsequently had DR, most within 1 year of LR. Salvage therapy for initial LR was evenly divided between surgery only with amputation, surgery followed by RT (45–60 Gy), or RT only (40–55.8 Gy).
Table 4.
Patterns of initial and subsequent relapse in patients with ES treated with chemotherapy and R0 resection (N=66).
| Characteristic | Local (N=8) | Distant (N=16) | Both (N=4) |
|---|---|---|---|
| Time to recurrence, years | |||
| Median | 0.8 | 0.6 | 0.5 |
| Range | 0.4–1.3 | 0.2–7.3 | 0.1–1.0 |
| Salvage local therapy | |||
| Surgery alone | 2 (25%) | - | - |
| RT alone | 3 (38%) | - | 2 (50%) |
| Surgery + RT | 3 (38%) | - | - |
| Second relapse | |||
| Local | - | 3 (19%) | - |
| Distant | 5 (63%) | - | - |
| Time to second relapse, years | |||
| Median | 0.5 | 1.1 | - |
| Range | 0.2–1.9 | 0.2–5.0 | - |
The median PRS was 1.5 years, with a 2-year PRS of 28% (95% CI: 9–46%) and a 5-year PRS of 12% (95% CI: 0–27%) (Supplemental Figure 2A). On UVA, PRS was not associated with patient age, tumor location, initial tumor size, radiologic response by RECIST or volumetric reduction, or histologic response. The type of local salvage therapy was not associated with PRS when applied to all recurrences or to the subset with initial LR. The only independent factors associated with worse PRS were initial simultaneous LR and DR and shorter time to recurrence (Table 3). The 2-year PRS for patients who experienced recurrence less than 1 year after surgery was 6% (95% CI: 0–17%), while it was 88% (95% CI: 64–100%) in patients who experienced later recurrence (P=0.001, Supplemental Figure 2B). For the 12 patients with initial LR, the median post-LR survival was 1.2 years, with 1-year survival of 64% (95% CI: 35–93%) and a 2-year survival of 14% (95% CI: 0–39%) (Supplemental Figure 2C).
Discussion
Our first key finding was that disease progression occurred within 2 years of surgery in a predominantly distant pattern, including after initial local-only recurrence, and a strong association was found between DR and death. Consistent with prior reports[10,11,13,14], our study validated that poor histologic response, poor radiologic response, and metastasis on presentation predicted worse outcomes.
The most consistently described prognostic factors in ES are metastasis at presentation[13,14] and histologic response to chemotherapy. Early studies graded the histologic response as macroscopic, microscopic, or no viable tumor present[9,11,15], while later studies characterized the response as a percentage of tumor necrosis, using 5% or 10% remaining viable tumor as the cutoff between poor and good response[10,16,17]. Patients whose tumor expressed the non-Type1 EWS-FLI1 fusion oncogene previously also had a poorer prognosis, but this disparity has been eliminated with intensified treatment protocols[18]. Our results were consistent with the literature, with overt metastatic presentation and tumor necrosis ≤95% each independently conferring an approximately 7- or 6-fold risk of disease progression, respectively, and a 7- or 5-fold risk of death, respectively, despite R0 resection. Even with an apparently localized presentation, ES is considered a systemic disease, as evinced by the relapse rate of up to 90% when ES was treated with local therapy alone[19,20]. A poor histologic response is suggestive of resistant disease in which initial subclinical micrometastasis eventually progresses to overt metastasis.
Prior investigations have sought to identify radiologic response as either a proxy of histologic response or an independent predictor of outcome. Early reports of initial tumor volume as a prognostic factor were conflicting[15,17], while a study that examined volumetric response to therapy found soft tissue reduction <50% to denote poor prognosis[10]. Functional imaging such as positron emission tomography (PET) was studied with more promising results. In a series of 36 patients, absolute value of standard uptake value (SUV) of PET tracer after neoadjuvant chemotherapy was predictive of PFS[21], and a study of 14 patients demonstrated a correlation between the relative change in SUV and histologic response[22]. In our study, there was no correlation between radiologic response and histologic response, but different radiologic measures were associated with disease outcomes. In particular, small tumor size trended toward association with LC, PR (or better) by RECIST was an independent predictor of DR and PFS, and post-chemotherapy tumor volume was inversely correlated with OS. These radiologic measures have logical biological correlates of initial disease burden, chemotherapy sensitivity, and residual post-chemotherapy disease burden, respectively.
In our study cohort, all but one disease recurrence occurred within 2 years, with a 12-fold risk of death if relapse occurred less than 1 year after surgery. Importantly, systemic disease control was the primary driver of OS, as all deaths were preceded by DR. Our findings of a predominantly distant initial relapse pattern and poorer prognosis for patients with simultaneous LR and DR and shorter time to relapse are similar to a prior report of 71 cases of recurrent ES[23]. However, the prior study showed better PRS for patients who underwent salvage surgery, which was not observed in the current study. This may be due to differences in RT dose, as recurrences treated with RT in the current study received ≤40 Gy, while the prior study had 5 of 13 recurrences receiving <35 Gy and overlapping PRS CIs for >35 Gy of RT and surgery[24].
Recently completed prospective chemotherapy trials have investigated the effect of treatment intensification on ES. While dose-intensified VDC/IE did not improve overall outcome[25], interval-compressed VDC/IE increased EFS without additional treatment toxicity in pediatric cases[6]. Ongoing investigations of additional chemotherapy options include combining vincristine, topotecan, and cyclophosphamide (VTC) with the standard VDC/IE regimen (COG-AEWS1031) and using high-dose consolidation chemotherapy with busulfan and melphalan with autologous peripheral stem cell transplantation (Euro-EWING 99). In addition to these efforts to maximize the delivery of cytotoxic chemotherapy, other studies have investigated the role of biologically targeted therapies. Early clinical trials of inhibitors of insulin growth factor-1 receptor and mammalian target of rapamycin have shown promising results[26], and preclinical investigations of antagonists of EWS-FLI1 fusion protein are underway[27].
While the cooperative group ES trials have sought to primarily answer chemotherapy questions, the role of RT in ES has evolved through the combination of secondary analyses, retrospective series, and expert opinion. Surgery and RT have both been options for initial LC of ES, but it was unclear if historically better LC rates with surgery were attributable to selection bias. A recent comparative evaluation demonstrated a 2.4-fold increased risk of LR with RT when controlled for other risk factors, but was unable to take into account margin status, radiologic response, and histologic response[7]. The study also found no difference in EFS or OS by LC method, underscoring the importance of systemic therapies for long-term outcomes.
The next question, then, is the role of adjuvant RT after R0 resection, particularly in the case of poor histologic response, as our study showed 2-year LC rates of 91% vs 56% based on this factor. Adjuvant RT was recommended but not always administered for poor histologic response in prior European Intergroup Cooperative Ewing Sarcoma Study (EICESS) trials. A review of EICESS results showed that the LR rate decreased from 12% (3 of 25 patients) to 6% (3 of 59 patients) when postoperative RT was added to wide resection in patients with poor histologic response[28]. Separately, a review of the combined surgery and RT experience of 39 patients at a single institution revealed a 5-year LC rate of 89% that was not significantly altered by histologic response[29]. These results suggest that adjuvant RT may obviate the disadvantage of poor histologic response in LC outcomes.
While we found a 9-fold increased risk of death and a median survival of 1 year after LR, it is difficult to separate the effects of LR and DR in our study, as 9 of 12 patients with LR also developed simultaneous or subsequent DR. The overall pattern of relapse with all deaths preceded by DR suggest that the potential benefit of adjuvant RT must be balanced with the expense of delaying systemic therapy, which we recommend be discussed in a multidisciplinary tumor board. The current COG-AEWS1031 trial guidelines omit adjuvant RT if adequate surgical margins are obtained, regardless of degree of tumor necrosis. The primary rationale for this approach is to prioritize consolidative chemotherapy to address micrometastatic disease. Another concern is to minimize the risk of RT-related toxic effects, including secondary malignancy, previously estimated at about 9% over 20 years and related to dose [30].
A final scenario in which to consider RT is at the time of local recurrence. Our results show that initial LRs are often closely followed by DR. Furthermore, patients with initial recurrence less than 1 year after surgery had particularly poor outcomes. In these situations, RT might be favored over surgery for salvage therapy with the benefits of avoiding an invasive procedure in the presence of likely soon-to-be declared micrometastatic disease and removing the risk of postsurgical infection that can potentially delay the administration of chemotherapy needed to blunt the growth of multifocal metastases.
Comparison of the patient outcomes from this study with cooperative group trials is limited by differences in patient and treatment characteristics. The current study had a median patient age of 19 and included metastatic presentation (15%) and treatment with adjuvant high-dose ifosfamide [31,32]. In contrast, the AEWS0031 study was limited to localized presentations and had only 12% of patients aged ≥18[6]. With prior studies showing 5-year EFS of 43–47% for adult patients and 70–72% for pediatric patients[6,33], our 5-year PFS of 58% is comparable based on patient age distribution. Interestingly, among our 53 localized presentations, the incidence of 7 initial LR-only events (13%) is higher than the 7–8% in the two treatment arms of AEWS0031. Similar to prior reports [34], our study also included LR when it occurred simultaneously with initial DR for a total of 9 LR events (17%) among localized presentations.
Additional limitations to this study include its retrospective nature and associated biases. The sample size is relatively modest and there may be selection bias for patients who presented to our tertiary cancer center and for those who had available imaging to review. While all patients underwent R0 resection, the margin distance was not routinely recorded, which precluded an analysis of the effect of close margins. We await the results of COG-AEWS1031, as many of its secondary aims overlap with the questions of this study, including the prognostic values of initial tumor size, histologic response, PET response, and surgical margin status.
In conclusion, histologic response, radiologic response, and metastatic presentation are independent predictors of outcome in ES patients after R0 resection. The utility of adjuvant RT to improve LC after poor histologic response requires further study.
Supplementary Material
Supplemental Figure 1. Kaplan-Meier survival curves for patients with Ewing sarcoma treated with neoadjuvant chemotherapy and R0 resection. At 5 years after surgery, the local control, distant control, progression-free survival, and overall survival rates were 78%, 69%, 58%, and 65%, respectively. Thin lines denote 95% confidence intervals.
Supplemental Figure 2. Kaplan-Meier curves for patients with Ewing sarcoma treated with neoadjuvant chemotherapy and R0 resection showing post-recurrence survival for all recurrences (A) and then stratified by recurrence less than 1 year after surgery (B), followed by post-local failure survival (C). Thin lines denote 95% confidence intervals.
Summary.
This retrospective review of 66 patients treated with chemotherapy and surgery without radiation for Ewing sarcoma identified histologic response, radiologic response, and metastatic presentation as independent predictors of outcome. All deaths were preceded by distant failure, and initial local-only failures were often soon followed by distant failure. Additional study is needed to determine the role of adjuvant radiation for patients who have poor histologic response after R0 resection.
Acknowledgments
Funding: None
The authors would like to thank Kathryn Hale, MS, of MD Anderson’s Department of Scientific Publications, for her work in reviewing and editing this manuscript.
Footnotes
Abstract selected for oral presentation at the 2014 Annual Meeting of the American Society for Radiation Oncology (ASTRO) on 9/17/14 in San Francisco, CA.
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Esiashvili N, Goodman M, Marcus RB., Jr Changes in incidence and survival of ewing sarcoma patients over the past 3 decades: Surveillance epidemiology and end results data. J Pediatr Hematol Oncol. 2008;30:425–430. doi: 10.1097/MPH.0b013e31816e22f3. [DOI] [PubMed] [Google Scholar]
- 2.Herzog CE. Overview of sarcomas in the adolescent and young adult population. J Pediatr Hematol Oncol. 2005;27:215–218. doi: 10.1097/01.mph.0000161762.53175.e4. [DOI] [PubMed] [Google Scholar]
- 3.Smith MA, Seibel NL, Altekruse SF, et al. Outcomes for children and adolescents with cancer: Challenges for the twenty-first century. J Clin Oncol. 2010;28:2625–2634. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howlader N, Noone A, Krapcho M, et al. Seer cancer statistics review, 1975–2011. National Cancer Institute; 2014. [Google Scholar]
- 5.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 6.Womer RB, West DC, Krailo MD, et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized ewing sarcoma: A report from the children’s oncology group. J Clin Oncol. 2012;30:4148–4154. doi: 10.1200/JCO.2011.41.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DuBois SG, Krailo MD, Gebhardt MC, et al. Comparative evaluation of local control strategies in localized ewing sarcoma of bone: A report from the children’s oncology group. Cancer. 2014 doi: 10.1002/cncr.29065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merchant TE, Hodgson D, Laack NN, et al. Children’s oncology group’s 2013 blueprint for research: Radiation oncology. Pediatr Blood Cancer. 2013;60:1037–1043. doi: 10.1002/pbc.24425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bacci G, Ferrari S, Bertoni F, et al. Prognostic factors in nonmetastatic ewing’s sarcoma of bone treated with adjuvant chemotherapy: Analysis of 359 patients at the istituto ortopedico rizzoli. J Clin Oncol. 2000;18:4–11. doi: 10.1200/JCO.2000.18.1.4. [DOI] [PubMed] [Google Scholar]
- 10.Oberlin O, Deley MC, Bui BN, et al. Prognostic factors in localized ewing’s tumours and peripheral neuroectodermal tumours: The third study of the french society of paediatric oncology (ew88 study) Br J Cancer. 2001;85:1646–1654. doi: 10.1054/bjoc.2001.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picci P, Bohling T, Bacci G, et al. Chemotherapy-induced tumor necrosis as a prognostic factor in localized ewing’s sarcoma of the extremities. J Clin Oncol. 1997;15:1553–1559. doi: 10.1200/JCO.1997.15.4.1553. [DOI] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the united states, national cancer institute of canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Cotterill SJ, Ahrens S, Paulussen M, et al. Prognostic factors in ewing’s tumor of bone: Analysis of 975 patients from the european intergroup cooperative ewing’s sarcoma study group. J Clin Oncol. 2000;18:3108–3114. doi: 10.1200/JCO.2000.18.17.3108. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Galindo C, Liu T, Krasin MJ, et al. Analysis of prognostic factors in ewing sarcoma family of tumors: Review of st. Jude children’s research hospital studies. Cancer. 2007;110:375–384. doi: 10.1002/cncr.22821. [DOI] [PubMed] [Google Scholar]
- 15.Rosito P, Mancini AF, Rondelli R, et al. Italian cooperative study for the treatment of children and young adults with localized ewing sarcoma of bone: A preliminary report of 6 years of experience. Cancer. 1999;86:421–428. doi: 10.1002/(sici)1097-0142(19990801)86:3<421::aid-cncr10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 16.Lin PP, Jaffe N, Herzog CE, et al. Chemotherapy response is an important predictor of local recurrence in ewing sarcoma. Cancer. 2007;109:603–611. doi: 10.1002/cncr.22412. [DOI] [PubMed] [Google Scholar]
- 17.Paulussen M, Ahrens S, Dunst J, et al. Localized ewing tumor of bone: Final results of the cooperative ewing’s sarcoma study cess 86. J Clin Oncol. 2001;19:1818–1829. doi: 10.1200/JCO.2001.19.6.1818. [DOI] [PubMed] [Google Scholar]
- 18.van Doorninck JA, Ji L, Schaub B, et al. Current treatment protocols have eliminated the prognostic advantage of type 1 fusions in ewing sarcoma: A report from the children’s oncology group. J Clin Oncol. 2010;28:1989–1994. doi: 10.1200/JCO.2009.24.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dahlin DC, Coventry MB, Scanlon PW. Ewing’s sarcoma. A critical analysis of 165 cases. J Bone Joint Surg Am. 1961;43-A:185–192. [PubMed] [Google Scholar]
- 20.Falk S, Alpert M. Five-year survival of patients with ewing’s sarcoma. Surg Gynecol Obstet. 1967;124:319–324. [PubMed] [Google Scholar]
- 21.Hawkins DS, Schuetze SM, Butrynski JE, et al. [18f]fluorodeoxyglucose positron emission tomography predicts outcome for ewing sarcoma family of tumors. J Clin Oncol. 2005;23:8828–8834. doi: 10.1200/JCO.2005.01.7079. [DOI] [PubMed] [Google Scholar]
- 22.Gupta K, Pawaskar A, Basu S, et al. Potential role of fdg pet imaging in predicting metastatic potential and assessment of therapeutic response to neoadjuvant chemotherapy in ewing sarcoma family of tumors. Clin Nucl Med. 2011;36:973–977. doi: 10.1097/RLU.0b013e31822f684b. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Galindo C, Billups CA, Kun LE, et al. Survival after recurrence of ewing tumors: The st jude children’s research hospital experience, 1979–1999. Cancer. 2002;94:561–569. doi: 10.1002/cncr.10192. [DOI] [PubMed] [Google Scholar]
- 24.Donaldson SS. Ewing sarcoma: Radiation dose and target volume. Pediatr Blood Cancer. 2004;42:471–476. doi: 10.1002/pbc.10472. [DOI] [PubMed] [Google Scholar]
- 25.Granowetter L, Womer R, Devidas M, et al. Dose-intensified compared with standard chemotherapy for nonmetastatic ewing sarcoma family of tumors: A children’s oncology group study. J Clin Oncol. 2009;27:2536–2541. doi: 10.1200/JCO.2008.19.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz GK, Tap WD, Qin LX, et al. Cixutumumab and temsirolimus for patients with bone and soft-tissue sarcoma: A multicentre, open-label, phase 2 trial. Lancet Oncol. 2013;14:371–382. doi: 10.1016/S1470-2045(13)70049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erkizan HV, Kong Y, Merchant M, et al. A small molecule blocking oncogenic protein ews-fli1 interaction with rna helicase a inhibits growth of ewing’s sarcoma. Nat Med. 2009;15:750–756. doi: 10.1038/nm.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuck A, Ahrens S, Paulussen M, et al. Local therapy in localized ewing tumors: Results of 1058 patients treated in the cess 81, cess 86, and eicess 92 trials. Int J Radiat Oncol Biol Phys. 2003;55:168–177. doi: 10.1016/s0360-3016(02)03797-5. [DOI] [PubMed] [Google Scholar]
- 29.Krasin MJ, Rodriguez-Galindo C, Davidoff AM, et al. Efficacy of combined surgery and irradiation for localized ewings sarcoma family of tumors. Pediatr Blood Cancer. 2004;43:229–236. doi: 10.1002/pbc.20095. [DOI] [PubMed] [Google Scholar]
- 30.Kuttesch JF, Jr, Wexler LH, Marcus RB, et al. Second malignancies after ewing’s sarcoma: Radiation dose-dependency of secondary sarcomas. J Clin Oncol. 1996;14:2818–2825. doi: 10.1200/JCO.1996.14.10.2818. [DOI] [PubMed] [Google Scholar]
- 31.Ferrari S, del Prever AB, Palmerini E, et al. Response to high-dose ifosfamide in patients with advanced/recurrent ewing sarcoma. Pediatr Blood Cancer. 2009;52:581–584. doi: 10.1002/pbc.21917. [DOI] [PubMed] [Google Scholar]
- 32.Patel SR, Vadhan-Raj S, Papadopolous N, et al. High-dose ifosfamide in bone and soft tissue sarcomas: Results of phase ii and pilot studies--dose-response and schedule dependence. J Clin Oncol. 1997;15:2378–2384. doi: 10.1200/JCO.1997.15.6.2378. [DOI] [PubMed] [Google Scholar]
- 33.Gupta AA, Pappo A, Saunders N, et al. Clinical outcome of children and adults with localized ewing sarcoma: Impact of chemotherapy dose and timing of local therapy. Cancer. 2010;116:3189–3194. doi: 10.1002/cncr.25144. [DOI] [PubMed] [Google Scholar]
- 34.Yock TI, Krailo M, Fryer CJ, et al. Local control in pelvic ewing sarcoma: Analysis from int-0091--a report from the children’s oncology group. J Clin Oncol. 2006;24:3838–3843. doi: 10.1200/JCO.2006.05.9188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Kaplan-Meier survival curves for patients with Ewing sarcoma treated with neoadjuvant chemotherapy and R0 resection. At 5 years after surgery, the local control, distant control, progression-free survival, and overall survival rates were 78%, 69%, 58%, and 65%, respectively. Thin lines denote 95% confidence intervals.
Supplemental Figure 2. Kaplan-Meier curves for patients with Ewing sarcoma treated with neoadjuvant chemotherapy and R0 resection showing post-recurrence survival for all recurrences (A) and then stratified by recurrence less than 1 year after surgery (B), followed by post-local failure survival (C). Thin lines denote 95% confidence intervals.