Abstract
The purpose of this study was to understand the autoimmune response and immunogenicity of a tumor-associated antigen IMP2/p62 in breast cancer. Autoantibody responses to IMP2/p62 were evaluated by enzyme-linked immunosorbent assay (ELISA), western blotting and indirect immunofluorescence assay in sera from patients with breast cancer, benign breast tumor and normal human individuals. Immunohistochemistry (IHC) study with breast cancer tissues was also performed to analyze protein expression of IMP2/p62. The results have demonstrated that IMP2/p62 can induce a relatively higher frequency of autoantibody response in breast cancer (14.3%, 7/49) compared to patients with benign breast tumor (5.6%, 2/36) and normal individuals (2.2%, 1/44). The frequency of IMP2/p62 expression in breast cancer tissues was significantly higher than that in normal tissues (P<0.01). The data suggest that autoantibody against IMP2/p62 may be a useful serum biomarker for early stage breast cancer screening and diagnosis.
Keywords: IMP2/p62, breast cancer, immunodiagonsis, tumor-associated antigen, autoantibody
Introduction
Breast cancer is one of the most common malignant tumors in women. In recent years, the incidence of breast cancer increased rapidly [1]. As with all cancer, early diagnosis and appropriate therapy can reduce the mortality rate of breast cancer and early detection is one of the most significant predictors of survival. Cancer has long been recognized as a highly complex disorder that involves not only the interplay of genomic changes but also factors including the immunological, hormonal, environmental, and other disease [2]. There is persuasive evidence that the immune system of the cancer patients have the capability of sensing abnormalities in structure, function, intracellular location, and other alterations of cellular participants in carcinogenesis. Many studies have demonstrated that cancer sera contain antibodies that react with a unique group of autologous cellular antigens called tumor-associated antigens (TAAs) [3–5]. These antigens are probably cellular proteins, such as p16, IMP2/p62, whose aberrant regulation or overexpression is capable of leading to tumorigenesis [6–7]. Therefore, these anti-TAA antibodies likely can be regarded as reporters, which can identify the antigenic changes in cellular factors involved in the transformation process [8]. We have made use of such autoantibodies from patients with hepatocellular carcinoma to immunoscreen a cDNA expression library and have identified a cytoplasmic mRNA-binding protein IMP2/p62 [9]. IMP2/p62 is a member of a family of proteins which were proposed to call RRM-KH proteins. These proteins have two types of RNA-binding motifs, the RNA recognition motif (RRM) and hnRNP K homology (KH) motif arranged in a characteristic order [4]. Several proteins of this family bind to insulin-like growth factor II (IGF-II) mRNA, which is currently known as IMPs (IGF-II mRNA-binding proteins). The latter finding is of special interest, because of many studies showing the association of increased IGF-II expression with carcinogenesis [10–11]. Autoantibodies to IMP2/p62 have been detected in up to 21% of a cohort of patients with hepatocellular carcinoma (HCC) [9]. Furthermore, IMP2/p62 has been demonstrated as a novel oncoprotein and a growing number of reports have shown its overexpression in many human malignancies. Many investigators have been interested in the use of autoantibodies as serological markers for cancer diagnosis, especially because of the general absence of these autoantibodies in normal individuals and non-cancer conditions. In this study, we aimed to investigate whether serum anti-IMP2/p62 autoantibody can be used as a biomarker in immunodiagnosis of breast cancer.
Materials and Methods
Patients and Serum Samples
In this study, 49 sera from breast cancer, 36 sera from patients with benign breast tumor and also 44 normal human sera (NHS) were derived from the sera bank in the Cancer Autoimmunity Research Laboratory at the University of Texas, El Paso (UTEP). All cancer sera were collected at one time of cancer diagnosis when the patients had not yet received treatment with any chemotherapy or radiotherapy, 44 normal human sera were collected from adults during annual health examination in people who had no obvious evidence of malignancy. Due to regulations concerning studies of human subjects, the patient’s name and identification number were blinded to investigators.
Enzyme-linked Immunosorbent Assay (ELISA)
Recombinant IMP2/p62, which is same to the IGF2BP2 isoform b, has been expressed from a clone isolated from cDNA expression library by immunoscreening with serum antibody from a patient with HCC [9]. Purified recombinant protein IMP2/p62 was diluted in PBS to a final concentration of 0.5μg/ml, and 100μl were added into each well to coat microtitre plates (Fisher Scientific, Houston, TX, USA) overnight at 4°C. Plates were blocked with gelatin post-coating solution for 2h at room temperature. The human serum samples were diluted at 1:200, incubated with the antigen-coated wells at 37°C for 90 min followed by washing with PBS containing 0.05% Tween 20 (PBST), then incubated with goat antihuman IgG-HRP (Invitrogen, NY) diluted 1:4000 for 90 min followed by washing with PBST. The substrate 2,2′-azino-bis-3-ethylbenzo-thiazoline-6-sulfonic acid (ABTS, Invitrogen) were used as detecting reagents. The optical density (OD) value of each well was read at 405nm. Each sample was tested in duplicate. The cut-off value for determining a positive reaction was designated as the mean OD value of the 44 normal human sera plus 3 standard deviations (mean + 3SD).
Western blotting
Purified recombinant IMP2/p62 proteins was electrophoresed on 10% SDS-PAGE and subsequently transferred onto a nitrocellulose membrane. After blocking with PBST containing 3% nonfat dry milk for 1h at room temperature, the nitrocellulose papers were incubated for 60 min at room temperature with a 1:200 dilution of serum. HRP-conjugated goat antihuman IgG was applied as secondary antibodies at a 1:20,000 dilution. Immunoreactive bands were detected using the ECL kit (Thermo Scientific) according to the manufacturer’s instructions.
Indirect immunofluorescence (IIF) assay
Commercially available Hep-2 cell slides (MBL International Corporation, MA) were used in IIF for identification of autoantibodies in cancer sera. Sera with1:80 dilution and anti-IMP2/p62 antibody with 1:50 dilution were incubated for 1h at room temperature. FITC-conjugated goat anti-human IgG and anti-rabbit IgG Fab2 were used as secondary antibodies at a 1:200 and 1:100 dilution, respectively. Confocal fluorescence images were acquired with a laser scanning microscope (LSM 700; Zeiss, New York, NY), using a×20 objective and processed with ZEN 2009 software (Zeiss, CA).
Absorption of antibodies with recombinant protein
The diluted human sera (1:80) were incubated with recombinant protein (final concentration of recombinant protein was 0.03μg/μl) overnight at 4°C and then centrifuged at 10,000×g for 10 min. The supernatant was used for immunofluorescence assay.
Immunohistochemistry (IHC) with tissue array slides
In this study, the tissue array slide with 50 breast cancer tissues and 11 normal breast tissue specimens were purchased (US Biomax, Inc., Rockville, MD) and used to detect the expression of the IMP2/p62 protein. Tissue array slides were deparaffinized with xylene and dehydrated with ethanol of different strengths. Antigen retrieval was performed by microwave-heating methods in Trilogy pretreatment solution for 20min. Avidin/biotin blocking solution was used to prevent nonspecific binding of antibodies. The sections were incubated with polyclonal anti-IMP2/p62 antibody (1:400 dilution) overnight at 4°C. The p62/IMP2 (IGF2BP2) polyclonal antibody was originally made in our lab, which was raised against p62-C-terminal peptide. The specificity of the p62/IMP2 (IGF2BP2) polyclonal antibody was first tested using Western blots. Anti-p62C specifically recognized recombinant p62/IMP2 (IGF2BP2) protein, but had no cross-reactivity with recombinant IMP1 (IFG2BP1) and IMP3/koc (IGF2BP3) [7]. The HRP Detection System (HRP streptavidin label and polyvalent biotinylated link) and DAB Substrate Kit were used as detecting reagents. After counterstaining with hematoxylin, the sections were dehydrated and mounted. The slides were observed by light microscopy (Leica DM1000, Germany).
Statistical Analysis
Statistical analysis was performed using SPSS13.0. The data were analyzed using ROC curve, the Chi-square (χ2) tests and represented as the mean ± 3 SD from ELISA. Two statistical significant levels (0.05 and 0.01) were used and p<0.05 was considered to indicate statistically significant differences.
Results
Prevalence of antibodies to IMP2/p62 in breast cancer
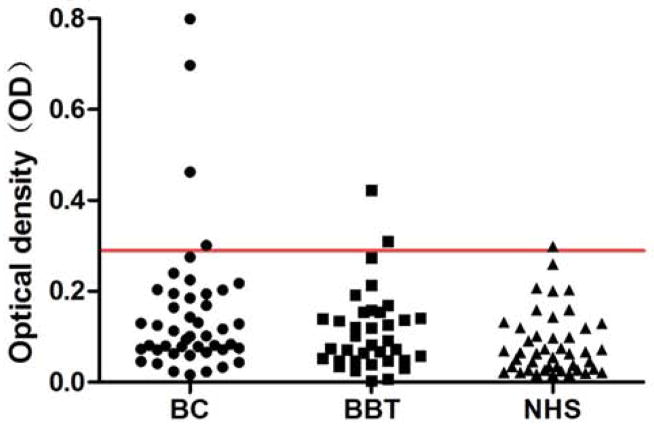
In the present study, full-length recombinant IMP2/p62 protein was used as coating antigen in ELISA to detect antibodies against this protein in sera from patients with different conditions. Sera from 49 patients with breast cancer and 36 patients with benign breast tumor as well as 44 normal human sera were available for this study. As showed in Table 1, the frequency of autoantibody to IMP2/p62 was 14.3% (7/49) in breast cancer sera, which was significantly higher than that in benign breast tumor sera (5.6%) and NHS (2.2%) (P < 0.05). Titer of anti-IMP2/p62 autoantibody in human sera is shown in Figure 1. The average titer of autoantibody against IMP2/p62 in breast cancer sera was higher than that in benign breast tumor and NHS (P < 0.01). Compared the antibody level of IMP2/p62 in patients with breast cancer to patients with benign breast tumor and to normal individuals through receiver operating characteristic (ROC) curve, the area under curve of each TAA is more than 0.5 (see Figure 2). These data indicated that there has a statistically significant difference of the antibody level in sera between breast cancer group and the others. The ELISA results were also confirmed by Western blot analysis. Figure 3 shows that representative breast cancer sera which have positive reaction to IMP2/p62 in ELISA, and also have strong reactivity in Western blotting analysis.
Table 1.
Frequency of autoantibody against IMP2/p62 in breast cancer
| Type of sera | No. tested | Autoantibody to IMP2/p62(%) |
|---|---|---|
| BC | 49 | 7(14.3)* |
| BBT | 36 | 2(5.6) |
| NHS | 44 | 1(2.2) |
BC: breast cancer; BBT: benign breast tumor; NHS: normal human sera;
Cut-off value: mean + 3 SD of NHS; P value relative to NHS:
P <0.05
Figure 1.
Titer of autoantibody against IMP2/p62 in human sera by ELISA. The range of antibody titers to IMP2/p62 was expressed as optical density (OD) obtained from ELISA. The mean + 3 SD of NHS were shown in relationship to all serum samples. Titer of anti-IMP2/p62 in breast cancer was much higher than that in other types of sera (P < 0.01). The cutoff value line for positive samples is indicated with red color.
Figure 2.
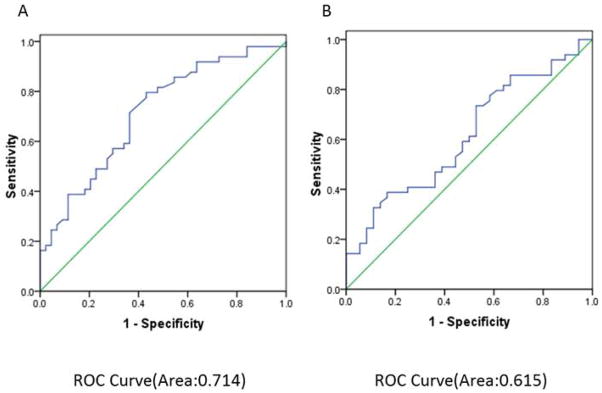
ROC analyses for IMP2/p62. Receiver Operating Characteristic curve for IMP2/p62 levels between breast cancer patients and benign breast tumor patients (A) or breast cancer patients and normal individuals (B).
Figure 3.
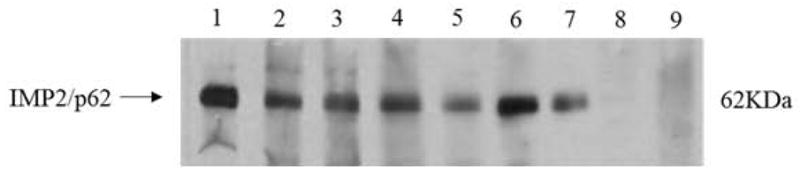
Western blot analysis with representative sera in ELISA. Lane 1, the monoclonal anti-IMP2/p62 antibody was used as positive control. Lanes 2–7, six representative breast cancer sera which were positive in ELISA and also had strong reactivity with IMP2/p62 recombinant protein in western blot. Lanes 8 and 9, normal human sera were used as negative control.
Indirect immunofluorescence analysis result of IMP2/p62 in Hep2 cells
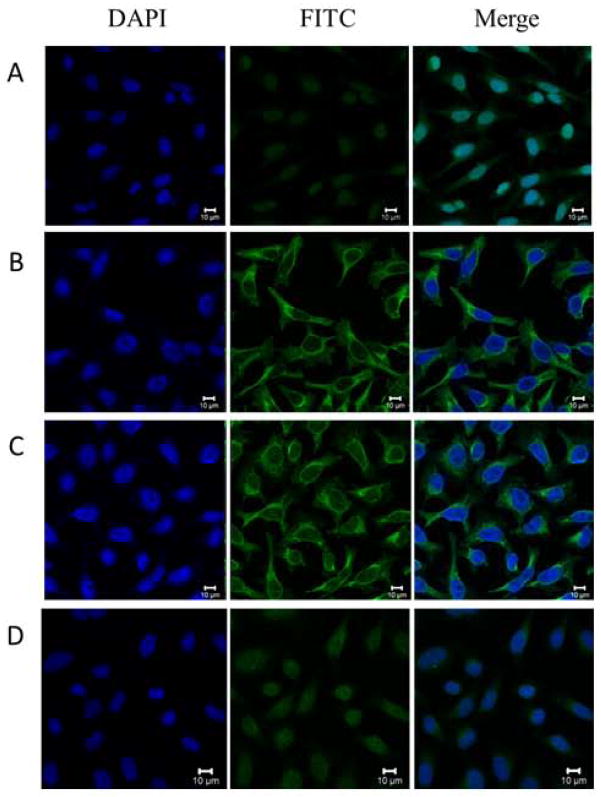
To further confirm the reactivity of autoantibody against IMP2/p62 in breast cancer sera and the intracellular location of IMP2/p62, commercially purchased Hep2 cell slides were used in indirect immunofluorescence assay to detect breast cancer sera with anti-IMP2/p62 positive in ELISA. As shown in Figure 4, a representative anti-IMP2/p62 positive breast cancer serum had cytoplasmic staining pattern. The fluorescent staining was significantly reduced when the same breast cancer serum was pre-absorbed with recombinant IMP2/p62 protein.
Figure 4.
Representative immunofluorescence staining pattern of anti-IMP2/p62 antibody positive breast cancer serum. A: a normal human serum (NHS) was used as negative control; B: monoclonal anti-62/IMP2 antibody that showed a cytoplasmic immunofluorescence staining pattern was used as positive control; C: a representative anti-IMP2/p62 antibody positive breast cancer serum demonstrated cytoplasmic immunofluorescence staining pattern; D: the same breast cancer serum used in panel C was post- absorbed with recombinant IMP2/p62 protein. The fluorescent staining was remarkably reduced.
Expression of IMP2/p62 in breast cancer tissues by immunohistochemistry with tissue array
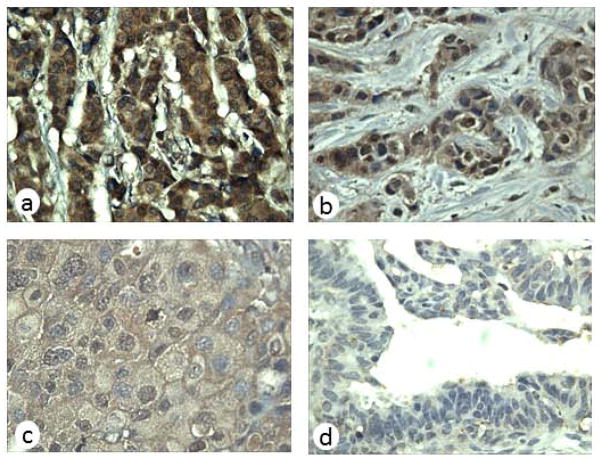
In this study, immunohistochemical staining was performed to detect the protein expression of IMP2/p62 in breast cancer and normal breast tissue. As shown in Table 2, 66% (33/50) breast cancer tissues were positively stained and 18% (2/11) normal tissues were positively stained. The frequency of IMP2/p62 expression in breast cancer tissue was significantly higher than that in normal breast tissue (p<0.01). The expression of IMP2/p62 in breast cancer and normal breast tissues is shown in Figure 5.
Table 2.
Expression of IMP2/p62 in malignant tumor tissues and normal tissues
| Score
|
||||||
|---|---|---|---|---|---|---|
| Type of Tissues | No. tested | Positive (%) | 0 | + | ++ | +++ |
| Malignant tumors | 50 | 33 (66%) | 17 | 6 | 12 | 15 |
| Normal tissues | 11 | 2 (18%) | 9 | 2 | 0 | 0 |
Scoring criteria: 0, negative staining; +, weak positive staining; ++, moderate positive staining; +++, strong positive staining.
P value of malignant tumor tissues relative to normal tissues: *P <0.01
Figure 5.
Expression of IMP2/p62 in breast cancer tissues examined by immunohistochemistry. Breast cancer tissue slides were stained with monoclonal anti-IMP2/p62 antibody at 1:400 dilution. (a) Breast cancer tissue (+++); (b) Breast cancer tissue (++); (c) Breast cancer tissue (+); (d) normal breast tissue had negative staining (magnification, ×400).
Discussion
Breast cancer is the most prevalent form of cancer among women. Early diagnosis and effective therapy are essential for the breast cancer. Tumor formation is a complex process. Many studies have demonstrated that cancer sera contain antibodies, which react with a unique group of autologous cellular antigens. These autoantibodies can be used as reporters identifying aberrant de novo or dysregulated cellular mechanisms in tumorigenesis, and therefore these autoantibodies may have important roles in the monitoring of tumor development [3–4]. IMP2/p62 is a member of insulin-like growth factor II mRNA-binding proteins (IMPs). The IMP family (IMP1, IMP2/p62 and IMP3/koc) have been found to regulate translation of insulin-like growth factor II (IGF2) mRNA in a post-transcriptional manner [9–10]. Members of this protein family are oncofetal proteins [12–13], which have been implicated in RNA localization, stability, and translation. These are essential for normal embryonic growth and development. The expression of these proteins disappears from all tissues soon after birth but frequently has appeared again during the process of malignant transformation. The overexpression of these proteins has been detected in many types of tumors, and it has been hypothesized that these proteins can mediate cell motility and invasion and might be closely related to cancer [14–15]. In order to understand the immunogenicity of IMP2/p62 and evaluate whether anti-IMP2/p62 autoantibody can be used as serological diagnostic marker in breast cancer, we detect anti-IMP2/p62 autoantibody in sera from patients with breast cancer and the expression of IMP2/p62 in breast cancer tissue specimens. In the present study, the result indicated that there was a relative higher frequency of anti-IMP2/p62 autoantibody response in breast cancer sera (14.3%) compared with benign breast tumor sera (5.6%) and normal human sera (2.2%). Immunohistochemistry study with breast cancer tissue array slides was also performed to evaluate IMP2/p62 protein expression in breast cancer. Of 50 breast cancer tissue specimens examined, 33 tissues (66%) had high level of IMP2/p62 expression, which was significantly higher than that in normal breast tissues (18%). This might indicate that IMP2/p62 is associated with breast cancer, and it can be also used as a potential marker in the detection of breast cancer.
Serum biomarkers have become increasingly important to diagnosis of breast cancer for its simple collection and non-invasiveness. CA153 and CEA are the most widely used markers in monitoring patients with breast cancer, and elevated serum levels are associated with breast cancer relapse, but both lack of sensitivity for early-stage disease and specificity [16]. In addition, CEA is a non-specific tumor marker and has a lower positive rate in early diagnosis of breast cancer. It cannot serve as an indicator of early diagnosis of breast cancer. One of our previous studies showed other TAAs (such as Imp1, p16, Koc, survivin, cyclinB1 and c-myc) had higher autoantibody response in breast cancer [17]. However, diagnosis based on single tumor marker detection of either a TAA or an anti-TAA antibody usually lacks sensitivity and specificity as noticed in most studies. Combination of these cancer-related autoantibodies resulted in increased diagnostic power of the assay suggesting that circulating antibodies could potentially be valuable diagnostic markers. Further research is underway to analyze the role of multiple circulating antibodies for early detection and prognosis of breast cancer. In this study, Immunohistochemical analysis of breast cancer tissues showed that cytoplasmic IMP2/p62 staining was significantly higher in malignant cells than that in normal breast cells. Due to the limited number of serum samples and tissues specimens, it is hard to establish a statistical association between IMP2/p62 and tumor grade, and also whether the autoantibody response to IMP2/p62 in breast cancer sera is correlated with the clinical significance remains to be established.
In conclusion, the data in this study showed IMP2/p62 can induce autoantibody response in sera from patients with breast cancer and indicated that IMP2/p62 might play an important role in breast cancer tumorigenesis. The anti-IMP2/p62 autoantibody might be a potential biomarker for clinical serologic screening in the diagnosis of breast cancer. Although the function of IMP2/p62 is not very clear, but this preliminary data does provide a very good clue for us to further understand the mechanism underlining the production of anti-IMP2/p62 antibody in patients with cancer.
Acknowledgments
This work was supported by a grant (SC1CA166016) from the National Institutes of Health (NIH). We also thank the Border Biological Research Center (BBRC) Core Facilities at The University of Texas at El Paso (UTEP) for their support, which were funded by NIH grant (2G12MD007592).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Tan HT, Lee YH, Chung MC. Cancer proteomics. Mass Spectrom Rev. 2012;31:583–605. doi: 10.1002/mas.20356. [DOI] [PubMed] [Google Scholar]
- 3.Tan EM. Autoantibodies as reporters identifying aberrant cellular mechanisms in tumorigenesis. J Clin Invest. 2001;108:1411–5. doi: 10.1172/JCI14451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan EM, Zhang J. Autoantibodies to tumor-associated antigens: reporters from the immune system. Immunol Rev. 2008;222:328–40. doi: 10.1111/j.1600-065X.2008.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Old LJ, Chen YT. New paths in human cancer serology. J Exp Med. 1998;187:1163–7. doi: 10.1084/jem.187.8.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang JY, Chan EK, Peng XX, et al. Autoimmune responses to mRNA binding proteins IMP2/p62 and koc in diverse malignancies. Clin Immunol. 2001;100:149–56. doi: 10.1006/clim.2001.5048. [DOI] [PubMed] [Google Scholar]
- 7.Lu M, Nakamura RM, Dent ED, et al. Aberrant expression of fetal RNA-binding protein p62 in liver cancer and liver cirrhosis. Am J Pathol. 2001;159:945–953. doi: 10.1016/S0002-9440(10)61770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan EM. Autoantibodies as reporters identifying aberrant cellular mechanisms in tumorigenesis. J Clin Invest. 2001;108:1411–5. doi: 10.1172/JCI14451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang JY, Chan EKL, Peng XX, Tan EM. A novel cytoplasmic protein with RNA-binding motifs is an autoantigen in human hepatocellular carcinoma. J Exp Med. 1999;189:1101–10. doi: 10.1084/jem.189.7.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen J, Christiansen J, Lykke-Andersen J, Johnsen AH, Wewer UM, Nielsen FC. A family of insulin-like growth factor II mRNA binding proteins represses translation in late development. Mol Cell Biol. 1999;19:1262–70. doi: 10.1128/mcb.19.2.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller-Pillasch F, Lacher U, Wallrap C, et al. Cloning of a gene highly expressed in cancer coding for a novel KH-domain containing protein. Oncogene. 1997;14:2729–33. doi: 10.1038/sj.onc.1201110. [DOI] [PubMed] [Google Scholar]
- 12.Liao B, Hu Y, Herrick DJ, Brewer G. The RNA-binding protein IMP-3 is a translational activator of insulin-like growth factor II leader-3 mRNA during proliferation of human K562 leukemia cells. Journal of Biological Chemistry. 2005;280:18517–24. doi: 10.1074/jbc.M500270200. [DOI] [PubMed] [Google Scholar]
- 13.Himoto T, Kuriyama S, Zhang JY, Chan EKL, Nishioka M, Tan EM. Significance of autoantibodies against insulin-like growth factor II mRNA-binding proteins in patients with hepatocellular carcinoma. International Journal of Oncology. 2005;26:311–17. [PubMed] [Google Scholar]
- 14.Ross J, Lemm I, Berberet B. Overexpression of an mRNA-binding protein in human colorectal cancer. Oncogene. 2001;20:6544–50. doi: 10.1038/sj.onc.1204838. [DOI] [PubMed] [Google Scholar]
- 15.Mueller-Pillasch F, Lacher U, Wallrapp C, et al. Cloning of a gene highly overexpressed in cancer coding for a novel KH-domain containing protein. Oncogene. 1997:142729–33. doi: 10.1038/sj.onc.1201110. [DOI] [PubMed] [Google Scholar]
- 16.Samy N, Ragab HM, El Maksoud NA, Shaalan M. Prognostic significance of serum Her2/neu, BCL2, CA15-3 and CEA in breast cancer patients: A short follow-up. Cancer Biomark. 2010;6:63–72. doi: 10.3233/CBM-2009-0119. [DOI] [PubMed] [Google Scholar]
- 17.Liu W, De La Torre IG, Gutiérrez-Rivera MC, Wang B, Liu Y, Dai L, Qian W, Zhang JY. Detection of autoantibodies to multiple tumor-associated antigens (TAAs) in the immunodiagnosis of breast cancer. Tumour Biol. 2014 Oct 30; doi: 10.1007/s13277-014-2756-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]