Abstract
Purpose
A new form of functional imaging has been proposed in the form of 4DCT-ventilation. Because 4DCTs are acquired as part of routine care for lung cancer patients, calculating ventilation maps from 4DCTs provides spatial lung function information without added dosimetric or monetary cost to the patient. Before 4DCT-ventilation is implemented it needs to be clinically validated. Pulmonary function tests (PFTs) provide a clinically established way of evaluating lung function. The purpose of our work was to perform a clinical validation by comparing 4DCT-ventilation metrics with PFT data.
Methods and Materials
Ninety-eight lung cancer patients with pre-treatment 4DCT and PFT data were included in the study. PFT metrics used to diagnose obstructive lung disease were recorded: forced expiratory volume in 1 second (FEV1) and FEV1/forced vital capacity (FEV1/FVC). 4DCT data sets and spatial registration were used to compute 4DCT-ventilation images using a density-change based and a Jacobian-based model. The ventilation maps were reduced to single metrics intended to reflect the degree of ventilation obstruction. Specifically, we computed the coefficient of variation (CoV) (standard deviation/mean), ventilation V20 (volume of lung ≤20% ventilation), and correlated the ventilation metrics with PFT data. Regression analysis was used to determine whether 4DCT-ventilation data could predict for normal versus abnormal lung function using PFT thresholds.
Results
Correlation coefficients comparing 4DCT-ventilation to PFT data ranged from 0.63–0.72 with the best agreement between FEV1 and CoV. 4DCT-ventilation metrics were able to significantly delineate between clinically normal versus abnormal PFT results.
Conclusions
Validation of 4DCT-ventilation with clinically relevant metrics is essential. We demonstrate good global agreement between PFTs and 4DCT-ventilation, indicating that 4DCT ventilation provides a reliable assessment of lung function. 4DCT-ventilation enables exciting opportunities to assess lung function and create functional avoidance radiotherapy plans. The current work presents supporting evidence for the integration of 4DCT-ventilation into clinical trials.
Introduction
A new and exciting form of lung functional imaging has been proposed that uses 4-dimensional computed tomography (4DCT) (1, 2) data to calculate ventilation maps (3–7). Because 4DCTs are acquired as part of routine clinical care for lung cancer patients, calculating ventilation maps from 4DCTs provides clinicians the ability to evaluate spatial lung function without the added dosimetric or monetary cost to the patient. 4DCT-ventilation also has attractive imaging characteristics including good spatial resolution (compared to nuclear medicine ventilation) and by definition 4DCT-ventilation provides both anatomical information (from the 4DCT) and functional information (from the 4DCT-ventilation) in one scan. Several authors have proposed potential clinical uses for 4DCT-ventilation (5, 8–14). Yamamoto et al and others (11, 14) proposed using 4DCT-ventilation for functional avoidance radiotherapy treatment planning. The hypothesis is that by avoiding the more functional portions of the lung (as defined by the 4DCT-ventilation), the rate of thoracic clinical toxicity could be reduced for lung cancer patients. _______ tested the ability of dose and dose-function to predict clinical radiation pneumonitis and found that 4DCT-ventilation can improve prediction of clinical toxicity. 4DCT-ventilation has been used to assess changes in lung function throughout (8) and after (13) radiotherapy. Outside of the scope of oncology, several authors have also shown the ability of 4DCT-ventilation to detect non-oncologic lung conditions such as emphysema and chronic obstructive pulmonary disease (COPD) (12, 15).
4DCT-ventilation must be properly validated before being put into clinical practice. Studies have attempted to validate 4DCT-ventilation by comparing it against other ventilation imaging modalities such as nuclear medicine ventilation-perfusion (VQ) imaging (16–18), xenon-CT (6, 19), positron emission tomography (20), and magnetic resonance imaging (21). The studies generally found good agreement on a global level with worsening results locally. Although the validation studies have provided promising results, more work is needed to demonstrate that 4DCT-ventilation is able to reliably depict patient lung function information.
Pulmonary function tests (PFTs) are routinely used by pulmonologists and provide an established way of evaluating lung function (22). In oncology, surgeons use PFTs to assess whether patients will be able to withstand lung surgery (23) and radiation oncologists can use PFTs to evaluate patients with poor lung function prior to radiation therapy (24). In this work we propose to use PFTs to further clinically validate 4DCT-ventilation in a large lung cancer patient dataset. The purpose of our work was to retrospectively compare 4DCT-ventilation with PFT data in 98 lung cancer patients.
Methods and Material
Patient population
Ninety-eight lung cancer patients from __________________ were used for the study. Patients were chosen retrospectively and included if they had 4DCT simulation and PFTs acquired prior to radiation treatment and within 100 days of each other. Patients were excluded if they had any thoracic interventions (surgery, chemotherapy, or radiation) between the 4DCT and PFT acquisitions. Patient and clinical characteristics are shown in Table 1. Patients with disease stages I–IV were included in the study which provided a patient database with a wide array of PFT lung function results. Of the 98 patients, 28 (29%) had pre-existing COPD.
Table 1.
Patient and clinical characteristics for the patient population used for the study.
| Parameter | Median (Range) or Number (%) |
|---|---|
| Age | 68 (43–87) |
| Sex | |
| F | 54 (55%) |
| M | 44 (45%) |
| COPD | |
| yes | 28 (29%) |
| no | 70 (71%) |
| Tumor location | |
| Right | 54 (55%) |
| Left | 42 (43%) |
| Both | 2 (2%) |
| Tumor Stage | |
| I | 32 (33%) |
| II | 4 (4%) |
| III | 60 (61%) |
| IV | 2 (2%) |
PFT data
PFTs use spirometry to measure air flow and are an established way of measuring lung function. Patients with poor PFT results have been shown to have clinically significant lung function deterioration and more respiratory complaints (25). Pulmonologists routinely use PFTs to diagnose lung disease such as asthma and COPD. Standard PFT metrics used to diagnose obstructive lung disease were recorded (22). For each patient we noted the Forced Expiratory Volume in 1 second (FEV1) and the ratio of the FEV1 and the Forced Vital Capacity (FEV1/FVC). PFT metrics were reported as percentage of predicted value which is based on healthy subjects with the same anthropomorphic characteristics (height, age, gender, and others) as the patient being tested (22). Generally, lower PFT metrics indicate worse lung function. In addition to evaluating the raw PFT data we separated the data into 2 groups (normal and abnormal lung function) and performed a binary analysis. Due to the uncertainties in spirometry testing pulmonologists often delineate normal versus abnormal lung function using a PFT threshold, rather than interpreting PFT data as a continuous variable. A PFT value of 70% (26) is a commonly used threshold to separate normal versus abnormal results. To mimic the pulmonologist’s binary interpretation of PFT results, we used a 70% threshold of FEV1 and FEV/FVC to delineate normal versus abnormal lung function for the binary analysis.
4DCT-ventilation
The patient’s pre-treatment simulation 4DCT scan was used to calculate 4DCT-ventilation images. The 4DCT scans were acquired either on a Brilliance Big Bore scanner (Phillips Healthcare, Andover, MA) using a bellows belt to track breathing motion or a Discovery PET/CT (GE Healthcare, Waukesha, WI) system with a Varian RPM to track breathing motion. The lungs were then segmented on the inhale and exhale phases of the breathing cycle (7). As part of the segmentation any voxel with a Hounsfield Unit (HU) greater than −250 was excluded. A deformable registration algorithm based on compressible flow that was previously presented (27) was used to map lung voxel elements from inhale to exhale. The accuracy of the registration was shown to be 1.25 mm in the lung (27). 4DCT-ventilation was calculated using the HU-based model (3, 4, 8) and the Jacobian model (5, 6, 13). The HU (3, 4, 8) model uses the following equation to calculate ventilation
| Equation 1 |
where Vin and Vex are the inhale and exhale volumes and HUin and HUex are the inhale and exhale Hounsfield units of the individual lung voxels. Applying Equation 1 on a voxel-by-voxel basis produces a 3D spatial map of ventilation function throughout the lung (Figure 1). Smoothing was applied to produce final ventilation voxel sizes of 9×9×3 mm3. The HU model is derived from the idea that CT numbers are composed of a linear combination of water-like material and air-like material (3,4). The Jacobian-based ventilation was calculation by directly taking the Jacobian of the deformable registration results (5, 6, 13) and is based on the idea that local partial derivatives are related to the volume change of a given voxel. For each patient we manually reviewed the 4DCT scans for image artifacts and the deformation fields for anomalies and discontinuities. Review of the registration entailed overlaying the deformation vectors over the CT image and qualitatively evaluating whether the voxel movement was consistent with expected respiratory patterns and whether the magnitude and direction of any registration vectors significantly deviated from the patterns of the surrounding voxels. Six patients had to be excluded due to image artifacts (lung cut–off or volume averaging artifacts) and no patients had to be excluded due to their deformation fields.
Figure 1.
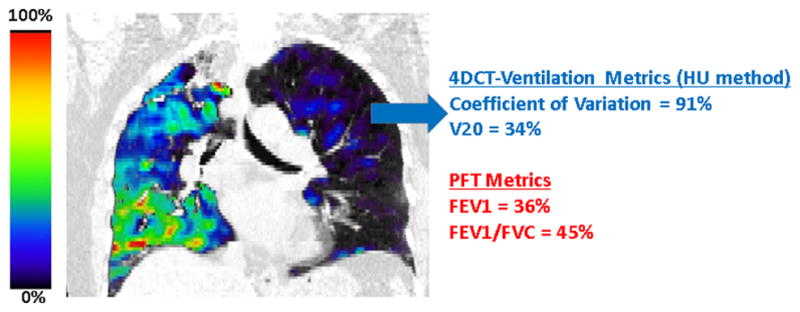
A representative example of a patient with poor lung function. The ventilation defects in the 4DCT-ventilation image, 4DCT-ventilation derived metrics (using the HU method), and PFT data all indicate poor lung function for the presented patient.
In order to compare the 4DCT-ventilation to PFT metrics, the 4DCT-ventilation images had to be reduced to single descriptive metrics. We derived single metrics from the 4DCT-ventilation that were intended to reflect the degree of ventilation obstruction and heterogeneity of the ventilation image. We computed the coefficient of variation (CoV) defined as the ratio of the standard deviation and the mean (20), the percentage of lung with 20% ventilation or less (V20) (15, 18), and an observer based binary metric of whether a ventilation defect was present or not. Larger CoV and V20 values indicated a more heterogeneous ventilation image and consequently worsening lung function. The observation of defect presence was done using consensus between 2 observers. Each observer reviewed cases independently using the 4DCT-ventilation image overlaid with the CT. Cases where the observers independently disagreed were discussed to produce a consensus observation of defect presence or absence.
The 4DCT-ventilation derived metrics (CoV, V20) were compared to the PFT metrics (FEV1, FEV1/FVC) using correlation coefficients and linear regression analysis. Two separate binary end-point analyses were performed. First, PFT metrics were compared among the group of patients with and without noted ventilation defects using t-tests (significance set at 0.05). Finally, we used logistic regression analysis to determine whether normal versus abnormal lung function (as defined by a PFT threshold of 70%) could be predicted using the 4DCT-ventilation and performed receiver operator characteristic (ROC) analysis to test model fit with area under the curve (AUC) metrics.
Results
A representative patient example is shown in Figure 1. The 4DCT-ventilation image, 4DCT-ventilation derived heterogeneity metrics, and the PFT data all indicate poor patient lung function. The 4DCT-ventilation image shows major ventilation defects in both lungs, the CoV indicates that the standard deviation of the image is nearly as large as the mean (indicating greater image heterogeneity), and the FEV1 is 36% of what is expected for that patient; all indications of poor lung function status.
The correlation of the 4DCT-ventilation derived metrics (CoV and V20) and the PFT metrics (FEV1, FEV1/FVC) were on the order of 0.7 for the HU method (Table 2). The lowest correlation occurred between the V20 and FEV1 (correlation=0.63, p<0.01) and the highest correlation occurred between the CoV and FEV1 (correlation=0.72, p<0.01). Correlation for the Jacobian-based ventilation metrics were on the order of 0.4 with the best correlation between FEV1/FVC and V20 (correlation=0.46, p<0.01). The scatter plot comparing the FEV1 as a function of the 4DCT-ventilation derived CoV shows that as the CoV decreases (lung function gets worse) the FEV1 congruently indicates worsening lung function (Figure 2).
Table 2.
Correlation coefficients and area under the curve from the ROC analysis comparing PFT metrics and 4DCT-ventilation derived metrics.
| 4DCT-Ventilation HU Metrics | 4DCT-Ventilation Jacobian Metrics | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CoV | V20 | CoV | V20 | ||||||
| CC | AUC | CC | AUC | CC | AUC | CC | AUC | ||
| PFT Metrics | FEV1 % of reference | 0.72 | 0.86 | 0.72 | 0.82 | 0.40 | 0.72 | 0.40 | 0.67 |
| FEV1/FVC | 0.67 | 0.83 | 0.67 | 0.81 | 0.38 | 0.64 | 0.46 | 0.67 | |
Abbreviations: PFT: Pulmonary function test, FEV1 = Forced expiratory volume in 1 second, FVC = Forced vital capacity, CoV = Coefficient of variation defined as the ratio of the standard deviation and the mean, V20 = volume of lung with 20% ventilation or less, CC=correlation coefficient, AUC = area under the curve.
Figure 2.
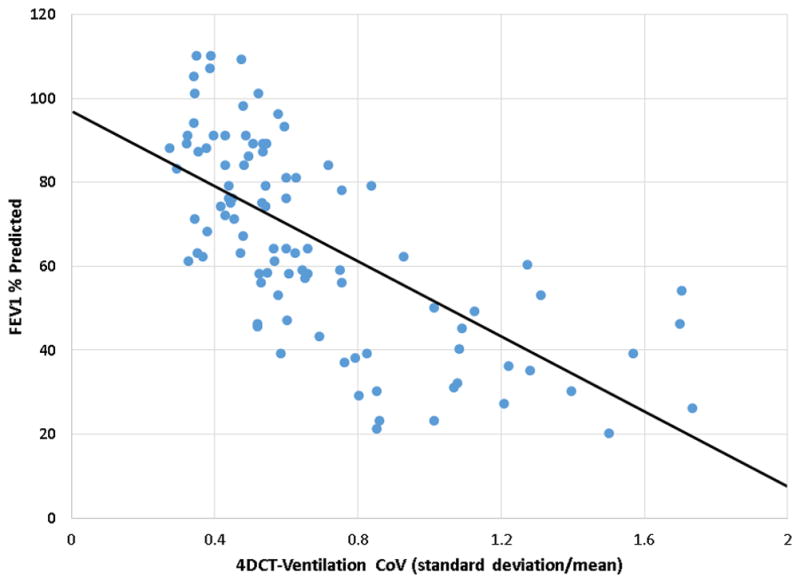
Scatter plot showing the relationship between the FEV1 and the 4DCT-ventilation derived CoV (using the HU method). As the CoV increases (lung function gets worse) the FEV1 is congruently reduced.
The patients were grouped according to whether they had observed ventilation defects and the PFT values of the 2 groups were compared (Table 3). The group that had no observed ventilation defects had a mean FEV1 of 70.8 while the group that had ventilation defects had a significantly (p=0.034) worse FEV1 mean value of 60.3.
Table 3.
A comparison of mean PFT values for patients with and without noted ventilation defects.
| Ventilation defect present (mean ± standard deviation) | No ventilation defect present (mean ± standard deviation) | ttest p value | |
|---|---|---|---|
| FEV1 | 60.3±22.8 | 70.8±25.1 | 0.034 |
| FEV1/FVC | 61.0±15.3 | 66.5±15.6 | 0.086 |
Abbreviations: PFT: Pulmonary function test, FEV1 – Forced expiratory volume in 1 second, FVC = Forced vital capacity.
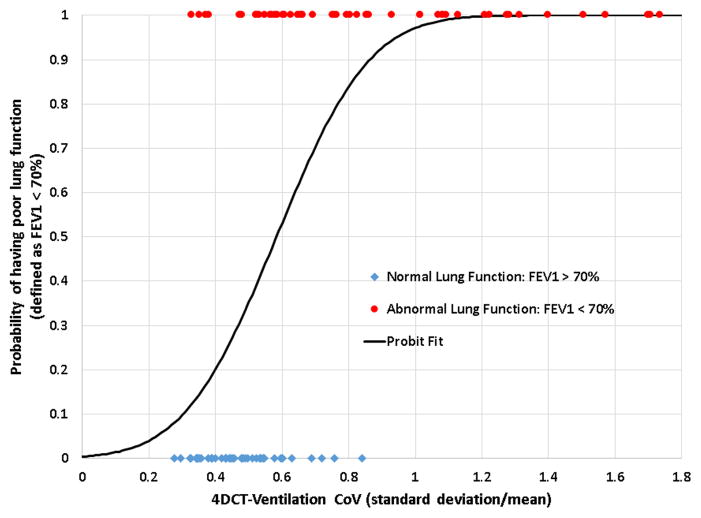
A threshold value of 70% was used for the PFT data to delineate normal versus abnormal lung function and the average ventilation-based CoV and V20 values were compared between the 2 groups. All comparisons produced statistically significant differences in ventilation-derived metrics (for both HU and Jacobian methods) for the normal versus abnormal groups. As an example, the average CoV value for the poor lung function group (FEV/FVC < 70%) was 0.83, while the average for the normal lung function group (FEV1/FVC > 70%) was 0.53 with the difference being significant (p<0.01). Similarly the FEV1/FVC differences between the groups with and without ventilation defects approached statistically significant differences (p=0.086). The sigmoid model fit converged (Figure 3) with a statistically significant fit (p<0.01) indicating the ability of the CoV to delineate between normal versus abnormal lung function. Model fit between the ventilation and PFT metrics was assessed using ROC analysis. The AUC values assessing model fit ranged from 0.81–0.86 for the HU based ventilation metrics and were 0.64–0.72 for the Jacobian based metrics (Table 2).
Figure 3.
A PFT threshold of 70% was used to delineate between normal versus abnormal lung function. The graph shows raw data points and a sigmoidal curve fit to the binary results (normal/abnormal) as a function of the ventilation-derived coefficient of variation. The significant model fit of the curve indicates the ability of 4DCT-ventilation (using the HU method) to delineate between normal versus abnormal lung function.
Discussion
The patient example (Figure 1), correlation coefficients on the order of 0.7, AUC values on the order of 0.8, and scatter plot (Figure 2) all indicate good overall agreement between 4DCT-ventilation and PFT data. In general, HU-based ventilation metrics produced better correlations and AUC results when compared to Jacobian-based ventilation metrics. When the data were categorized according to whether patient’s had observed ventilation defects, the group with observed defects had significantly worse FEV1 and FEV1/FVC values when compared to the group that did not have observed ventilation defects (Table 2). Ventilation metrics indicated worse lung function for the group with PFT results less than 70%. These data suggest that 4DCT-ventilation provides reliable global lung function information when compared to PFT data.
Our results are in line with previously reported validation of 4DCT-ventilation for patients with lung cancer (18). Yamamoto et al (18) compared 4DCT-ventilation derived metrics to FEV1 and FEV1/FVC and in a prospective trial with 18 patients and found correlation coefficients on the order of 0.5–0.73, which are similar to our correlation coefficients which ranged from 0.6–0.7. Murphy et al (15) compared CT-derived measures with spirometry results in 216 COPD patients and found correlation coefficient in the 0.85–0.90 range. A possible reason for the noted improvement in correlations reported by Murphy et al (15) is that they analyzed COPD patients while our study and the work by Yamamoto et al reports on lung cancer patients who may have airway occlusion due to the tumor as well as COPD. Another possibility to explain the lower correlation values for our study compared to Murphy et al (15) is that the relationship between 4DCT-ventilation and PFT metrics may not be linear (a saturation effects appears in Figure 2) and other mathematical relationships need to be investigated in future work. Our data can be also be compared to other validation work comparing 4DCT-ventilation imaging with other forms of ventilation imaging modalities (6, 16–21). For example, Ding et al (19) and Reinhardt et al (6) compared 4DCT-ventilation to xenon CT measures of ventilation with correlation ranging from 0.52 to 0.94, while Castillo et al (16) compared 4DCT-ventilation with nuclear medicine imaging and found dice similarity coefficients in the range of 0.10 to 0.60 with the best agreement occurring in the areas of ventilation defects. Recently, Kipritidis et al (20) compared 4DCT-ventilation to PET-Galligas ventilation and found correlation in the range of 0.22 to 0.76. An important question to be addressed that the current work along with others (7, 15–18, 20, 21) have struggled with is what constitutes a good enough correlation in order for 4DCT-ventilation to be a reliable predictor of global lung function. Mathematically, a good correlation value is taken to be on the order of 0.8; however, in the clinical setting, where the conditions are less controlled, the authors believe a value of 0.7 can be viewed as an indicator of correlation. Correlation is further complicated because PFT signal can be due to tumor occlusion as well as other conditions such as COPD. In future work, our goal is to attempt to isolate the effects of the tumor from other thoracic conditions to improve the confidence in the ability of 4DCT-ventilation to predict specific thoracic conditions.
Our study builds on prior work (15, 18) and present several important advancements in the validation of 4DCT-ventilation. Our study of 98 lung cancer patients presents the largest study set to date to validate 4DCT-ventilation with PFT data or any other imaging modality. In addition, we performed analysis treating PFT data as a binary variable delineating between normal versus abnormal lung function. Although the results are very preliminary, the idea that 4DCT-ventilation can differentiate between normal versus abnormal lung function (Figure 3), not only supports the validation of 4DCT-ventilation but presents the exciting idea that 4DCT-ventilation can be used to evaluate population-based metrics of lung function. Most suggested uses of 4DCT-ventilation propose to use 4DCT-ventilation for intrapatient comparisons but the work presented here suggests that certain 4DCT-ventilation metrics can be used to compare lung function among different patients.
Although PFTs present a single metric (rather than a 3D spatial map) and have their own shortcomings, they are currently an established gold standard of evaluating lung function. Clinical applications of 4DCT-ventilation such as radiotherapy functional avoidance and lung function assessment are based on the idea that 4DCT-ventilation provides clinically meaningful lung function information (5, 8, 9, 11, 12, 14) and this work along with others suggests that 4DCT-ventilation is able to provide an accurate assessment of lung function.
Because the data were collected retrospectively the PFTs and 4DCTs were generally collected at different time points. Patient breathing effort was also different between PFT and 4DCT data collection. PFT data collections are taken with the patient in forced breathing states while 4DCT data is generally taken under free breathing or abdominal compression conditions. In future prospective studies, correcting for patient breathing effort could improve correlation between the 2 methods of assessing lung function. There were uncertainties in both the PFT and 4DCT-ventilation data. PFTs are subject to uncertainties due to patient effort and co-operation, determination of reference values, and test interpretation. 4DCT-ventilation calculations are still subject to uncertainties due to the quality of the 4DCT (29) and the deformation algorithm (30), differences in the calculation metrics used (7), and reproducibility of the imaging (31, 32). An attempt was made to mitigate the uncertainties of the 4DCT-ventilation by reviewing all 4DCTs, deformations, and 4DCT-ventilation images. One of the challenges of the proposed work was to convert 3D ventilation images into single metrics. We chose 4DCT-ventilation metrics that we deemed clinically meaningful and metrics that were previously proposed (15, 18). However, the used metrics can have their shortcomings. For example, with certain thoracic conditions homogenous ventilation may not necessarily equate to normal patient lung function. Evaluating lung function can be complex and in some cases multiple evaluation tools (PFTs, imaging, clinical interpretation) may be needed for a full characterization. Another uncertainty lies in the observer reading of 4DCT-ventilation images. There are currently no established guidelines for the interpretation of 4DCT-ventilation and future work needs to be performed to establish quantitative and qualitative criteria for evaluation. In addition to V20, CoV, and defect presence, we analyzed the ipsilateral to contralateral ratio and minimum ventilation in each lung third which did not produce any meaningful correlations. Future development of 4DCT-ventilation metrics, including novel normalization strategies, is needed. We believe with continually improving 4DCT-ventilation techniques and refinement of 4DCT-ventilation derived metrics the accuracy in predicting lung function will only improve.
Conclusion
The current work attempted to validate 4DCT-ventilation by comparing 4DCT-ventilation derived metrics of lung function with PFT data. We found fairly good correlation coefficients on the order of 0.7 comparing 4DCT-ventilation to PFTs. 4DCT-ventilation metrics were able to delineate between normal versus abnormal lung function patients as defined by PFT thresholds (AUC values on the order of 0.8). PFTs provide an established way of measuring lung function and our validation data suggest that 4DCT-ventilation can provide an accurate assessment of lung function. 4DCT-ventilation enables exciting opportunities to assess lung function and create functional avoidance radiotherapy plans for lung cancer patients. The current work presents important supporting evidence towards thoracic clinical trial assessment of 4DCT-ventilation.
Summary.
An exciting form of lung functional imaging has been proposed that uses 4DCT data to calculation ventilation maps. The purpose of our work was to validate 4DCT-ventilation by comparing it to pulmonary function test data (PFT). We found good agreement between 4DCT-ventilation derived metrics of lung function and PFT data. Our results suggest that 4DCT-ventilation can provide an accurate assessment of lung function, supporting the design of 4DCT-ventilation clinical trials.
Acknowledgments
National Institutes of Health through an NIH Director’s New Innovator Award DP2OD007044 (EC, RC, TG)
National Institutes of Health Research Scientist Development Award K01-CA181292 (RC
State of Colorado Advanced Industries Accelerator Grant (YV)
Footnotes
Conflict of interest: This work was partially funded by:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rietzel E, Pan TS, Chen GTY. Four-dimensional computed tomography: Image formation and clinical protocol. Med Phys. 2005;32:874–889. doi: 10.1118/1.1869852. [DOI] [PubMed] [Google Scholar]
- 2.Vedam SS, Keall PJ, Kini VR, Mostafavi H, Shukla HP, Mohan R. Acquiring a four-dimensional computed tomography dataset using an external respiratory signal. Phys Med Biol. 2003;48:45. doi: 10.1088/0031-9155/48/1/304. [DOI] [PubMed] [Google Scholar]
- 3.Guerrero T, Sanders K, Castillo E, et al. Dynamic ventilation imaging from four-dimensional computed tomography. Phys Med Biol. 2006;51:777–791. doi: 10.1088/0031-9155/51/4/002. [DOI] [PubMed] [Google Scholar]
- 4.Simon BA. Non-invasive imaging of regional lung function using X-ray computed tomography. J Clin Monit Comput. 2000;16:433–442. doi: 10.1023/a:1011444826908. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto T, Kabus S, von Berg J, et al. Four-dimensional computed tomography-based pulmonary ventilation imaging for adaptive functional guidance in radiotherapy. J Thor Oncol. 2009;4:S959–S960. [Google Scholar]
- 6.Reinhardt JM, Ding K, Cao K, Christensen GE, Hoffman EA, Bodas SV. Registration-based estimates of local lung tissue expansion compared to xenon CT measures of specific ventilation. Med Image Anal. 2008;12:752–763. doi: 10.1016/j.media.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castillo R, Castillo E, Martinez J, Guerrero T. Ventilation from four-dimensional computed tomography: density versus Jacobian methods. Phys Med Biol. 2010;55:4661–4685. doi: 10.1088/0031-9155/55/16/004. [DOI] [PubMed] [Google Scholar]
- 8. [Google Scholar]
- 9. [Google Scholar]
- 10.Yamamoto T, Kabus S, von Berg J, Lorenz C, Keall PJ. Impact of Four-dimensional CT-derived Pulmonary Ventilation Images on Radiotherapy Treatment Planning for Lung Cancer. Int J Radiat Oncol Biol Phys. 2009;75:S443–S443. doi: 10.1016/j.ijrobp.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto T, Kabus S, von Berg J, Lorenz C, Keall PJ. Impact of four-dimensional Computed Tomography Pulmonary Ventilation Imaging-based Functional Avoidance for Lung Cancer Radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:279–288. doi: 10.1016/j.ijrobp.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto T, Kabus S, Klinder T, et al. Investigation of four-dimensional computed tomography-based pulmonary ventilation imaging in patients with emphysematous lung regions. Phys Med Biol. 2011;56:2279–2298. doi: 10.1088/0031-9155/56/7/023. [DOI] [PubMed] [Google Scholar]
- 13.Bayouth J, Du K, Christensen G, Smith B, Buatti J, Reinhardt J. Establishing a relationship between radiosensitivity of lung tissue and ventilation. Int J Radiat Oncol Biol Phys. 2012;84:S31–S32. [Google Scholar]
- 14.Yaremko BP, Guerrero TM, Noyola-Martinez J, et al. Reduction of normal lung irradiation in locally advanced non-small-cell lung cancer patients, using ventilation images for functional avoidance. Int J Radiat Oncol Biol Phys. 2007;68:562–571. doi: 10.1016/j.ijrobp.2007.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy K, Pluim JPW, van Rikxoort EM, et al. Toward automatic regional analysis of pulmonary function using inspiration and expiration thoracic CT. Med Phys. 2012;39:1650–1662. doi: 10.1118/1.3687891. [DOI] [PubMed] [Google Scholar]
- 16.Castillo R, Castillo E, McCurdy M, et al. Spatial correspondence of 4D CT ventilation and SPECT pulmonary perfusion defects in patients with malignant airway stenosis. Phys Med Biol. 2012;57:1855–1871. doi: 10.1088/0031-9155/57/7/1855. [DOI] [PubMed] [Google Scholar]
- 17. [Google Scholar]
- 18.Yamamoto T, Kabus S, Lorenz C, et al. Pulmonary Ventilation Imaging Based on 4-Dimensional Computed Tomography: Comparison With Pulmonary Function Tests and SPECT Ventilation Images. Int J Radiat Oncol Biol Phys. 2014 doi: 10.1016/j.ijrobp.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding K, Cao KL, Fuld MK, et al. Comparison of image registration based measures of regional lung ventilation from dynamic spiral CT with Xe-CT. Med Phys. 2012;39:5084–5098. doi: 10.1118/1.4736808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kipritidis J, Siva S, Hofman MS, Callahan J, Hicks RJ, Keall PJ. Validating and improving CT ventilation imaging by correlating with ventilation 4D-PET/CT using 68Ga-labeled nanoparticles. Med phys. 2014;41:011910. doi: 10.1118/1.4856055. [DOI] [PubMed] [Google Scholar]
- 21.Mathew L, Wheatley A, Castillo R, et al. Hyperpolarized He-3 Magnetic Resonance Imaging: Comparison with Four-dimensional X-ray Computed Tomography Imaging in Lung Cancer. Acad Rad. 2012;19:1546–1553. doi: 10.1016/j.acra.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Tafuro F, Corradi M, Mutti A. Interpretative strategies of lung function tests: obstructive pattern. Med Lavoro. 2014;105:197–213. [PubMed] [Google Scholar]
- 23.Beckles MA, Spiro SG, Colice GL, Rudd RM. The physiologic evaluation of patients with lung cancer being considered for resectional surgery. CHEST J. 2003;123:105S–114S. doi: 10.1378/chest.123.1_suppl.105s. [DOI] [PubMed] [Google Scholar]
- 24.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J clin oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 25.Anthonisen NR, Wright EC, Hodgkin JE, Hopewell PC, Levin DC, Stevens PM. prognosis in chronic obstructive pulmonary-disease. Am Rev Respir Dis. 1986;133:14–20. doi: 10.1164/arrd.1986.133.1.14. [DOI] [PubMed] [Google Scholar]
- 26.Celli BR, MacNee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 27.Castillo E, Castillo R, White B, Rojo J, Guerrero T. Least median of squares filtering of locally optimal point matches for compressible flow image registration. Phys Med Biol. 2012;57:4827–4843. doi: 10.1088/0031-9155/57/15/4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kutcher GJ, Burman c. calculation of complication probability factors for non-uniform normal tissue irradiation - the effective volume method. Int J Radiat Oncol Biol Phys. 1989;16:1623–1630. doi: 10.1016/0360-3016(89)90972-3. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto T, Kabus S, Lorenz C, et al. 4D CT lung ventilation images are affected by the 4D CT sorting method. Med Phys. 2013:40. doi: 10.1118/1.4820538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto T, Kabus S, Klinder T, et al. Four-dimensional computed tomography pulmonary ventilation images vary with deformable image registration algorithms and metrics. Med Phys. 2011;38:1348–1358. doi: 10.1118/1.3547719. [DOI] [PubMed] [Google Scholar]
- 31.Du KF, Bayouth JE, Cao KL, Christensen GE, Ding K, Reinhardt JM. Reproducibility of registration-based measures of lung tissue expansion. Med Phys. 2012;39:1595–1608. doi: 10.1118/1.3685589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto T, Kabus S, von Berg J, et al. Reproducibility of Four-dimensional Computed Tomography-based Lung Ventilation Imaging. Acad Rad. 2012;19:1554–1565. doi: 10.1016/j.acra.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]