Abstract
Objectives
This research sought to assess racial and SES differences in level and change in allostatic load (AL) over time in midlife women and to test whether psychosocial factors mediate these relationships. These factors were: discrimination, perceived stress, and hostility.
Methods
Longitudinal data obtained from the Study of Women’s Health Across the Nation SWAN were used (n = 2063; mean age at baseline = 46.0). Latent growth curve (LGC) models evaluated the impact of demographic, menopausal, and psychosocial variables on level and change in AL over 8 years.
Results
Direct effects: High levels of discrimination and hostility significantly predicted higher AL (path coefficients 0.05, 0.05 respectively). High perceived stress significantly predicted a faster rate of increase of AL (path coefficient 0.06). Racial and socioeconomic status (SES) differentials were present, with African American race (path coefficient 0.23), low income (path coefficient −0.15), and low education (path coefficient −0.08) significantly predicted high AL level. Indirect effects: Significant indirect effects were found for African American race, less income, and lower education through higher discrimination, perceived stress, and hostility on level and rate of AL.
Conclusion
This was one of the first studies that investigated AL over multiple time periods and results supported AL as a cumulative phenomenon, affected by multiple psychosocial and demographic factors. The results suggest the complex ways in which race, SES, and psychosocial factors operate to influence AL.
Keywords: allostatic load, race, socioeconomic status, discrimination, perceived stress, hostility
INTRODUCTION
Health differences among racial and socioeconomic groups are a function of a complex set of inter-related environmental, social, economic, and personal influences (1–3). Recent biopsychosocial theories have proposed integrated models of these influences that specify psychosocial mediators linking race and socioeconomic status (SES) on the one hand and to health outcomes on the other hand (1, 3, 4). Allostatic load (AL) is a useful outcome for studying how person-environment interactions “get under the skin” because it captures the cumulative physiological wear and tear of stressful circumstances relatively early in poor health outcomes (5, 6). However, studies of race and SES effects on health typically only control for gender. In contrast, the current study emphasizes heterogeneity in these factors among women and focuses on midlife, the time in the life course when indicators of poor health become more apparent (7, 8). Specifically, longitudinal data from a multi-ethnic cohort of midlife women are used to assess racial and SES differences in AL level and change over time, and to test the extent to which these difference are explained by a set of psychosocial factors that include discrimination, perceived stress, and hostility.
The health outcome we study is AL, which is conceptualized as a multi-system, cumulative burden of physiological dysregulation (9, 10). A multi-system approach is preferred over a single biological system when the interest is in ascertaining the multiple influences of racial, SES, and psychosocial factors on health (1–3). Moreover, studies of a single system are not good proxies for overall impact of stressors because the effects of exposure to stressors are commonly nonspecific (11).
We examine two indicators of social structural predictors of AL: SES and race, conceptualized as fundamental causes of health differences (1–3). The stresses of lower SES reflect the lack of multiple resources that include knowledge, money, power, prestige, and beneficial social connections, which in turn contribute to health disadvantages (1, 3, 4). Lower SES is associated with higher AL (12–16) and AL is thought to be an indicator of the adverse health effects of low SES (12, 17, 18). Therefore, AL warrants investigation in its own right. Independent of SES, race is associated with AL, with African Americans having higher AL than other groups (12, 19, 20). Chronic exposure to social adversity produces long-term stress responses leading to earlier health deterioration among African Americans (or “weathering) (19–21). These forces are distinct from the resources linked to SES because they additionally constrain opportunities on the basis of race (22).
A large literature identifies relations between psychosocial factors and AL (6, 12, 14, 15, 23–30). Research attention has increasingly focused on discrimination as a link between structural disadvantage and poor health (31, 32, 33), but to date, few studies have examined its effect on AL. In assessing the distinctive impact of discrimination on AL, is important to take into consideration exposure to other sources of stressors. For this reason we include perceive stress. Lower SES individuals report higher levels of perceived stress (29, 30). Similarly, it is also relevant to evaluate whether structural disadvantage results in higher AL because it is associated with higher hostility (14, 15).
The effects of structural disadvantage, as mediated by psychosocial factors, is conceptualized as being cumulative over time and is the key to selection of the data used for this study and our analytic approach. Unlike many AL studies (34), we utilize 8 years of annual longitudinal data. Our analytic approach models the relationships among structural disadvantage, psychosocial factors, and AL as a system of relationships that is operationalized as a set of simultaneous equations. Importantly, structural equation models (SEMs) estimate the indirect effects of race and SES on AL that operate through the intervening psychosocial variables, also known as mediators. Thus, race and SES are thought to affect AL at least in part because they affect discrimination, perceived stress, and hostility, which in turn affect AL. The direct effects of race and SES are equivalent to the effects captured in a general linear model of AL as the single dependent variable. The Latent Growth Curve (LGC) model is an extension of SEM but estimates trajectories of change in the outcome (here AL), as distinct from estimating only the level of the outcome at a particular time (35). This approach enables us to quantify change in AL over time and to identify factors that are predictive of change in AL, as distinct from identifying factors that are predictive of level of AL only.
The current study incorporates a dynamic biopsychosocial model to investigate racial and SES effects on AL and to estimate the extent to which discrimination, perceived stress, and hostility mediate their effects. Guided by the theoretical perspectives developed above, we hypothesize racial and SES differences in AL, with African American women and lower SES women having higher AL. We also hypothesize greater discrimination, perceived stress, and hostility will be predictive of higher AL. Last, we hypothesize that racial and SES differences in AL will, in part, be explained by related differences in discrimination, perceived stress, and hostility. We also examine the effects of age and menopausal transition stage on AL because there is good evidence that biomarker profiles are affected such that progression through menopause is related to less salutary profiles (36, 37). Additionally, we test whether the psychosocial variables also contribute to a more rapid increase in AL. Such findings would underscore the value of a latent growth approach.
METHODS
Study Design
The Study of Women Across the Nation (SWAN) is a community-based, multi-ethnic sample of midlife women designed to investigate the biological and psychosocial characteristics of the menopausal transition. Details of recruitment procedures and study design have been described elsewhere (38). From 1995 through 1997, 16,065 women were randomly selected from sampling frames established at each site and screened for inclusion in the longitudinal cohort. Each site screened one racial/ethnic minority population (African Americans in Pittsburgh, PA, Boston, MA, Detroit, MI, and Chicago, IL; Japanese in Los Angeles, CA; Chinese in Oakland, CA; and Hispanics in Newark, NJ) and one white population. The SWAN protocol was approved by each site’s institutional review board. All participants provided written informed consent.
Women were eligible for the longitudinal study if they: were ages 42–52 years, had an intact uterus and at least one ovary, were not currently using exogenous hormones affecting ovarian functioning, had at least one menstrual period in the previous 3 months, and self-identified with one of each site’s racial/ethnic groups (38). Of eligible women, the overall participation rate was 51% (n = 3306). Participation rates did not vary by age, marital status, parity, or menopausal status but did by SES and race (38).1 The current study included 8 time periods of annual data from baseline through follow-up visit 07. Data collection at the NJ site was temporarily halted, therefore Hispanics were not included. SWAN retention rates remain very high (82–94% depending on the site and 81% overall) as of follow-up 07. The final analytic sample included women who had valid values of all 11 biomarkers at baseline (n = 2743) and at least 2 additional AL summary scores available during the 8 years of assessment (n = 2063).
Study visits were conducted by trained, certified staff, who supervised the collection of the self-reported data, administered interviews, conducted the physical measures, and performed biological specimen collection (38). Blood samples were collected annually. Phlebotomy protocols and laboratory assays have been detailed elsewhere (39). Blood pressure was taken seated after a 5-minute rest. Two assessments were taken and averaged. Waist circumference was measured in undergarments at the natural waist. Hip circumference was measured in undergarments at the maximum extension of the buttocks. Height and weight were measured without shoes and in light indoor clothing.
Single Biomarker Measures and Composite AL Score
Eleven biomarkers were used to create the summary AL score. They were selected from available data based on their representation of multiple physiological systems, use in prior AL research, and pertinent to disease risk (10, 12, 17, 28, 40, 41). Cardiovascular markers were systolic blood pressure (SBP) and diastolic blood pressure (DBP). Metabolic markers were total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, body mass index (BMI), waist-to-hip ratio, and fasting serum glucose. Inflammatory markers included C-reactive protein (CRP) and fibrinogen. Dehydroepiandrosterone sulfate (DHEA-S) was the neuroendocrine marker.
Operationalization of AL was based on an algorithm developed by Seeman and colleagues (41) and has been used extensively (e.g., 19, 20, 26, 27, 41–44). For each of the 11 biomarkers, the highest risk quartile value was determined based on its baseline distribution of within the SWAN sample (75th quartile for all biomarkers except HDL and DHEA-S, for which the 25th quartile represents high risk). AL is the sum of the number of biomarkers in the high risk quartiles.2 AL was computed for baseline and each of 7 follow-up visits.3 These procedures use empirically-driven cutoffs based on sample values. Although alternative methods for summarizing biomarker scores have been investigated, comparable results have been found regardless of method (12, 17, 45, 46).
Distributional qualities of each of the 11 biomarkers and cutoff points, based on baseline values, and AL score are shown in Table 1. AL scores varied from 0 to 11 and the mean was 2.57 (SD = 2.27). We also assessed the distributional qualities of the 11 biomarkers at each follow-up visit. Means shifted toward less healthy values over the 8 years. Last, we examined single biomarker correlations with each of the 3 psychosocial variables at each follow-up. Correlations and significance levels were comparable regardless of specific biomarker or follow-up visit. (See appendices available in Supplemental Digital Content 1 and Supplemental Digital Content for additional detail.)
Table 1.
Biomarker and AL distributions at baseline, SWAN (n = 2063)a
| Range | Mean | Standard Deviation | 25% | Median | 75% | Cutoff point | |
|---|---|---|---|---|---|---|---|
| Cardiovascular | |||||||
| SBP (mm Hg) | (80, 227) | 116.26 | 17.33 | 104 | 113 | 125 | >125 |
| DBP (mm Hg) | (41,143) | 74.31 | 10.46 | 68 | 73 | 80 | >80 |
| Metabolic | |||||||
| Total cholesterol (mg/dL) | (92, 338) | 193.41 | 34.14 | 171 | 191 | 213 | >213 |
| HDL (mg/dL) | (18, 138) | 56.86 | 14.21 | 47 | 55 | 66 | <47 |
| Triglycerides (mg/dL) | (31, 1185) | 109.17 | 76.32 | 34 | 89 | 125 | >127 |
| Glucose (mg/dL) | (52, 439) | 97.35 | 29.81 | 86 | 91 | 98 | >98 |
| BMI (kg/m2) | (14.99, 59.13) | 27.84 | 7.18 | 22.49 | 26.01 | 31.76 | >31.76 |
| Waist-Hip-Ratio | (0.51, 1.14) | 0.80 | 0.07 | 0.75 | 0.79 | 0.84 | >0.84 |
| Inflammatory | |||||||
| CRP (mg/L) | (0.04, 9.90) | 2.19 | 2.38 | 0.5 | 1.2 | 3.0 | >3.0 |
| Fibrinogen (mg/dL) | (122, 722) | 291.72 | 66.16 | 246 | 282 | 325 | >325 |
| Neuroendocrine | |||||||
| DHEA-S (μg/dL) | (0.29, 621.5) | 133.75 | 81.13 | 76.9 | 117.7 | 172.7 | <76.9 |
| AL | (0, 11) | 2.57 | 2.27 | 1 | 2 | 4 | |
mm=millimeter; Hg=mercury; mg=milligrams; dL=deciliter; kg=kilogram; m=meter; μg=microgram; L=liter
Demographic Measures and Menopausal Transition Stage
Baseline demographic indicators
Race was coded with two dichotomous variables (African American 1=yes, 0=no; white 1=yes, 0=no). Japanese or Chinese was the combined reference category because of similar and lowest AL scores. Educational attainment was scored on a 1–5 scale (<12 years; high school graduate; some college; college graduate; and post-college). Household income was scored on a 1–8 scale (<$10,000; $10,000–19,999; $20,000–34,999; $35,000–49,999; $50,000–74,999; $75,000–99,999; $100,000–149,999; and >$150,000). Marital status was coded as a dichotomy in the structural model (married/cohabiting versus not). Age was coded as a continuous variable (ages 42–52).
Menopausal transition stage was coded at each of the 8 time periods and scaled 0–4. Definitions of stage followed standard guidelines (47). Categories were: 0 = premenopausal (bleeding in the previous 3 months, no change in cycle predictability in past year); 1 = early perimenopausal (bleeding in the previous 3 months, decrease in cycle predictability in past year); 2 = late perimenopausal (3–11 months amenorrhea); 3 or 4 = postmenopausal (>12 months amenorrhea). The postmenopausal stage was divided into those who were current users of hormone therapy (HT) (3 = HT) and those who were not (4 = no HT). Women who used HT before postmenopause were excluded in the follow-up visits when it was used but reinstated in those who stopped HT after an 18 month HT wash-out period (as established by the SWAN Coordinating Center).4 At baseline all women were scored either premenopausal or early perimenopause.
Psychosocial Mediating Measures
Mediating measures were collected over multiple follow-up visits. Our original intent was to include them as time-varying latent constructs. However, there was no upward or downward trajectory observed for these variables (see Table 3). Therefore, they were not partitioned into intercept and slope components; rather, the mean indicators described below were used. Initial analyses ascertained no within-woman variance in these measures. Discrimination was assessed with a modified version of the Detroit Area Study Everyday Discrimination Scale (31). This 10-item scale asked participants to rate the frequency they experienced various types of interpersonal mistreatment over the past 12 months (e.g., “You are treated with less respect than other people.”) using a 1–4 response scale. The scale has demonstrated high levels of internal consistency (31, 48). Scale items collected at baseline and 3 years of follow-up were used. Items were averaged within each year and then used as 4 indicators of a simple latent variable of discrimination.
Table 3.
Means or percentages, standard deviations, ranges, and factor loadings of measured variables in the CFA, SWAN (n = 2063).
| Latent and Measured Variables (range) | Mean (S. D.)/percentage | Factor Loadinga |
|---|---|---|
| Baseline demographic variables | ||
| African-American | 29% | NAb |
| Caucasian | 51% | NA |
| Asian (Japanese and Chinese) | 20% | |
| Age (range = 42–52 years) | 46.00 (2.70) | NA |
| Income (1–8) | 4.71 (1.71) | NA |
| Education (1–5) | 3.53 (1.13) | NA |
| Married or cohabiting (yes/no) | 68% | NA |
| Mediating psychosocial variables | ||
| Discrimination (1–4) | ||
| Baseline | 1.76 (0.47) | .79 |
| Year 1 | 1.75 (0.47) | .84 |
| Year 2 | 1.70 (0.48) | .85 |
| Year 3 | 1.67 (0.49) | .84 |
| Stressc (4–20) | ||
| Baseline | 8.34 (2.86) | .55 |
| Year 2 | 7.70 (2.86) | .74 |
| Year 3 | 7.69 (2.88) | .75 |
| Year 4 | 7.59 (2.86) | .81 |
| Year 5 | 7.63 (2.91) | .79 |
| Year 6 | 7.61 (2.99) | .74 |
| Hostility (0–13) | 3.77 (2.91) | NA |
| Menopausal Transition Stage Variables (0–4) | ||
| Baseline | 0.45 (0.50) | .32 |
| Year 1 | 0.83 (0.66) | .50 |
| Year 2 | 1.05 (0.85) | .66 |
| Year 3 | 1.35 (1.10) | .83 |
| Year 4 | 1.72 (1.28) | .98 |
| Year 5 | 2.08 (1.39) | .85 |
| Year 6 | 2.46 (1.44) | .71 |
| Allostatic Load Latent Growth Variables | ||
| Baseline | 2.57 (2.27) | .88 |
| Year 1 | 2.57 (2.26) | .88 |
| Year 2 | 2.68 (2.29) | .88 |
| Year 3 | 2.67 (2.28) | .89 |
| Year 4 | 2.73 (2.19) | .90 |
| Year 5 | 2.81 (2.21) | .89 |
| Year 6 | 2.80 (2.13) | .88 |
| Year 7 | 2.76 (2.13) | .84 |
All factor loadings significant, p ≤ .001. Factor loadings are standardized.
NA = Not applicable.
Not available Year 1, Year 7.
Factor loadings reported for latent growth variables before imposition of latent growth fixed structure.
Perceived stress was measured with the 4-item shortened version of the Perceived Stress Scale (49). The items assessed stress in the past 2 weeks (e.g., “Felt unable to control important things in your life.”) using a 1–5 response scale. Because of substantial missing data at the first follow-up visit, we used the averages of the baseline and subsequent scores for follow-up visits 2–6 as 6 mean indicators of a single latent variable representing perceived stress. Hostility was measured at baseline from a subscale of 13-items with dichotomous 0–1 responses from the Cooke-Medley Questionnaire (50). A sum score was used.5
Analysis
Preliminary baseline analyses
The distributional qualities including mean, quartiles, range, standard deviations, and the empirical cutoff values evaluated at baseline for each of the 11 biomarkers were computed. Baseline percentage distributions of the demographic and menopausal transition stage variables were estimated. Standard χ2 was used to test associations between each covariate and AL.
A preliminary confirmatory factor analysis (CFA) was conducted prior to testing a LGC model to simplify the process of fitting the model by focusing first only on the measurement portion and to test the adequacy of the measurement model. The CFA contained 7 measured variables: African American, white, education, income, age, married, and hostility. The 4 latent variables are multiply-indicated and include discrimination, perceived stress, menopausal transition stage, and AL. As noted above, none of the psychosocial variables varied over time so these variables were used as indicators of a standard latent variable without a time-varying component. For the CFA only, no time structure is imposed on menopausal transition stage or AL.
The main analysis consists of a LGC model which is ideal for studying longitudinal change, as it provides a means of studying individual differences. Compared to the CFA, the LGC model has two important changes. First, a time structure was imposed on menopausal transition stage and on AL. The intercept corresponds to the initial status (or level) of the individual at baseline. The intercept is a constant for any individual across time and represents information concerning the mean of the collective individual intercepts that characterize each individual’s growth curve. The slope, represents the rate of change in AL over the period of study. Second, the LGC differs from CFA by specifying relationships among the variables: the background and demographic variables predict the psychosocial variables which predict AL (both intercept and slope). Covariances were allowed between age and menopausal transition stage intercept and slope. Correlations (covariances) between adjacent error residuals within the latent intercept and slope variables (autocorrelations) were considered for addition to the model using recommendations from the La Grange Multiplier (LM) test (53) to improve model fit.
The EQS structural equations program (54) was used to assess CFA and the LGC models and provided information on the relationships among AL, demographic, menopausal, and psychosocial variables. Goodness-of-fit of the models were assessed with the robust Yuan-Bentler scaled chi-square (Y-B) χ2, Comparative Fit Index (CFI), and Root Mean Square Error Approximation (RMSEA) (54, 55). Y-B robust statistics were used due to the non-normality of the data ((56) normalized estimate = 65.36) and the missingness. The RMSEA ideally should be < 0.06, and values > .95 for the CFI are desirable (55).
Due to multiple assessments over many years, not all women had complete data. Thus, the Full Information Maximum Likelihood (FIML) missing data method available in EQS that uses an expectation and maximization (EM) algorithm was employed (54). In EM, imputation parameter estimates are obtained by iterating an expectation step and a maximization step. FIML is the recommended data imputation method when using the EQS structural equations modeling program. Diagnostics indicated that the missing data points were missing completely at random (MCAR).
RESULTS
Baseline Descriptive Results
Baseline demographics and menopausal transition stage are presented in Table 2. Mean age was 46. Over half of the cohort was white, 29% African American, and the remaining was Chinese or Japanese. Women were well-educated (49% were college graduates or higher) and lived in relatively affluent households (31% lived in households with annual incomes of $75,000 or higher). Two-thirds were married. Each demographic variable was significantly associated with mean AL (p < .001). Age was positively associated with higher AL. African American women had the highest AL, followed by white, last Chinese and Japanese (lowest and similar means). Higher education and income were significantly associated with lower AL. Married/cohabiting women had lower AL than other marital statuses. Women who were in early perimenopause at baseline had higher AL than premenopausal women.
Table 2.
Demographic characteristics at baseline and mean AL, SWAN (n = 2063)
| Characteristic | Percentage | Mean AL |
|---|---|---|
| Age | ||
| 42–45 years | 45.95 | 2.37*** |
| 46–49 years | 42.90 | 2.65 |
| 50–52 years | 11.15 | 2.95 |
| Race/ethnicity | ||
| Caucasian | 50.75 | 2.32*** |
| African American | 28.79 | 3.69 |
| Chinese | 9.45 | 1.59 |
| Japanese | 11.00 | 1.67 |
| Education | ||
| <12 years | 3.12 | 3.27*** |
| High school graduate | 16.07 | 3.18 |
| Some college | 32.05 | 2.84 |
| College graduate | 22.65 | 2.12 |
| Post-college | 26.11 | 2.17 |
| Household income | ||
| <$10,000 | 4.23 | 3.88*** |
| $10,000 – 19,999 | 5.33 | 4.20 |
| $20,000 – 34,999 | 14.93 | 2.94 |
| $35,000 – 49,999 | 18.72 | 2.68 |
| $50,000 – 74,999 | 25.73 | 2.40 |
| $75,000 – 99, 999 | 14.34 | 2.23 |
| $100,000 – 149,999 | 11.75 | 1.97 |
| ≥$150,000 | 4.98 | 1.55 |
| Marital status | ||
| Single/Never Married | 13.90 | 3.04*** |
| Married/Cohabiting | 68.09 | 2.38 |
| Separated/Divorced/Widowed | 18.01 | 3.01 |
| Menopausal transition stage | ||
| Premenopausal | 54.55 | 2.42*** |
| Early perimenopausal | 45.45 | 2.77 |
| Late perimenopausal | -- | |
| Postmenopausal, no HRT | -- | |
| Postmenopausal, HRT | -- | |
| Site | ||
| Detroit | 18.18 | 3.63*** |
| Boston | 15.90 | 2.55 |
| Chicago | 14.83 | 3.25 |
| Davis | 17.16 | 1.81 |
| Los Angeles | 19.15 | 1.65 |
| Pittsburgh | 14.78 | 2.71 |
p < .001. Bivariate chi-square.
Preliminary CFA
Table 3 reports the means (or percentages), standard deviations, ranges, and factor loadings of the measured variables used in the CFA. Factor loadings reported for latent growth variables before imposition of latent growth factor structure. All factor loadings were highly significant (p < .001). Fit indexes for the CFA model are reasonable: Y-B χ2 = 1538.38/411 df; CFI = .96, RMSEA = .043. Note also that mean levels of AL increase over the study period. Correlations among the variables in the model are reported in Table 4. Of note among the correlations in Table 4, all variables included in the hypothesized model were significantly associated with the AL latent variable.
Table 4.
Correlations among variables in model, SWAN (n = 2063)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. AL | --- | ||||||||||
| 2. African-Amer. | .34*** | — | |||||||||
| 3. Age | .14*** | −.02 | — | ||||||||
| 4. Income | −.28*** | −.30*** | .07*** | — | |||||||
| 5. Education | −.21*** | −.19*** | −.02 | .39*** | — | ||||||
| 6. Married | −.16*** | −.27*** | .01 | .47*** | .05* | — | |||||
| 7. Caucasian | −.13*** | −.65*** | −.01 | .16*** | .21*** | .14*** | — | ||||
| 8. Discrimination | .17*** | .24*** | −.04 | −.19*** | −.04 | −.15*** | −.20*** | — | |||
| 9. Stress | .11*** | .02 | −.03 | −.22*** | −.12*** | −.08*** | −.11*** | .39*** | — | ||
| 10. Hostility | .21*** | .28*** | .00 | −.28*** | −.23*** | −.16*** | −.24*** | .38*** | .28*** | -- | |
| 11. Menopausal transition stage | .16*** | .10*** | .49*** | −.03 | −.12*** | −.04 | −.07*** | .03 | .03 | .11*** | -- |
= p < .05,
= p < .01,
= p < .001.
LGC Results
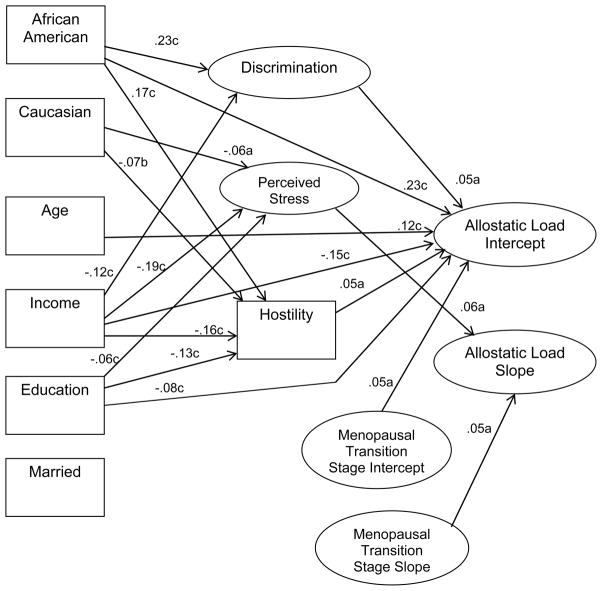
Figure 1 presents the significant predictive paths in the final trimmed LGC model. For readability, the figure does not depict the significant relationships among the predictors. They are similar to those reported in Table 4. The fit indexes of the path model are highly acceptable: Y-B χ2 = 1449.46/438 df; CFI = .97, RMSEA = .039.
Figure 1.
Latent growth curve analysis of demographic variables, menopausal transition stage, mediating variables and pathways of AL, SWAN (n = 2063).
Note: a = p < .05; b = p < .01; c = p < .001. Variables in rectangles are single items. Variables in ovals are latent variables.
Direct effects
Two of the mediating variables, discrimination and hostility, were predictive of a higher AL intercept. African American race predicted a higher AL intercept compared to Chinese/Japanese (there was no difference for White race). Older age, lower income, less education, and menopausal transition stage at baseline were predictive of a higher AL intercept. Perceived stress and menopausal transition stage slope were predictive of higher AL slope.
Among the mediating variables, higher discrimination was predicted by African American race and lower income. Higher stress was predicted by less income and lower education and was lower among white women. Higher hostility was predicted by African American race, less income, lower education, and was lower among white women. Marital status was included in the model but was not significant.
Indirect effects
The AL intercept was indirectly and significantly predicted by African American race (p < .001; standardized total effect = .25, indirect effect = .02), less income (p < .001; total effect = −.165, indirect effect = −.013), and lower education (p < .05; total effect = −.08, indirect effect = −.01). The AL slope was predicted by less income (p < .05; no direct effect; indirect effect = −.01). (The other possible indirect pathways depicted in Figure 1 were not significant.)
DISCUSSION
This is one of the first longitudinal studies to investigate racial and SES differences in AL over time and to identify pathways involving multiple psychosocial factors. Specifically, we find persistent racial and SES differentials in AL, with African American women and women of lower SES having higher AL. Also, we find support for the influences of discrimination, perceived stress, and hostility on level and change in AL among of midlife women. Our results identify several significant pathways through which race and SES indirectly predict level and change of AL through these psychosocial mediators.
A distinctive contribution of this work is that it models AL as a dynamic process. The majority of the covariates are predictive of the AL intercept versus the slope. The intercept reflects a lifetime of exposure to social and environmental stressors and the slope reflects the change over a much shorter duration. Importantly, the finding that one mediating variable is predictive of rate of change of AL underscores the value of our LGC model approach. Consistent with theories linking stressful experiences to AL accumulation (9, 10, 40, 57), women reporting higher perceived stress experience a faster rate of increase in AL. Perceived stress represents individuals’ interpretation of life events and environmental demands. It reflects not only these ‘objective’ stressors but also personal experiences, resources, coping strategies, and availability of social supports (58). Prior studies also found a significant relationship between higher perceived stress and higher AL (24, 28). Our findings support and extend this earlier work by demonstrating that differences in perceived stress represent a significant pathway affecting the rate at which AL accumulates over time. As our model also indicates, such perceptions are strongly linked to central characteristics that affect individuals’ positions and experiences within society, especially lower SES.
As expected, women reporting higher levels of discrimination have higher AL levels. There is a large literature linking higher discrimination to poorer health (for a review see (33)), but little work has focused specifically on AL. One exception is a recent study of adolescents (23). The authors found higher level of discrimination predictive of higher AL, as we show. Moreover, our results demonstrate that two demographic factors (being African American and lower income) are predictive of higher discrimination, suggesting the need to further investigate the intersections of how multiple dimensions of social placement result in discrimination. As we hypothesized, hostility is predictive of higher AL, confirming work from earlier studies (14, 15). Prior research shows the contribution of hostility for specific biological systems, such as cardiovascular (59) and metabolic (60). Our findings demonstrate hostility is also predictive of a measure of cumulative dysregulation. Contrary to our expectations, discrimination and hostility did not predict change in AL. The construct of discrimination refers to chronic, lifetime, exposure to adversity (31–33) so it is reasonable that it only affected level of AL. Also, there was no change in levels of discrimination or perceived stress over the study period pointing again to their cumulative and chronic nature. It is this stable disposition that is harmful with respect to AL.
Although three psychosocial mediator variables were significant, important and significant racial and SES direct effects persist. African American women and women of lower SES have higher levels of AL. These findings are comparable to earlier studies of AL (14, 15, 16, 19, 20, 42, 43). These results point to the need to cast a wider net in future work with respect to potential mediating pathways and to consider persistent structural disadvantage in program development. From a fundamental cause perspective (2, 3), emphases for public health policy and programs shift from an individually based risk factor approach to one that contextualizes risk factors. In particular, intervention strategies need to first identify factors that put individuals at risk of “being at risk” (2). For example, characterizing neighborhood environments that preclude individuals from engaging in beneficial health practices, such as exercise, which has known benefits in reducing stress, and then intervening at the neighborhood level (e.g., improved street lighting) (61, 62).
The strengths of this research have been highlighted; however, there are limitations. SWAN is a community-based sample and not nationally representative. A recent systematic review of AL concluded that regardless of specific biomarkers used or samples considered, there is empirical support for consistent relationships between AL and SES, race/ethnicity, and stress exposure (12). Namely, lower SES, African American race, and higher levels of stress were predictive of higher AL. These findings align with our own. Ancillary analysis we conducted provide further substantive support (see online appendices, Supplemental Digital Content 1 and Supplemental Digital Content 2, for additional analyses). Nevertheless, we acknowledge potential biases in our findings. Data limitations precluded analysis of Hispanic women and we only considered baseline income. In addition, we did not find social support to be predictive of AL, which may in part be due to the measures included in SWAN. Although we employed a specification of AL widely used, we were limited by available biomarkers, especially primary mediators of the stress response (34). However, findings from prior research and a recent systematic review suggest that despite differences in operationalization of AL, substantive findings are robust (12, 17, 45, 46). Last, we acknowledge the possibility of moderating effects of psychosocial factors, but did not examine them because of the complexity of the mediation model we present.
The results highlight some of the multiple ways in which race and SES impact AL and indicate several significant indirect effects. For African American women, indirect effects through higher discrimination and higher hostility are predictive of higher level of AL. For lower income women, indirect effects through increased discrimination and hostility are predictive of higher AL level and higher perceived stress predictive of more rapid increases in AL. Finally, for women with lower education, indirect effects through hostility are predictive of higher level of AL. Taken together, these results suggest the complex ways in which race, SES, and psychosocial factors operate to influence AL.
Supplementary Material
Acknowledgments
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Supplemental funding from:
The National Institute on Aging and the Office of Research on Women’s Health R01AG038467 awarded to Dr. Upchurch is also gratefully acknowledged. The National Institute on Drug Abuse DA 01070-38 provided support to Dr. Stein. The National Institute of Aging and the UCLA/USC Biodemography Center 5P30AG017265-13 provided support for Dr. Seeman and an earlier pilot study conducted by Drs. Upchurch and Greendale.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Winifred Rossi 2012 - present; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
SWAN Repository: University of Michigan, Ann Arbor – Siobán Harlow 2013 - present; Dan McConnell 2011 – 2013; MaryFran Sowers 2000 – 2011.
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Acronyms
- AL
allostatic load
- BMI
body mass index
- CFA
confirmatory factor analysis
- CFI
comparative fit index
- CRP
C-reactive protein
- DBP
diastolic blood pressure
- DHEA-S
dehydroepiandrosterone sulfate
- EM
expectation and maximization
- FIML
full information maximum likelihood
- HDL
high-density lipoprotein
- HT
hormone therapy
- LGC
latent growth curve
- LM
LaGrange multiplier
- MCAR
missing completely at random
- RMSEA
root mean square error of approximation
- SES
socioeconomic status
- SEM
structural equation models
- SWAN
Study of Women’s Health Across the Nation
- SBP
systolic blood pressure
- Y-B χ2
Yuan-Bentler scaled chi-square
Footnotes
To substantively assess possible biases in the SWAN data with respect to demographics and the AL outcome, we conducted additional analysis using comparable coding (to the extent possible) with a similar age group of midlife women using data from the National Health and Nutrition Examination Survey (NHANES), 1999–2004, a nationally representative sample of adult Americans. A higher percentage of the SWAN sample was black (28.8% vs. 12.0%), and a higher percentage in SWAN had higher education (81% with some college or more vs. 61%) and a lower percentage at the lowest but not the highest income levels (9.5% <$20,000 versus 24.4%). However, even with a slightly different specification in AL for the two samples (SWAN 11 vs. NHANES 10 biomarkers), overall mean AL were similar (2.57 vs. 2.30) and mean AL by demographics were comparable, but not identical. Significant findings were: blacks had higher AL than whites, AL increased with age, and decreased with education and income. Multivariate results for analysis of demographics and AL showed similar substantive results across the two samples and mirrored the bivariate findings. Results available from authors.
There is a question of how to score AL for individuals on medications that might impact biomarker values. Following previous studies and because AL theory is concerned with actual physiological regulation, we did not make an adjustment of AL values according to medication status.
At follow-up 02, total cholesterol, HDL, triglycerides, glucose, CRP, and fibrinogen were not assessed. At follow-up 07, fibrinogen was assessed for 50% of the sample. We imputed missing fibrinogen values using a linear mixed effects model where fibrinogen was a linear function of age. The mixed effect model also included a random intercept and slope. If a participant had ≥ 2 observations for fibrinogen, the missing value was imputed by the predictor based on the mixed effects model (correlation of observed vs. predicted = 0.74, p≤0.001). For the remaining missing biomarkers in follow-up 02, the values were imputed by averaging values at follow-up 01 and follow-up 03. Analyses conducted using the non-imputed sample produced similar results.
Women who had hysterectomy without bilateral oophorectomy before postmenopause were dropped at the follow-up visit when it occurred. Premenopausal women who had bilateral oophorectomy (with or without hysterectomy) were coded as surgically postmenopausal. Women who were pregnant or breastfeeding were excluded in the follow-up visits it occurred and were reinstated once they were no longer pregnant or breastfeeding.
The original model specification also included latent variables for social support and stressful life events. Social support included 4 measures selected from the Medical Outcomes Study Social Support Survey indicating instrumental and emotional support (51). It was collected annually through follow-up 06. Stressful life events included 20 items modified from the Psychiatric Epidemiology Research Interview (52). Stressful life events was examined as a total number and also categorized based on items that were ‘very stressful.’ None of these variables were significant in earlier model development and so were dropped from the final model.
Conflicts of Interest: None Declared.
References
- 1.Diez Roux A. Conceptual approaches to the study of health disparities. Annu Rev Public Health. 2012;33:41–58. doi: 10.1146/annurev-publhealth-031811-124534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Link B, Phelan J. Social conditions as fundamental causes of disease. J Health Soc Behav. 1995;35:80–94. [PubMed] [Google Scholar]
- 3.Phelan J, Link B, Tehranifar P. Social conditions as fundamental causes of health inequalities. J Health Soc Behav. 2010;51:S28–40. doi: 10.1177/0022146510383498. [DOI] [PubMed] [Google Scholar]
- 4.Matthews K, Gallo L, Taylor S. Are psychosocial factors mediators of socioeconomic status and health connections? Ann N Y Acad Sci. 2010;1186:146–73. doi: 10.1111/j.1749-6632.2009.05332.x. [DOI] [PubMed] [Google Scholar]
- 5.McEwen B. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- 6.Taylor S, Repetti R, Seeman T. Health psychology: what is an unhealthy environment and how does it get under the skin? Annu Rev Psychol. 1997;48:411–47. doi: 10.1146/annurev.psych.48.1.411. [DOI] [PubMed] [Google Scholar]
- 7.House J, Lantz P, Herd P. Continuity and change in the social stratification of aging and health over the life course: Evidence from a nationally representative longitudinal study from 1986 to 2001/2002 (Americans’ Changing Lives Study) J Gerontol B Psychol Soc Sci. 2005;60:S15–S26. doi: 10.1093/geronb/60.special_issue_2.s15. [DOI] [PubMed] [Google Scholar]
- 8.Adler N, Ostrove J. Socioeconomic status and health: what we know and what we don’t. Ann N Y Acad Sci. 1999;896:3–15. doi: 10.1111/j.1749-6632.1999.tb08101.x. [DOI] [PubMed] [Google Scholar]
- 9.McEwen B. Stress, adaptation, and disease: allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 10.McEwen B, Stellar E. Stress and the individual: mechanisms leading to disease. Arch Intern Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- 11.Aneshensel C, Rutter C, Lachenbruch P. Social structure, stress, and mental health: competing conceptual and analytic models. Am Sociol Rev. 1992;56:166–78. [Google Scholar]
- 12.Beckie T. A systematic review of allostatic load, health, and health disparities. Biol Res Nurs. 2012;14:311–46. doi: 10.1177/1099800412455688. [DOI] [PubMed] [Google Scholar]
- 13.Gustafsson P, Janlert U, Theorell T, Westerlund H, Hammarstrom A. Socioeconomic status over the life course and allostatic load in adults: results from the Northern Swedish Cohort. J Epidemiol Community Health. 2011;65:986–92. doi: 10.1136/jech.2010.108332. [DOI] [PubMed] [Google Scholar]
- 14.Hawley L, Lavelle L, Berntson G, Cacioppo J. Mediators of the relationship between socioeconomic status and allostatic load in the Chicago Health, Aging, and Social Relations Study (CHASRS) Psychophysiology. 2011;48:1134–45. doi: 10.1111/j.1469-8986.2011.01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubzansky L, Kawachi I, Sparrow D. Socioeconomic status, hostility, and risk factor clustering in the normative aging study: any help from the concept of allostatic load? Ann Behav Med. 1999;21:330–8. doi: 10.1007/BF02895966. [DOI] [PubMed] [Google Scholar]
- 16.Seeman T, Merkin S, Crimmins E, Koretz B, Charette S, Karlamangla A. Education, income, and ethnic differences in cumulative biological risk profiles in a national sample of US adults: NHANES III (1988–1994) Soc Sci Med. 2008;66:72–87. doi: 10.1016/j.socscimed.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlson E, Chamberlain R. Allostatic load and health disparities: a theoretical orientation. Res Nurs Health. 2005;28:305–15. doi: 10.1002/nur.20084. [DOI] [PubMed] [Google Scholar]
- 18.Szanton S, Gill J, Allen J. Allostatic load: a mechanism of socioeconomic health disparities? Biol Res Nurs. 2005;7:7–15. doi: 10.1177/1099800405278216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geronimus A, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96:826–33. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peek M, Cutchin M, Salinas J, Sheffield K, Eschbach K, Stowe R, Goodwin J. Allostatic load among non-Hispanic whites, non-Hispanic blacks, and people of Mexican origin. Am J Public Health. 2010;100:940–6. doi: 10.2105/AJPH.2007.129312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geronimus A, Hicken M, Pearson J, Seashols S, Brown K, Cruz T. Do US black women experience stress-related accelerated biological aging? Human Nature. 2010;21:19–38. doi: 10.1007/s12110-010-9078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braveman P, Egerter S, Willams D. The social determinants of health: coming of age. Annu Rev Public Health. 2011;32:381–98. doi: 10.1146/annurev-publhealth-031210-101218. [DOI] [PubMed] [Google Scholar]
- 23.Fuller-Rowell T, Evans G, Ong A. Poverty and health. Psychol Science. 2012;23:734–9. doi: 10.1177/0956797612439720. [DOI] [PubMed] [Google Scholar]
- 24.Goldman N, Glei D, Seplaki C, Liu I, Weinstein M. Perceived stress and physiological dysregulation in older adults. Stress. 2005;8:95–105. doi: 10.1080/10253890500141905. [DOI] [PubMed] [Google Scholar]
- 25.Schnorpfeil P, Noll A, Schulze R, Ehlert U, Frey K, Fischer J. Allostatic load and work conditions. Soc Sci Med. 2003;57:647–56. doi: 10.1016/s0277-9536(02)00407-0. [DOI] [PubMed] [Google Scholar]
- 26.Seeman T, Singer B, Ryff C, Dienberg Love G, Levy-Storms L. Social relationships, gender, and allostatic load across two age cohorts. Psychosom Med. 2002;64:395–406. doi: 10.1097/00006842-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Weinstein M, Goldman N, Hedley A, Yu-Hsuan L, Seeman T. Social linkages to biological markers of health among the elderly. J Biosoc Sci. 2003;35:433–53. doi: 10.1017/s0021932003004334. [DOI] [PubMed] [Google Scholar]
- 28.Glei D, Goldman D, Chuang Y, Weinstein M. Do chronic stressors lead to physiological dysregulation? Testing the theory of allostatic load. Psychosom Med. 2007;69:769–76. doi: 10.1097/PSY.0b013e318157cba6. [DOI] [PubMed] [Google Scholar]
- 29.Cohen S, Tyrrell D, Smith A. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325:606–12. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 30.McLeod J, Kessler R. Socioeconomic status differences in vulnerability to undesirable life events. J Health Soc Behav. 1990;31:162–72. [PubMed] [Google Scholar]
- 31.Williams K, Yan Y, Jackson J, Anderson N. Racial differences in physical and mental health: socioeconomic status, stress, and discrimination. J Health Psychol. 1997;2:335–51. doi: 10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]
- 32.Lewis T, Everson-Rose S, Powell L, Matthew D, Brown C, Karavolos K, Sutton-Tyrrell K, Jacob E, Wesley D. Chronic exposure to everyday discrimination and coronary heart calcification in African-American women: the SWAN Heart Study. Psychosom Med. 2006;68:362–8. doi: 10.1097/01.psy.0000221360.94700.16. [DOI] [PubMed] [Google Scholar]
- 33.Williams D, Sternthal M. Understanding racial-ethnic disparities in health: sociological contributions. J Health Soc Behav. 2010;51:S15–S27. doi: 10.1177/0022146510383838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallo L, Fortman A, Mattei J. Allostatic load and the assessment of cumulative biological risk in biobehavioral medicine: challenges and opportunities. Psyschosom Med. 2014;76:478–80. doi: 10.1097/PSY.0000000000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duncan T, Duncan S, Strycker L, Li F, Alpert A. An introduction to latent variable growth curve modeling: concepts, issues, and applications. Mahwah: Lawrence Erlbaum Associates, Inc; 1999. [Google Scholar]
- 36.Chae C, Derby C. The menopausal transition and cardiovascular risk. Obstet Gynecol Clin North Am. 2011;38:477–88. doi: 10.1016/j.ogc.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Polotsky H, Polotsky A. Metabolic implications of menopause. Semin Reprod Med. 2010;28:426–34. doi: 10.1055/s-0030-1262902. [DOI] [PubMed] [Google Scholar]
- 38.Sowers M, Crawford S, Sternfield B, Morganstein D, Greendale G, Evans D, Neer R, Matthews K, Sherman S, Lo A, Weiss G, Kelsey J. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo R, Kelsey J, Marcus R, editors. Menopause: biology and pathobiology. NewYork: Academic Press; 2000. pp. 175–88. [Google Scholar]
- 39.Standard wording. ( http://www.edc.gsph.pitt.edu/swan/research/Documents/PublicationsPresentations/StandardWording/). Retrieved August 15, 2014.
- 40.McEwen B, Seeman T. Protective and damaging effects of mediators on stress: elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- 41.Seeman T, Singer B, Rowe J, Horwitz R, McEwen B. Price of adaptation – allostatic load and its health consequences: MacArthur Studies of Succeessful Aging. Arch Intern Med. 1997;157:2259–68. [PubMed] [Google Scholar]
- 42.Chyu L, Upchurch D. Racial and ethnic patterns of allostatic load among adult women in the United States: findings from the National Health and Nutrition Examination Survey 1999–2004. J Women’s Health. 2011;20:575–83. doi: 10.1089/jwh.2010.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crimmins E, Johnston M, Hayward M, Seeman T. Age differences in allostatic load: an index of physiological dysregulation. Exp Gerontol. 2003;38:731–34. doi: 10.1016/s0531-5565(03)00099-8. [DOI] [PubMed] [Google Scholar]
- 44.Crimmins E, Kim J, Alley D, Karlamangla A, Seeman T. Hispanic paradox in biological risk profiles. Am J Public Health. 2007;97:1305–10. doi: 10.2105/AJPH.2006.091892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seeman T, McEwen B, Rowe J, Singer B. Allostatic load as a marker of cumulative biological risk: MacArthur Studies of Successful Aging. Pro Natl Acad Sci USA. 2001;98:4770–5. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seplaki C, Goldman N, Glei D, Weinstein M. A comparative analysis of measurement approaches for physiological dysregulation in an older population. Exp Gerontol. 2005;40:438–49. doi: 10.1016/j.exger.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 47.WHO scientific group. Research on the menopause in the 1990s. World Health Organ Tech Rep Serv. 1996;866:1–107. [PubMed] [Google Scholar]
- 48.Barnes L, Mendes De Leon C, Wilson R, Bienias J, Bennett D, Evans D. Racial differences in perceived discrimination in a community population of older blacks and whites. J Aging Health. 2004;16:315–37. doi: 10.1177/0898264304264202. [DOI] [PubMed] [Google Scholar]
- 49.Cohen S, Karmarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 50.Barefoot J, Dodge K, Peterson B, Dahlstrom W, Williams R. The Cook-Medley hostility scale: item content and ability to predict survival. Psychosom Med. 1989;51:46–57. doi: 10.1097/00006842-198901000-00005. [DOI] [PubMed] [Google Scholar]
- 51.Sherbourne C, Stewart A. The MOS social survey. Soc Sci Med. 1991;31:731–7. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 52.Dohrenwend B, Krasnott L, Askenasy A, Dohrenwend B. Exemplification of a method for scaling life events: the Peri Life Events Scale. J Health Soc Behav. 1978;19:205–29. [PubMed] [Google Scholar]
- 53.Chou C, Bentler P. Model modification in covariance structure modeling: a comparison among likelihood ratio, LaGrange multiplier, and Wald tests. Multivariate Behav Res. 1990;25:115–36. doi: 10.1207/s15327906mbr2501_13. [DOI] [PubMed] [Google Scholar]
- 54.Benter P. EQS6 structural equations program manual. Encino: Multivariate Software Inc; 2006. [Google Scholar]
- 55.Hu L, Bentler P. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling a Multidisc J. 1999;6:1–55. [Google Scholar]
- 56.Yuan K, Lambert P, Fouladi R. Mardia’s multivariate kurtosis with missing data. Multivariate Behav Res. 2004;39:413–37. [Google Scholar]
- 57.Seeman T, McEwen b. Impact of social environment characteristics on neuroendocrine regulation. Psychosom Med. 1996;58:459–71. doi: 10.1097/00006842-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 58.Cohen S, Kessler R, Gordon L. Measuring stress: a guide for health and social scientists. New York: Oxford Press; 1995. [Google Scholar]
- 59.Fredrickson B, Maynard K, Helms M, Haney T, Siegler I, Barefoot J. Hostility predicts magnitude and duration of blood pressure response to anger. J Behav Med. 2000;23:229–43. doi: 10.1023/a:1005596208324. [DOI] [PubMed] [Google Scholar]
- 60.Raikkonen K, Matthews K, Salomon K. Hostility predicts metabolic syndrome risk factors in children and adolescents. Health Psychol. 2003;22:279–86. doi: 10.1037/0278-6133.22.3.279. [DOI] [PubMed] [Google Scholar]
- 61.Krieger J, Rabking J, Sharify D, Song L. High point walking for health: creating built and social environments that support walking in a public housing community. Am J Public Health. 2009;99:S593–99. doi: 10.2105/AJPH.2009.164384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hamer M. Psychosocial stress and cardiovascular disease risk: the role of physical activity. Psychosom Med. 2013;75:721–28. doi: 10.1097/PSY.0b013e31827457f4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.