Abstract
The objective of this study was to determine the extent to which a low level of trans-10, cis-12 (10,12) conjugated linoleic acid (CLA) decreases adiposity and increases browning in overweight mice, its dependence on inflammatory signaling, and potential synergistic effects of daily exercise. Young, Sv129 male mice were fed a high fat diet for 5 wk to make them fat and glucose intolerant, and then switch them to a low fat diet with or without 0.1% 10,12 CLA, sodium salicylate, or exercise for another 7 wk. 10,12 CLA decreased white adipose tissue (WAT) and brown adipose tissue mass, and increased the mRNA and protein levels, and activities of enzymes associated with thermogenesis or fatty acid oxidation in WAT. Mice fed 10,12 CLA had lower body temperatures compared to controls during cold exposure, which coincided with decreased adiposity. Although sodium salicylate decreased 10,12 CLA-mediated increases in markers of inflammation in WAT, it did not affect other outcomes. Exercise had no further effect on the outcomes measured. Collectively, these data indicate that 10,12 CLA-mediated reduction of adiposity is independent of inflammatory signaling, and possibly due to up-regulation of fatty acid oxidation and heat production in order to regulate body temperature. Although this low level of 10,12 CLA reduced adiposity in overweight mice, hepatomegaly and inflammation are major health concerns.
Keywords: adipose tissue, obesity, inflammation, fatty acid oxidation, thermogenesis, uncoupling protein, steatosis, exercise, sodium salicylate
1. Introduction
The prevalence of overweight and obesity have reached epidemic proportions over the past two decades. In 2010, 35.7% of American adults and 17% of children or adolescents were obese [1]. In 2012, 13 states in the U.S. had obesity rates greater than 30% [2]. The direct annual economic loss due to disability, injury, and death from being overweight and obese in the U.S. was ~170 billion dollars [3]. Indirect medical costs from obesity-related chronic diseases including cardiovascular disease, renal disease, and diabetes further increased heath care costs [3]. Therefore, developing long-term, effective strategies to decrease the prevalence of obesity and its comorbidities are urgently needed.
Conjugated linoleic acid (CLA) supplementation has become a popular method for weight management, especially after it was approved by the FDA for Generally Recognized as Safe status in 2008. Proposed mechanisms by which CLA, particularly the trans-10, cis-12 (10,12) isomer, reduces adiposity include regulation of energy metabolism, adipogenesis, lipid metabolism, white adipose tissue (WAT) apoptosis, and inflammation [reviewed in 4]. Studies have linked 10,12 CLA-mediated inflammatory signaling to the suppression of adipogenesis [5, 6], lipogenesis [5–7], and insulin sensitivity [5, 6, 8], and induction of lipolysis [5] and apoptosis [9].
We previously demonstrated that 10,12 CLA increased the expression of several G-coupled receptor proteins (GPR) such as GPR56 and GPRC5A [10], and activated phospholipase c [11] and diacyglycerol kinase [12], resulting in increased calcium release from endoplasmic reticulum (ER) [13] in human primary adipocytes. By chemically-blocking calcium release from the ER, 10,12 CLA-mediated activation of extracellular signal-regulated kinase (ERK)1/2 [6, 14], cJun-NH2-terminal kinase [15], and nuclear factor kappa B (NFκB) [8] were attenuated [13]. Notably, these 10,12 CLA-activated proteins antagonized peroxisome proliferator-activated receptor (PPAR) γ and its target genes [5, 6, 8, 14, 15], which contributed to CLA’s reduction of adipocyte triglyceride (TG) content. 10,12 CLA-mediated increase in intracellular calcium also impaired insulin sensitivity [13], and impacted prostaglandin synthesis [13, 16].
Notably, increased thermogenesis driven by inflammatory signaling has recently been reported to prevent diet-induced obesity [17–20]. For example, cyclooxygenase (COX)-2 transgenic mice had an increased abundance of mitochondria in WAT, expression of uncoupling protein (UCP) 1 in WAT, and systemic energy expenditure [17]. By knocking out COX-2, UCP1 expression in WAT was suppressed and the defense of body temperature during chronic cold exposure was impaired in mice [17]. Notably, overexpressing NFκB was shown to prevent high fat- induced obesity by elevated energy expenditure through thermogenesis [19, 20].
Our previous study demonstrated that a low dose of 10,12 CLA (0.1%) prevented an accumulation of body fat in young Sv129 mice fed a low fat diet for 7 wk [21]. These CLA-mediated changes in body fat were associated with increased mRNA and protein levels of markers associated with browning and inflammation in epididymal WAT [21]. Therefore, the objective of this study was to determine the extent to which this low dose of 10,12 CLA reduced adiposity in mice made overweight through feeding an American type, high fat diet and then transitioned to a low fat diet, and its dependence on inflammatory signaling. In addition, we wanted to determine if daily aerobic exercise could; (i) enhance 10,12 CLA’s decrease in adiposity; and (ii) decrease 10,12 CLA’s potential increase in hepatic TG levels.
2. Methods
2.1. Experimental design and diets
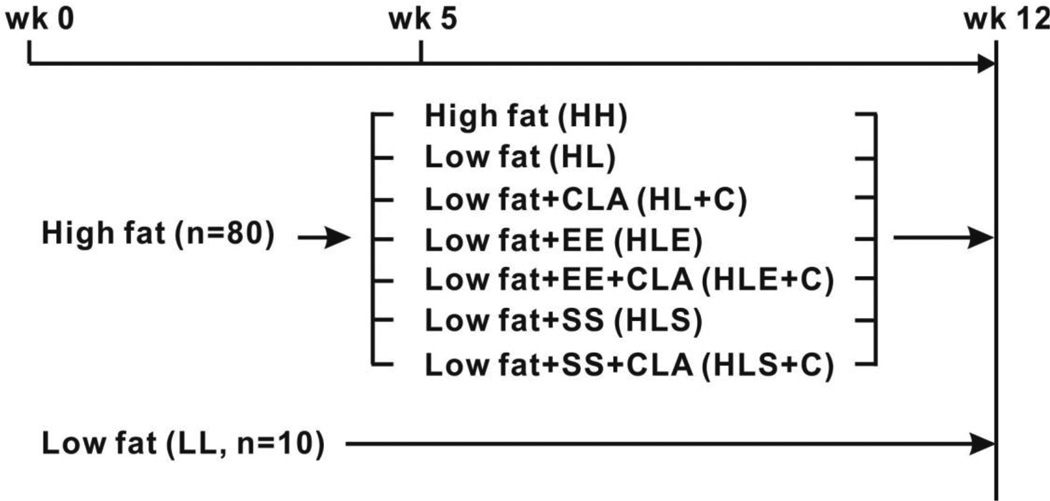
Ninety, 4–6 wk old, Sv129 male mice were obtained from Jackson Laboratories (Bar Harbor, ME) and housed in pairs in a 12 h light /12 h dark, temperature-controlled (22 °C) animal facility at University of North Carolina at Greensboro (UNCG). Ethical treatment of animals was assured by the campus Institutional Animal Care and Use Committee. Sv129 mice were used based on their capacity to develop beige adipocytes compared to C57BL6 mice [18]. After 1 wk of acclimation to the animal facility, 80 mice were fed a high fat diet (i.e., 35% kcals from fat, see Supplemental Table 1 and Fig. 1) for 5 wk (Phase 1 = wk 1–5, fattening period). After 5 wk of high fat-feeding, 10 mice were maintained on this high fat diet (HH) for the remaining 7 wk of the study. The other 70 mice were randomly assigned to either low fat (i.e., 10% kcals from fat, HL; Supplemental Table 1) with (HL+C, n=12) or without 0.1% 10, 12 CLA (HL, n=10), low fat combined with exercise (E) with (HLE+C, n=12) or without 10,12 CLA (HLE, n=12), or low fat combined with 4 g/kg sodium salicylate (S) with (HLS+C, n=12) or without 10,12 CLA (HLS, n=12) for another 7 wk. The remaining 10 mice were fed a low fat diet throughout the 12 wk study (LL; 10% kcals from fat, Supplemental Table 1). The experimental design is illustrated in Figure 1. The length of this study, as well as the dose and isomer of CLA, were chosen based on the results of our previous study [21]. 10,12 CLA (purity = 98%) was purchased from Matreya LLC (Pleasant Gap, PA). Sodium salicylate, an anti-inflammatory agent that has been shown to suppress NFκB signaling in vitro [22] and vivo [23, 24], was purchased from Sigma-Aldrich (St. Louis, MO). The dose of sodium salicylate was based on work by Shoelson’s group [23, 24]. Diets were prepared by Research Diets (New Brunswick, NJ), and stored at −20 °C until use. Mice had ad libitum access to both food and water, and food was changed every 3–4 days. Food intake and body weight were measured weekly.
Figure 1.
Experimental design. Eighty male Sv129 mice were fed a high fat diet for 5 wks and subsequently maintained on the high fat (HH, n=10) diet or switched to a (i) low fat (HL) diet with (HL+CLA, n=12) or without 0.1% 10,12 CLA (n=10), (ii) low fat diet plus exercise (HLE) with (HLE+CLA, n=12) or without 0.1% 10,12 CLA (n=12), (iii) or low fat diet plus 4 g/kg sodium salicylate (HLS) with (HLS+CLA, n=12) or without 0.1% 10,12 CLA (n=12) for another 7 wks. Ten mice were fed a low fat diet throughout the study (LL).
At the end of the study, fasted mice were euthanized with isoflurane vapor followed by cervical dislocation. Blood (serum), WAT depots (i.e., epididymal, inguinal, mesenteric, and retroperitoneal), brown adipose tissue (BAT), gastrocnemius muscle, and liver were harvested, weighted, frozen in liquid N2, and stored at −80 °C until analyses.
2.2. Intraperitoneal glucose tolerance tests (GTT)
Intraperitoneal GTT were conducted on non-anesthetized mice at wk 5 and wk 11. Mice were deprived of food for 8 h and given an intraperitoneal glucose injection (i.e., 20% glucose solution) at a dose of 1 g/kg body weight on the day of test. One drop of venous blood was taken from a small tail clip and blood glucose was measured using a Contour blood glucometer (Bayer Diabetes Care, Tarrytown, NY) at 0, 5, 15, 30, 60, and 120 min after glucose administration. Total area under the curve (AUC) for the GTT was calculated as described [25].
2.3. Dual-energy X-ray (DEXA)
In order to measure percentage body fat, DEXA measurements were performed using a GE Lunar Prodigy Advanced System (GE Healthcare, Milwaukee, WI) at wk 5 and wk 11. Mice were lightly anesthetized with isoflurane vapor using a SomnoSuite Small Animal Anesthesia System (Kent Scientific-Torrington, CT) with Integrated Digital Vaporizer isoflurane system and then positioned on the DEXA table with appendages extended away from the body. The system was calibrated daily according to manufacturer's instructions prior to scans. DEXA measurements were done in duplicate for each mouse. Data were analyzed using Encore 2007 Small Animal software (version 11.20.068).
2.4. Body temperatures
During the first week of acclimatization to the animal facility, mice were implanted subcutaneously with micro-transponders (BHMDS IPTT-300) purchased from Bio Medic Data Systems (BMDS, Seaford, DE) while under isoflurane vapor anesthesia. The sites of insertion were examined daily for 5 d post-implant to ensure that there were no signs of infection. At wk 8 and wk 11, mice from all treatments were exposed to cold temperature (7°C) for 4 h and body temperatures were measured at baseline and every hour for 4 h [26].
2.5. Exercise protocol
Exercise started on wk 6 on a 5 d/wk basis at room temperature. Mice were trained on a motor-driven rodent treadmill (Collins Instruments, Braintree, MA) equipped with a Coulbourne Precision Regulated Animal Shocker at the back. Very low current was used to encourage animals to run. Mice in the exercise groups were familiarized with the treadmill each day for 1 wk by gradually increasing running time and speed, so that at the end of 1 wk they were running for 30 min at a 10 m/min pace for the first 15 min and 12 m/min for the second 15 min up a 12 % grade. Following habituation, the length of each exercise session was gradually increased 5 min/day until the animals were running for 1 h per day at a 12 m/min pace up a 12 % grade. No fatigue was observed during the study as evidenced by not getting shocked more than four times in a minute or staying by the shocker for more than 5 sec [27]. This duration and intensity was maintained for the remainder of the exercise protocol. This moderate protocol has been reported to prevent high fat-mediated steatosis [28] and increase mitochondria content in leg muscles of C57BL/6 mice [29].
2.6. Cytochrome c oxidase and 3-hydroxyCoA dehydrogenase (3-HAD) activities
Cytochrome c oxidase, the final protein complex in the electron transport chain, and 3-HAD, a marker enzyme of β-oxidation, were used as indicators of mitochondria content. Epididymal and inguinal fat samples were homogenized and the activities of cytochrome c oxidase [30] and 3-HAD [31] were measured as previously described [21].
2.7. Liver TG content
Liver TG levels were measured as previously described [21]. Liver protein concentration was determined by using Lowry protein assay from delipidated liver.
2.8. Tissue RNA extraction and real-time quantitative (q) PCR
Total mRNA was extracted from WAT tissue using the RNeasy Lipid Tissue Kit (Qiagen, Valencia, CA), followed by using the DNase treatment (Qiagen). Single-strand RNA was reverse-transcribed into complementary DNA (cDNA) using the high-capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) and real-time qPCR was performed in 7500 FAST Real Time PCR System as previous described [21]. TATA-binding protein (TBP) was used as the internal control.
2.9. Apoptosis PCR Array
RT2 Profiler PCR Array kit (Qiagen) for apoptosis was conducted on epididymal mRNA samples. Procedures were followed using the company’s instructions. Briefly, total RNA samples were first tested for degradation on a 1% agarose gel, and then 1 µg of mRNA was reverse transcribed to cDNA using the Qiagen’s TR2First Strand Kit. Equal aliquots of cDNA were added to a PCR array plate which was coded with 84 genes at the bottom of each well. Real-time amplification data were collected using the 7500 FAST Real Time PCR System. Data analysis was conducted using web-based data analysis software provided by the company.
2.10. Immunoblotting
Immunoblotting was conducted as previously described (21) using primary antibodies of UCP-1 (#ab23841; Abcam, Cambridge, MA), carnitine palmitoyltransferase 1b (#sc20670; CPT1b, Santa Cruz Biotechnology Inc., Santa Cruz, CA), and β-actin (#sc1616; Santa Cruz) at 1:400, 1:200, and 1:1000 dilution, respectively. Horseradish peroxidase-conjugated secondary antibodies were probed at 1:1000 dilutions at room temperature for 2 h. Blots were exposed to chemiluminescence reagent and X-ray films were developed using a SRX-101A Konica Minolta film developer. Two samples from HL, HL+CLA, HLS, and HLS+CLA treatments were randomly selected and run on the same gel, and are representative of other samples within each treatment group. Densitometry was conducted using a Kodak 4400 CF Image Station as described previously [21].
2.11. Statistics
Student’s t test was performed to compare (i) the effects of high fat vs. low fat feeding during the first 5 wk period of the study, and (ii) the effects of HL vs. HL+CLA on the body temperature and body fat percentage data at wk 8 and 11 (p<0.05). A one-way ANOVA, Tukey’s HSD multi-comparison was conducted to detect significant treatment differences in (i) weights of adipose tissues, (ii) mRNA, protein and enzyme activity levels of browning markers, (iii) mRNA levels of inflammatory markers, and (iv) liver weight and TG content. We also used Bonferroni’s posthoc test to perform specific comparisons (i.e., HL vs HL+CLA, HLE vs HLE+CLA, HLS vs. HLS+CLA, HL+CLA vs HLE+CLA, and HL+CLA vs HLS+CLA groups), five groups in all using a family error rate of 0.05. Hence differences were considered significant at p<0.01 for Bonferroni’s adjustment. All statistical analyses were conducted by the JMP version 8.0 program (SAS, Cary, NC). Data are expressed as means ± SEM.
3. Results
3.1. High fat diet increases body weight, body fat, and insulin resistance at wk 5 (Phase 1-fattening period; wk 1–5)
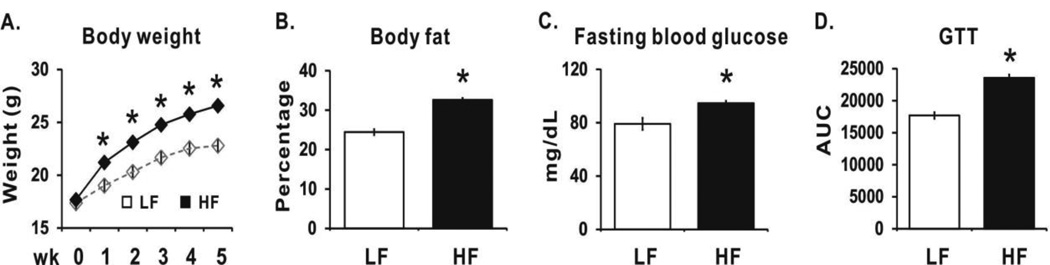
After 1 wk on the experimental diets, mice fed the high fat diet had higher body weights compared to mice fed the low fat diet (Fig. 2A). At wk 5, high fat-fed mice had a greater percentage of body fat (Fig. 2B), increased fasting blood glucose level (Fig. 2C), and GTT AUC (Fig. 2D) compared to the low fat fed mice. Collectively, these data demonstrated that the fattening period (phase 1) was of sufficient length to increase adipose tissue mass and cause glucose tolerance.
Figure 2.
High fat diet increases body weight, body fat, and blood glucose level. Panel A: Body weights of mice fed the high fat (HF) diet (n=80) or the low fat (LF) diet (n=10) for 5 wks. Panel B: Body fat percentages of mice that were randomly selected from HF (n=10) or the LF diet (n=10) for 5 wks. Panel C: Fasting blood glucose level from mice that were randomly selected from HF diet group (n=10) or the LF diet group (n=10). Panel D: Area under curve (AUC) from an intraperitoneal glucose tolerance test (GTT) from mice that were randomly selected from HF diet group (n=10) or the LF diet group (n=10). Mean ± SEM having an asterisks (*) are significantly different using the Student’s t test (p<0.05).
3.2. 10,12 CLA decreases adiposity without impacting glucose tolerance at wk 11–12 (Phase 2-CLA treatment period; wk 6–12)
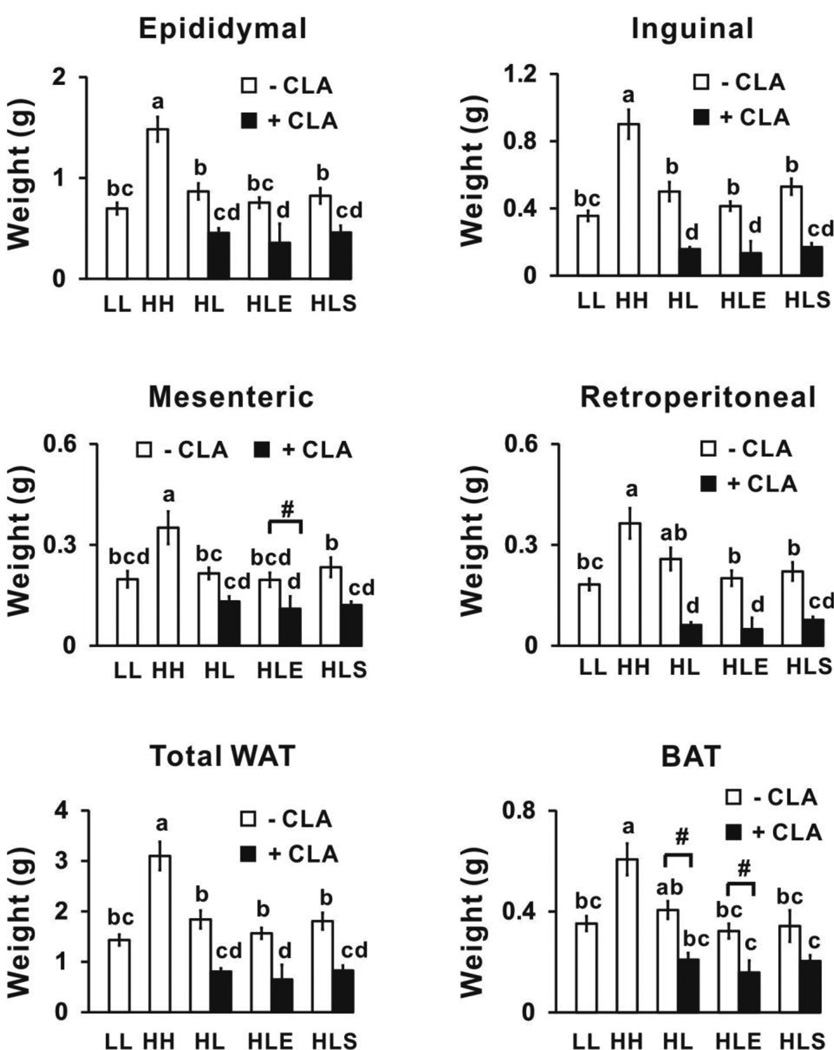
Mice fed the high fat (HH) diet for 12 wk had the highest body weights (Supplemental Table 2), and visceral (i.e., epididymal and mesenteric) and subcutaneous (i.e., inguinal) WAT depot weights (Fig. 3) compared to mice fed the low fat-fed mice (LL) for 12 wk and mice fed the high fat diet for 5 wk and then switched to the low fat diet for 7 wk (HL control group). All 10,12 CLA-treated mice had decreased weights of visceral (i.e., epididymal and retroperitoneal) and subcutaneous (i.e., inguinal) WAT and BAT compared to the mice fed the high fat diet for 5 wk and then switched to the low fat diet for 7 wk (HL controls; Fig. 3). Neither exercise nor sodium salicylate treatment affected 10,12 CLA’s reduction in adiposity. In addition, none of the treatments (i.e., 10,12 CLA, exercise, or sodium salicylate) impacted glucose tolerance levels compared to their controls (Supplemental Table 2).
Figure 3.
10,12 CLA decreases adiposity. At wk 12, epididymal, inguinal, retroperitoneal, and mesenteric white adipose tissue (WAT), and brown adipose tissue (BAT) were excised and weighed. Means ± SEM (n=10–12) not sharing a common letter differ (p<0.05) by one-way ANOVA. Means ± SEM sharing the symbol # differ using the Bonferroni’s adjustment (p<0.01). Weight of total WAT is the combined weights of the epididymal, inguinal, retroperitoneal, and mesenteric depots; LL, low fat throughout the 12 wk study; HH, high fat throughout the 12 wk study; HL, high fat for the first 5 wk and then switched to low fat for the following 7 wk; HLE, high fat diet for the first 5 wk, and then switched to low fat diet with daily exercise for the following 7 wk; HLS, high fat diet for the first 5 wk, and then switched to low fat diet with 4 g/kg sodium salicylate for the following 7 wk; CLA, 0.1% 10,12 conjugated linoleic acid.
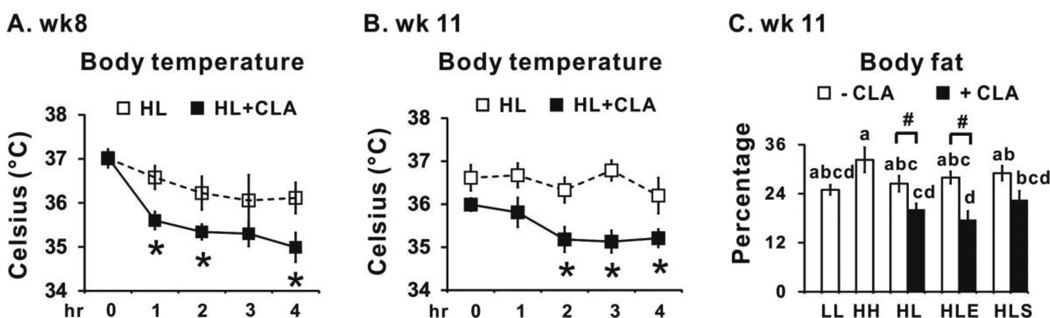
3.3. 10,12 CLA decreases body temperature during cold exposure (Phase 2-CLA treatment period; wk 6–12)
At wk 8 and wk 11, 10,12 CLA-treated mice had an impaired ability to maintain body temperature after exposure to 7 °C for 4 h compared to the mice fed the high fat diet for 5 wk and then switched to the low fat diet for 7 wk (HL control group; Figs. 4A and 4B). This reduction in body temperature was not observed in 10,12 CLA-treated mice that were exercised or supplemented with sodium salicylate (data not shown). Notably, the reduction in body temperature by 10,12 CLA at wk 11 coincided with the reduction in percentage of body fat during the same week (Fig. 4C), indicating that the loss of body fat may have impacted the mice’s ability to defend body temperature during cold exposure.
Figure 4.
10,12 CLA decreased body temperature during cold exposure. At wk 8 (Panel A) and wk 11 (Panel B), 10 mice from HL and HL+CLA groups were exposed to 7 °C for 4 h. Body temperatures were recorded at baseline and after 1, 2, 3, and 4 h of cold exposure. Panel C, body fat percentages using Dual-energy X-ray (DEXA) were measured in mice from all treatments (n=10–12 per group) at wk 11. Means ± SEM having an asterisks (*) are significant different using the Student’s t test (p<0.05). LL, low fat throughout the 12 wk study; HH, high fat throughout the 12 wk study; HL, high fat for the first 5 wk and then switched to low fat for the following 7 wk; HLE, high fat diet for the first 5 wk, and then switched to low fat diet with daily exercise for the following 7 wk; HLS, high fat diet for the first 5 wk, and then switched to low fat diet with 4 g/kg sodium salicylate for the following 7 wk; CLA, 0.1% 10,12 conjugated linoleic acid.
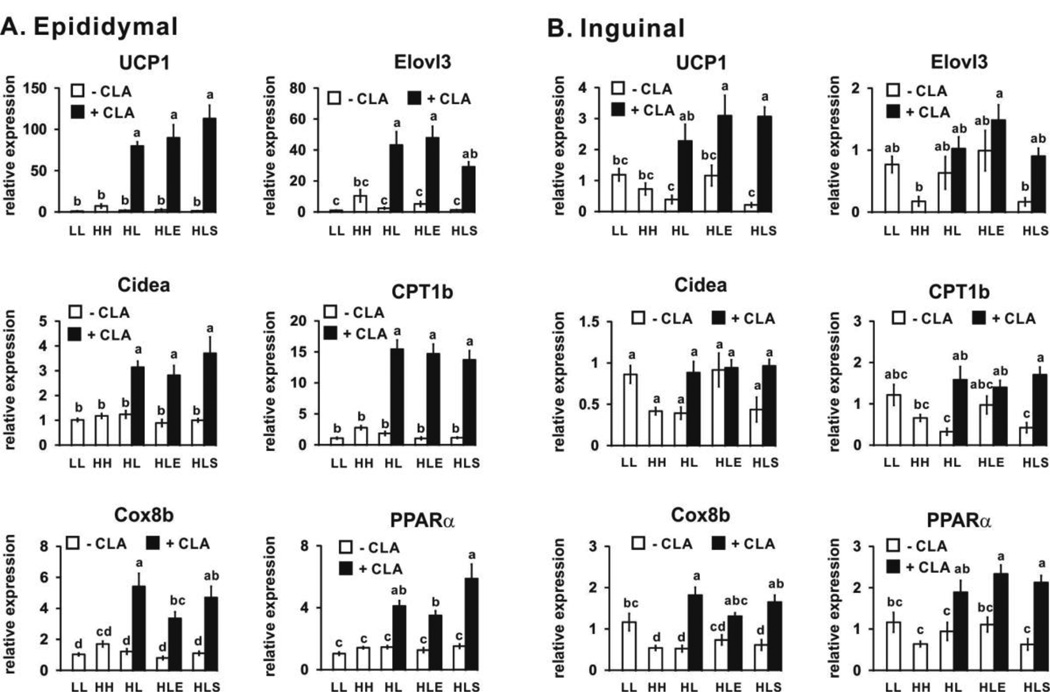
3.4. 10,12 CLA increases markers of browning and thermogenesis (Phase 2- CLA treatment period; wk 6–12)
Mice consuming the low fat and high fat diets for 12 wk (LL and HH, respectively) had similar mRNA levels of markers associated with browning in epididymal and inguinal fat depots (Figs. 5A and 5B). Consistent with our previous study [21], all 10,12 CLA-treated mice had increased mRNA levels of markers of thermogenesis and fatty acid oxidation in epididymal (Fig. 5A) and inguinal (Fig. 5B) WAT including UCP1 (uncouples phosphorylation of ADP to ATP), CPT1b (facilitates fatty acid transport into mitochondria), cytochrome c oxidase VIII b (Cox8b; promotes electron transfer during mitochondrial respiration), and PPARα (induces genes associated with fatty acid oxidation) compared to the HL controls. This 10,12 CLA-mediated induction was greatest in the epididymal depot, consistent with our previous study [21]. Exercise did not further affect markers of browning in control or 10,12 CLA-treated mice. In contrast to our hypothesis, sodium salicylate did not prevent 10,12 CLA’s induction of mRNA markers of browning in either WAT depot.
Figure 5.
10,12 CLA increases the mRNA levels of markers associated with browning in epididymal (Panel A) and inguinal (Panel B) WAT. mRNA levels were measured by real time qPCR. Means ± SEM (n=10–12) not sharing a common letter differ (p<0.05) by one-way ANOVA. UCP1, uncoupling protein 1; Elovl3, elongation of very long chain fatty acids 3; Cidea, cell death-induced DNA fragmentation factor-a-like effector A; CPT1b, carnitine palmitoyltransferase 1b; COX8b, cytochrome c oxidase subunit VIII b; PPARα, proliferator-activated receptor α; LL, low fat diet throughout the 12 wk study; HH, high fat diet throughout the 12 wk study; HL, high fat diet for the first 5 wk and then switched to low fat diet for the following 7 wk; HLE, high fat diet for the first 5 wk, and then switched to low fat diet with daily exercise for the following 7 wk; HLS, high fat diet for the first 5 wk, and then switched to low fat diet with 4 g/kg sodium salicylate for the following 7 wk; CLA, 0.1% 10,12 conjugated linoleic acid.
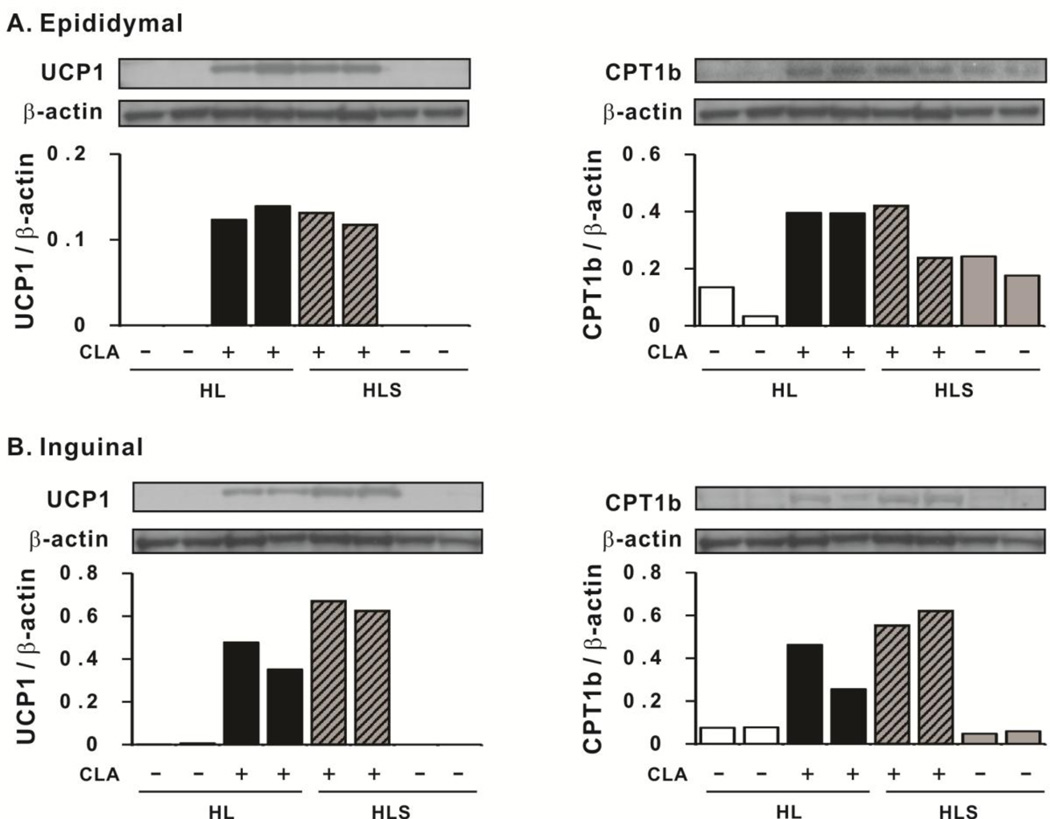
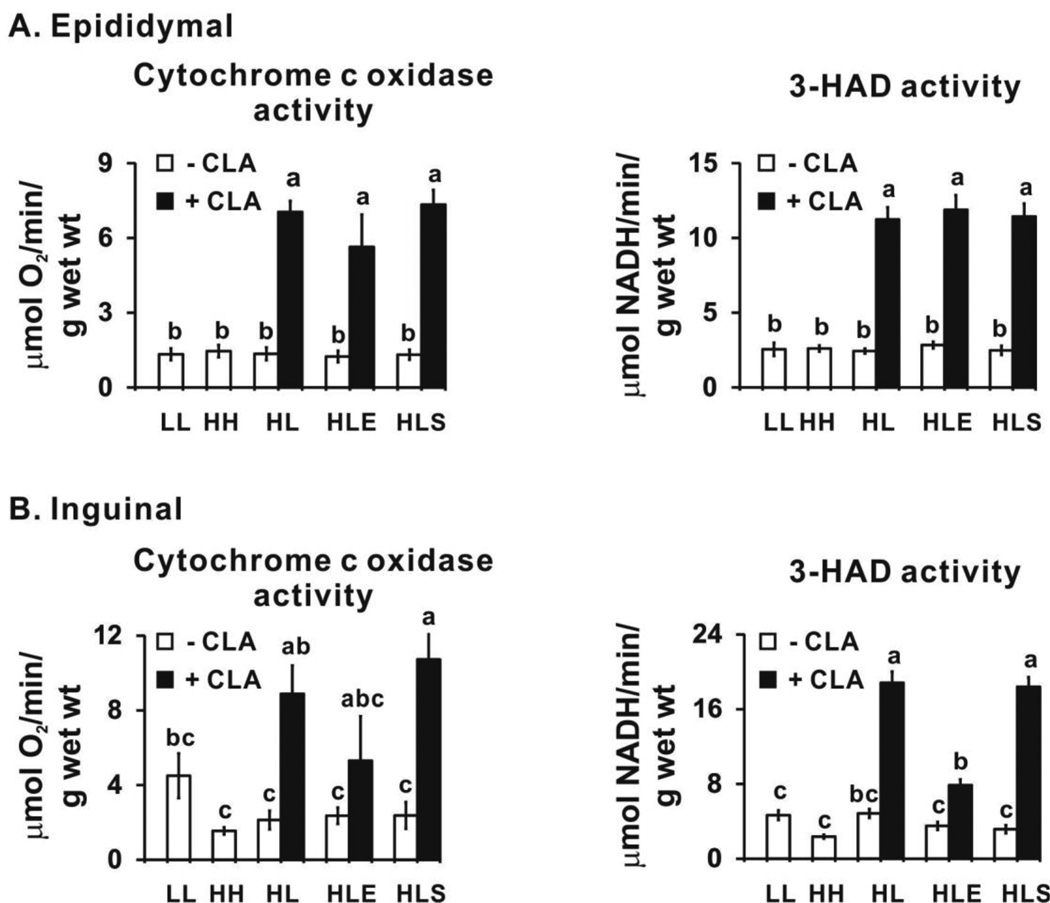
Consistently, protein levels of UCP1 and CPT1b were higher in 10,12 CLA-treated mice in visceral (epididymal) and subcutaneous (inguinal) depots compared to their respective controls, and sodium salicylate did not attenuate this increase by 10,12 CLA (Fig. 6). In agreement with mRNA and protein data, the activities of cytochrome c oxidase and 3-HAD (Fig. 7A) were elevated by 10,12 CLA treatment compared to the controls in the epididymal depot. Exercise did not further elevate the activity of these two enzymes, nor did sodium salicylate attenuate their activities. Notably, none of the treatments influenced the activities of cytochrome c oxidase or 3-HAD in gastronemius muscle (i.e., indicators of non-shivering thermogenesis; data not shown) or the mRNA levels of UCP1 in BAT (data not shown).
Figure 6.
10,12 CLA increases the protein levels of uncoupling protein 1 (UCP1) and carnitine palmitoyltransferase 1b (CPT1b) in epididymal (Panel A) and inguinal (Panel B) WAT. Protein levels were measured by immunoblotting. β-actin was used as a loading control. Two samples from HL, HL+CLA, HLS, and HLS+CLA treatments were randomly selected and ran on the same gel. We chose the most representative blots. LL, low fat diet throughout the 12 wk study; HH, high fat diet throughout the 12 wk study; HL, high fat diet for the first 5 wk and then switched to low fat diet for the following 7 wk; HLS, high fat diet for the first 5 wk, and then switched to low fat diet with 4 g/kg sodium salicylate for the following 7 wk; CLA, 0.1% 10,12 conjugated linoleic acid.
Figure 7.
10,12 CLA increases the activity of cytochrome c oxidase and 3-hydroxyCoA dehydrogenase (3-HAD) in epididymal (Panel A) and inguinal (Panel B) WAT. Means ± SEM (n=8–12) without a common letter differ (p<0.05) by one-way ANOVA. LL, low fat diet throughout the 12 wk study; HH, high fat diet throughout the 12 wk study; HL, high fat diet for the first 5 wk and then switched to low fat diet for the following 7 wk; HLE, high fat diet for the first 5 wk, and then switched to low fat diet with daily exercise for the following 7 wk; HLS, high fat diet for the first 5 wk, and then switched to low fat diet with 4 g/kg sodium salicylate for the following 7 wk; CLA, 0.1% 10,12 conjugated linoleic acid.
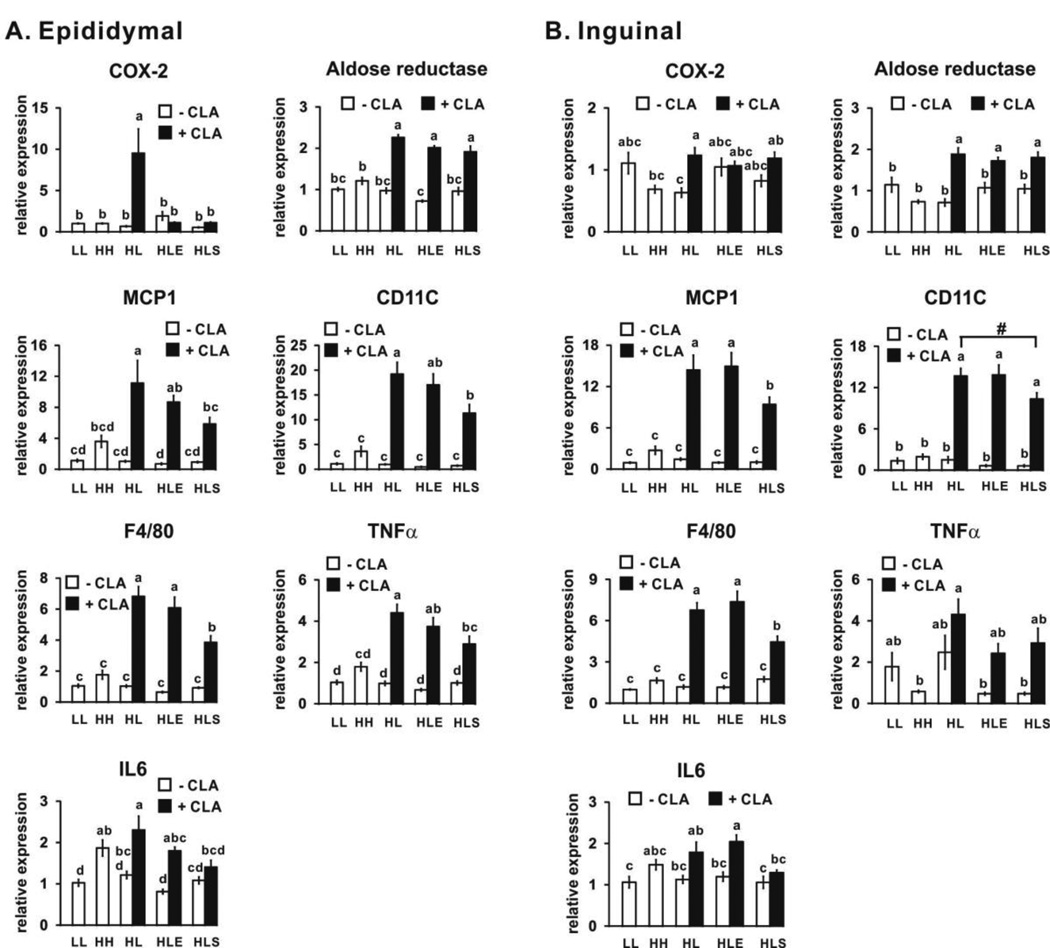
3.5. 10,12 CLA increases markers of inflammation (Phase 2- CLA treatment period; wk 6–12)
Mice consuming the low fat and high fat diets for 12 wk (LL and HH, respectively) had similar mRNA levels of markers associated with inflammation in epididymal (Fig. 8A) and inguinal (Fig. 8B) WAT depots. 10,12 CLA-treated (HL+CLA) mice had increased mRNA levels of markers of inflammation in epididymal and inguinal WAT depots, including those associated with prostaglandin synthesis (e.g., COX-2 and aldose reductase), M1 macrophage responses (e.g., chemoattractant protein 1 (MCP1), F4/80, and CD11c), and pro-inflammatory cytokines (e.g., IL6), compared to the low fat control mice (HL). Exercise did not further affect markers of inflammation in either depot. Sodium salicylate attenuated 10,12 CLA-mediated induction of markers of COX-2, MCP1, CD11C, F4/80, tumor necrosis factor (TNF) α, and IL6 in the epididymal depot (Fig. 8).
Figure 8.
10,12 CLA increases the mRNA levels of markers associated with inflammation in epididymal (Panel A) and inguinal (Panel B) WAT. mRNA levels were measured by real time qPCR. Means ± SEM (n=10–12) without a common letter differ (p<0.05) by one-way ANOVA. Means ± SEM sharing the symbol # differ using the Bonferroni’s adjustment (p<0.01). COX-2, cyclooxygenase 2; MCP1, monocyte chemoattractant protein 1; TNFα, tumor necrosis factor α; IL6, interleukin 6; LL, low fat diet throughout the 12 wk study; HH, high fat diet throughout the 12 wk study; HL, high fat diet for the first 5 wk and then switched to low fat diet for the following 7 wk; HLE, high fat diet for the first 5 wk, and then switched to low fat diet with daily exercise for the following 7 wk; HLS, high fat diet for the first 5 wk, and then switched to low fat diet with 4 g/kg sodium salicylate for the following 7 wk; CLA, 0.1% 10,12 conjugated linoleic acid.
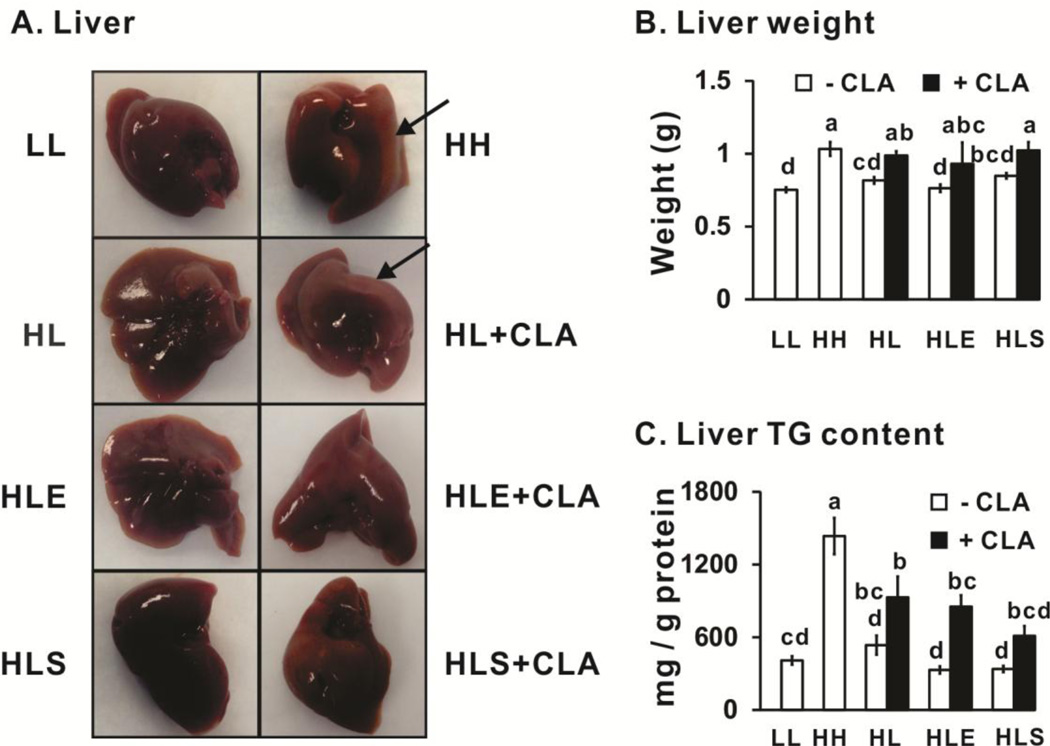
Mice fed the high fat diet for 12 wk (HH) had greater liver weights (Figs. 9A and 9B) and TG content (Fig. 9C) compared to the mice fed the low fat diet for 12 wk (LL). Mice fed 10,12 CLA (HL+CLA) had heavier livers compared to their controls (HL). Surprisingly, exercise (HLE+CLA) or sodium salicylate (HLS+CLA) did not prevent hepatomegaly (Fig. 9B) nor the increase in hepatic TG content (Fig. 9C) in 10,12 CLA-treated mice. These data indicate that even this low dose (0.1%, w/w) of 10,12 CLA may have deleterious effects in the liver, which has been consistently reported by others at higher doses of 10,12 CLA or mixed CLA isomers [32].
Figure 9.
Impact of 10,12 CLA on liver weight and triglyceride (TG) content. Panel A, representative pictures from each treatment group (n=6–8), with arrows pointing to areas of lipid accumulation. Panel B, liver weights from each group (n=10–12). Panel C, liver TG content was measured and normalized by per mg of protein (n=10–12). Mean ± SEM without a common letter differ (p<0.05) by one-way ANOVA. LL, low fat diet throughout the 12 wk study; HH, high fat diet throughout the 12 wk study; HL, high fat diet for the first 5 wk and then switched to low fat diet for the following 7 wk; HLE, high fat diet for the first 5 wk, and then switched to low fat diet with daily exercise for the following 7 wk; HLS, high fat diet for the first 5 wk, and then switched to low fat diet with 4 g/kg sodium salicylate for the following 7 wk; CLA, 0.1% 10,12 conjugated linoleic acid.
4. Discussion
4.1. 10,12 CLA-mediated loss of body fat and induction of browning is independent of COX-2 and inflammatory signaling
It has been consistently reported that COX-2 plays an important role in response to classic β3-adrenergic activation from cold exposure or agonists to promote mitochondrial biogenesis and increased UCP1 expression in WAT [17, 18, 33, reviewed in 34, reviewed in 35]. COX-2, the rate-determining enzyme in prostaglandin synthesis, has been shown to be up-regulated in WAT during exposure to cold or stimulation with a β3-adrenergic agonist [17]. For example, CL316243-mediated upregulation of UCP1, cell death-induced DNA fragmentation factor-a-like effector A (Cidea), and CPT1b mRNA levels in intra-abdominal WAT was attenuated by treatment with the selective COX-2 inhibitor celecoxib [17]. Our previous study [21] demonstrated that a 7 wk, low dose of 10,12 CLA prevented body fat accumulation and increased markers of browning in lean mice, which was accompanied by increased COX-2 mRNA and protein expression.
However, we did not know if this low dose of 10,12 CLA decreased adiposity in overweight mice to a greater extent than consuming a low fat diet alone, and if so, was it dependent on COX-2 signaling. To answer this question, mice were fed a high fat diet for 5 wk to get them overweight, and then switched to a low fat diet with or without CLA and sodium salicylate, a COX-2 inhibitor. Consistent with our hypothesis, all mice receiving 10,12 CLA had lower total WAT depot weights compared to their low fat-fed controls (Fig. 3). As anticipated, sodium salicylate blocked COX-2 gene expression and attenuated 10,12 CLA-mediated inflammatory gene expression in epididymal WAT (Fig. 8). However, it did not prevent 10,l2 CLA from decreasing body fat (Fig. 3), increasing markers of browning in epididymal or inguinal WAT (Fig. 5), or defending body temperature under cold exposure (data not shown). Collectively, these data advance the hypothesis that the induction or activation of COX-2, NFκB, or other inflammatory related markers may be a consequence, rather than a mediator, of 10,12 CLA-activated browning.
Consistent with the above hypothesis, Bruce Spiegelman’s group recently demonstrated that browning in WAT can be activated independent of β-adrenergic signaling [36]. For example, after 20 h exposure to 10°C, UCP1, Type II iodothyronine deiodinase, and peroxisome proliferator-activated receptor gamma coactivator 1 mRNA levels were not completely diminished compared to controls in inguinal WAT in mice lacking β-adrenergic receptors, although their expression levels were lower than in wild type mice [36]. These data provide further support for the hypothesis that WAT has the capacity to sense changes in external temperatures and respond to cold stimulus by uncoupling respiration from phosphorylation in mitochondria. In our study, we detected significant body fat loss after 6 wk of 10,12 CLA feeding, which was coincident with relatively lower, but not statistically significant basal body temperature (Supplemental Fig. 1) and impaired ability to maintain body temperature when exposed to cold environment, compared to its HL control (Fig. 4). A similar inability to regulate body temperature with 10,12 CLA supplementation during cold exposure was observed at an earlier time point (i.e., after 3 wk of 10,12 CLA supplementation, Fig. 4A). However, we did not measure the body fat percentage during that week. Interestingly, CLA supplementation (i.e., 1%; equal mixture of cis-9, trans-11 and 10,12 isomers, a.k.a. mixture CLA isomers) induced heat loss in C57Bl/6J mice, which coincided with significant loss of WAT mass on day 9 of treatment [37]. Consistent with these data, mixed CLA isomers increased energy expenditure and tended to lower respiratory quotient in AKR/J mice after wk 6 of treatment [38], suggesting CLA promoted fat oxidation.
Although 10,12 CLA did not increase UCP1 mRNA or protein in BAT (data not shown), or cytochrome c oxidase in muscle (an indicator of shivering thermogenesis; data not shown) after 7 wk of treatment, it is possible that BAT thermogenesis could be initially upregulated, because BAT mass was decreased by 50% following 10,12 CLA treatment for 7 wk (Fig 3). As BAT mass becomes compromised, WAT browning could be induced to provide heat to maintain body temperature. Thus, it is possible that BAT thermogenesis could have been initially upregulated by 10,12 CLA, and as it became depleted, browning of WAT was upregulated in an attempt to maintain body temperature when cold challenged. Future time course studies will need to be conducted to examine this hypothesis.
4.2. 10,12 CLA-mediated loss of body fat does not appear to be mediated by adipocyte apoptosis
Another proposed mechanism by which CLA reduces body fat is via activation of apoptosis [reviewed in 4]. Supplementation of 1% mixed CLA isomers induced apoptosis in WAT in female C57BL/6J mice [39] and mixed strains of mice [37] within 1 wk of treatment. Several apoptotic-related genes such as those in the TNF family, cell death factors, and anti-apoptotic genes were affected after 1 day of mixed CLA isomer feeding in WAT depots of male C57BL/6J mice [28]. Strikingly, mice from various genetic lines responded to 1% mixed CLA isomers or 0.5% 10,12 CLA differently [37]. For example, mice from low energy expenditure strains, but not from high energy expenditure lines, exhibited apoptosis in retroperitoneal WAT from either CLA treatment. Our study used a low dose of 10,12 CLA (0.1%) in male Sv129 mice for 7 wk and did not find a consistent pattern of 10,12 CLA-mediated apoptosis in epididymal WAT depot (Supplemental Fig. 2). We speculate that this lack of induction of apoptotic genes may be due to the higher basal metabolic rate background of Sv129 compared to the C57BI/6J strain [40].
4.3. 10,12 CLA causes hepatomegaly, which is not attenuated by exercise
Steatosis has been a notorious side effect associated with consuming high doses of CLA [32, 39, 41]. To our knowledge, there are no studies examining the ability of exercise to prevent or treat CLA-mediated steatosis. Several studies reported that exercise successfully prevented high fat-induced steatosis in rodents [28, 42]. For example, high fat (45% fat)-fed male C57BL/6 mice had elevated liver TG content at wk 6 [28]. Exercise (treadmill, 40 min/d, 12 m/min, 12% grade, 5 d/wk) for 6 wk prevented this high fat diet-mediated steatosis. This same intensity and duration of exercise also have been shown to improve blood lipid profiles and enhance oxidative capacity in leg muscles of male C57BL/6 mice [29]. Therefore, we adopted this exercise protocol for our study. However, exercise did not further attenuate 10,12 CLA mediated- (i) reduction of adiposity (Fig. 3), (ii) increase of liver weight (Fig. 9), or (iii) inflammatory gene expression in WAT (Fig. 8) in Sv129 mice. Furthermore, exercise did not affect 10,12 CLA-induced browning in WAT (Fig. 5). This could be due to different metabolic rates of these two strains or that the exercise protocol was not of sufficient intensity or duration.
4.4. Limitations
One major limitation of this study is that we did not measure energy expenditure, and thus we do not know if 10,12 CLA decreases body fat via increased heat loss. Another limitation is that we did not measure body fat percentage and body temperature of mice exposed to 4°C during the first few weeks of the study. A third limitation is that the DEXA instrument has not been validated for mice by the manufacturer. However, we compared the actual body weights (recorded weekly) to the DEXA instrument reads (bone mass + fat + lean tissue mass). Correlation analysis was conducted by SPSS (version 20). The Person’s correlation value is 0.938, and the significant p < 0.001 (data not shown). Lastly, we do not know if a low dose of 10,12 CLA reduces body fat in overweight mice consuming a high fat diet. Thus, future studies need to address these issues so as to gain further insights into the exact mechanism by which 10,12 CLA decreases body fat.
Supplementary Material
Acknowledgements
This work was supported by a grant from the National Institute of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases/Office of Dietary Supplements (NIDDK/ODS) (5R01-DK063070-09) to MM. We are grateful for all of the helpful advice and thoughtful discussions with Dr. Ron Morrison at the University of North Carolina at Greensboro.
Abbreviations
- AUC
area under the curve
- BAT
brown adipose tissue
- 10,12 CLA
trans-10, cis-12 conjugated linoleic acid
- Cidea
cell death-induced DNA fragmentation factor-a-like effector A
- Cox8b
cytochrome c oxidase subunit VIII b
- COX-2
cyclooxygenase-2
- CPT
carnitine palmitoyltransferase
- DEXA
dual-energy X-ray
- ER
endoplasmic reticulum
- Elovl3
elongation of very long chain fatty acids 3 protein
- ERK
extracellular signal-regulated kinase
- GPR
G protein receptor
- HAD
hydroxyCoA dehydrogenase
- HH
high fat throughout the study
- HL
high fat for the first 5 wk and then switched to low fat for the following 7 wk
- HLE
high fat for the first 5 wk, and then switched to low fat with daily exercise for the following 7 wk
- HLS
high fat for the first 5 wk, and then switched to low fat with 4 g/kg sodium salicylate for the following 7 wk
- IGTT
intraperitoneal glucose tolerance test
- IL
interleukin
- LL
low fat throughout the study
- MCP
monocyte chemoattractant protein
- NF-κB
nuclear factor kappa B
- PPAR
peroxisome proliferator activated receptor
- TG
triglyceride
- TNF
tumor necrosis factor
- UCP
uncoupling protein
- WAT
white adipose tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention. Overweight and Obesity. [Accessed on July 13, 2014]; http://www.cdc.gov/obesity/data/facts.html.
- 2.Centers for Disease Control and Prevention. Obesity prevalence in 2012 varies across states and regions. [Accessed July 13, 2014]; http://www.cdc.gov/obesity/data/adult.html#Prevalence.
- 3.Behan DF, Cox SH, Lin Y, Pai J, Pedersen HW, Yi M. Obesity and its relation to mortality and morbidity Costs. Society of Actuaries. 2010 [Google Scholar]
- 4.Kennedy A, Martinez K, Schmidt S, Mandrup S, LaPoint K, McIntosh M. Antiobesity mechanisms of action of conjugated linoleic acid. J Nutr Biochem. 2010 Mar;21(3):171–179. doi: 10.1016/j.jnutbio.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown JM, Boysen MS, Jensen SS, Morrison RF, Storkson J, Lea-Currie R, et al. Isomer-specific regulation of metabolism and PPARgamma signaling by CLA in human preadipocytes. J Lipid Res. 2003 Jul;44(7):1287–1300. doi: 10.1194/jlr.M300001-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown JM, Boysen MS, Chung S, Fabiyi O, Morrison RF, Mandrup S, et al. Conjugated linoleic acid induces human adipocyte delipidation: autocrine/paracrine regulation of MEK/ERK signaling by adipocytokines. J Biol Chem. 2004 Jun 18;279(25):26735–26747. doi: 10.1074/jbc.M401766200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obsen T, Faergeman NJ, Chung S, Martinez K, Gobern S, Loreau O, et al. Trans-10, cis-12 conjugated linoleic acid decreases de novo lipid synthesis in human adipocytes. J Nutr Biochem. 2012 Jun;23(6):580–590. doi: 10.1016/j.jnutbio.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung S, Brown JM, Provo JN, Hopkins R, McIntosh MK. Conjugated linoleic acid promotes human adipocyte insulin resistance through NFkappaB-dependent cytokine production. J Biol Chem. 2005 Nov 18;280(46):38445–38456. doi: 10.1074/jbc.M508159200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung S, Brown JM, Sandberg MB, McIntosh M. Trans-10,cis-12 CLA increases adipocyte lipolysis and alters lipid droplet-associated proteins: role of mTOR and ERK signaling. J Lipid Res. 2005 May;46(5):885–895. doi: 10.1194/jlr.M400476-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reardon M, Gobern S, Martinez K, Shen W, Reid T, McIntosh M. Oleic acid attenuates trans-10,cis-12 conjugated linoleic acid-mediated inflammatory gene expression in human adipocytes. Lipids. 2012 Nov;47(11):1043–1051. doi: 10.1007/s11745-012-3711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen W, Martinez K, Chuang CC, McIntosh M. The phospholipase C inhibitor U73122 attenuates trans-10, cis-12 conjugated linoleic acid-mediated inflammatory signaling and insulin resistance in human adipocytes. J Nutr. 2013 May;143(5):584–590. doi: 10.3945/jn.112.173161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez K, Shyamasundar S, Kennedy A, Chuang CC, Marsh A, Kincaid J, et al. Diacylglycerol kinase inhibitor R59022 attenuates conjugated linoleic acid-mediated inflammation in human adipocytes. J Lipid Res. 2013 Mar;54(3):662–670. doi: 10.1194/jlr.M031211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy A, Martinez K, Chung S, LaPoint K, Hopkins R, Schmidt SF, et al. Inflammation and insulin resistance induced by trans-10, cis-12 conjugated linoleic acid depend on intracellular calcium levels in primary cultures of human adipocytes. J Lipid Res. 2010 Jul;51(7):1906–1917. doi: 10.1194/jlr.M005447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy A, Chung S, LaPoint K, Fabiyi O, McIntosh MK. Trans-10, cis-12 conjugated linoleic acid antagonizes ligand-dependent PPARgamma activity in primary cultures of human adipocytes. J Nutr. 2008 Mar;138(3):455–461. doi: 10.1093/jn/138.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez K, Kennedy A, McIntosh MK. JNK inhibition by SP600125 attenuates trans-10, cis-12 conjugated linoleic acid-mediated regulation of inflammatory and lipogenic gene expression. Lipids. 2011 Oct;46(10):885–892. doi: 10.1007/s11745-011-3587-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez K, Kennedy A, West T, Milatovic D, Aschner M, McIntosh M. trans-10,cis-12-Conjugated linoleic acid instigates inflammation in human adipocytes compared with preadipocytes. J Biol Chem. 2010 Jun 4;285(23):17701–17712. doi: 10.1074/jbc.M109.043976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vegiopoulos A, Müller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, et al. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science. 2010 May 28;328(5982):1158–1161. doi: 10.1126/science.1186034. [DOI] [PubMed] [Google Scholar]
- 18.Madsen L, Pedersen LM, Lillefosse HH, Fjaere E, Bronstad I, Hao Q, et al. UCP1 induction during recruitment of brown adipocytes in white adipose tissue is dependent on cyclooxygenase activity. PLoS One. 2010 Jun 30;5(6):e11391. doi: 10.1371/journal.pone.0011391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang T, Zhang J, Yin J, Staszkiewicz J, Gawronska-Kozak B, Jung DY, et al. Uncoupling of inflammation and insulin resistance by NF-kappaB in transgenic mice through elevated energy expenditure. J Biol Chem. 2010 Feb 12;285(7):4637–4644. doi: 10.1074/jbc.M109.068007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiao P, Feng B, Ma J, Nie Y, Paul E, Li Y, Xu H. Constitutive activation of IKKβ in adipose tissue prevents diet-induced obesity in mice. Endocrinology. 2012 Jan;153(1):154–165. doi: 10.1210/en.2011-1346. [DOI] [PubMed] [Google Scholar]
- 21.Shen W, Chuang CC, Martinez K, Reid T, Brown JM, Xi L, et al. Conjugated linoleic acid reduces adiposity and increases markers of browning and inflammation in white adipose tissue of mice. J Lipid Res. 2013 Apr;54(4):909–922. doi: 10.1194/jlr.M030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994 Aug 12;265(5174):956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 23.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001 Aug 31;293(5535):1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 24.Herrero L, Shapiro H, Nayer A, Lee J, Shoelson SE. Inflammation and adipose tissue macrophages in lipodystrophic mice. Proc Natl Acad Sci U S A. 2010 Jan 5;107(1):240–245. doi: 10.1073/pnas.0905310107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potteiger JA, Jacobsen DJ, Donnelly JE. A comparison of methods for analyzing glucose and insulin areas under the curve following nine months of exercise in overweight adults. Int. J. Obes. Relat. Metab. Disord. 2002;26:87–89. doi: 10.1038/sj.ijo.0801839. [DOI] [PubMed] [Google Scholar]
- 26.Carmona MC, Hondares E, Rodríguez de la Concepción ML, Rodríguez-Sureda V, Peinado-Onsurbe J, Poli V, et al. Defective thermoregulation, impaired lipid metabolism, but preserved adrenergic induction of gene expression in brown fat of mice lacking C/EBPbeta. Biochem J. 2005 Jul 1;389(Pt 1):47–56. doi: 10.1042/BJ20050009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JH, Kim J, Park Y. trans-10,cis-12 conjugated linoleic acid enhances endurance capacity by increasing fatty acid oxidation and reducing glycogen utilization in mice. Lipids. 2012 Sep;47(9):855–863. doi: 10.1007/s11745-012-3698-6. [DOI] [PubMed] [Google Scholar]
- 28.Baynard T, Vieira-Potter VJ, Valentine RJ, Woods JA. Exercise training effects on inflammatory gene expression in white adipose tissue of young mice. Mediators Inflamm. 2012;2012:767953. doi: 10.1155/2012/767953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan H, Niu Y, Liu X, Yang F, Niu W, Fu L. Proteomic Analysis of Skeletal Muscle in Insulin-Resistant Mice: Response to 6-Week Aerobic Exercise. PLoS One. 2013;8(1):e53887. doi: 10.1371/journal.pone.0053887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell CR, Harris MB, Cordaro AR, Starnes JW. Effect of body temperature during exercise on skeletal muscle cytochrome c oxidase content. J Appl Physiol (1985) 2002 Aug;93(2):526–530. doi: 10.1152/japplphysiol.00536.2001. [DOI] [PubMed] [Google Scholar]
- 31.Hansford RG. Lipid oxidation by heart mitochondria from young adult and senescent rats. Biochem J. 1978 Feb 15;170(2):285–295. doi: 10.1042/bj1700285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clément L, Poirier H, Niot I, Bocher V, Guerre-Millo M, Krief S, et al. Dietary trans-10,cis-12 conjugated linoleic acid induces hyperinsulinemia and fatty liver in the mouse. J Lipid Res. 2002 Sep;43(9):1400–1409. doi: 10.1194/jlr.m20008-jlr200. [DOI] [PubMed] [Google Scholar]
- 33.Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab. 2010 Jun;298(6):E1244–E1253. doi: 10.1152/ajpendo.00600.2009. [DOI] [PubMed] [Google Scholar]
- 34.Ishibashi J, Seale P. Beige Can Be Slimming. Science. 2010;328:1113–1114. doi: 10.1126/science.1190816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 2013 Feb 1;27(3):234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye L, Wu J, Cohen P, Kazak L, Khandekar MJ, Jedrychowski MP, et al. Fat cells directly sense temperature to activate thermogenesis. Proc Natl Acad Sci U S A. 2013 Jul 23;110(30):12480–12485. doi: 10.1073/pnas.1310261110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miner JL, Cederberg CA, Nielsen MK, Chen X, Baile CA. Conjugated linoleic acid (CLA), body fat, apoptosis. Obes Res. 2001 Feb;9(2):129–134. doi: 10.1038/oby.2001.16. [DOI] [PubMed] [Google Scholar]
- 38.West DB, Delany JP, Camet PM, Blohm F, Truett AA, Scimeca J. Effects of conjugated linoleic acid on body fat and energy metabolism in the mouse. Am J Physiol. 1998 Sep;275(3 Pt 2):R667–R672. doi: 10.1152/ajpregu.1998.275.3.R667. [DOI] [PubMed] [Google Scholar]
- 39.Tsuboyama-Kasaoka N, Takahashi M, Tanemura K, Kim HJ, Tange T, Okuyama H, et al. Conjugated linoleic acid supplementation reduces adipose tissue by apoptosis and develops lipodystrophy in mice. Diabetes. 2000 Sep;49(9):1534–1542. doi: 10.2337/diabetes.49.9.1534. [DOI] [PubMed] [Google Scholar]
- 40.Almind K, Kahn CR. Genetic determinants of energy expenditure and insulin resistance in diet-induced obesity in mice. Diabetes. 2004 Dec;53(12):3274–3285. doi: 10.2337/diabetes.53.12.3274. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi Y, Kushiro M, Shinohara K, Ide T. Activity and mRNA levels of enzymes involved in hepatic fatty acid synthesis and oxidation in mice fed conjugated linoleic acid. Biochim Biophys Acta. 2003 Apr 8;1631(3):265–273. doi: 10.1016/s1388-1981(03)00038-6. [DOI] [PubMed] [Google Scholar]
- 42.Gauthier MS, Couturier K, Latour JG, Lavoie JM. Concurrent exercise prevents high-fat-diet-induced macrovesicular hepatic steatosis. J Appl Physiol (1985) 2003 Jun;94(6):2127–2134. doi: 10.1152/japplphysiol.01164.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.