Abstract
Objective
Patents with anxious bipolar disorder have worse clinical outcomes and are harder to treat with traditional medication regimens compared to those with non-anxious bipolar disorder. Ketamine has been shown to rapidly and robustly decrease symptoms of depression in depressed patients with bipolar disorder. We sought to determine whether baseline anxiety status reduced ketamine’s ability to decrease symptoms of depression.
Methods
Thirty-six inpatients with anxious (n = 21) and non-anxious (n = 15) treatment-resistant bipolar depression (types I and II; concurrently treated with either lithium or valproate) received a single infusion of ketamine (0.5 mg/kg) over 40 minutes. Post-hoc analyses compared changes in the Montgomery–Åsberg Depression Rating Scale (MADRS) and Hamilton Depression Rating Scale (HDRS) in anxious versus non-anxious depressed patients with bipolar disorder through 14 days post-infusion. Anxious bipolar depression was defined as DSM-IV bipolar depression plus ≥ 7 on the HDRS Anxiety/Somatization Factor Score.
Results
A linear mixed model revealed a significant effect of anxiety group on the MADRS (p = 0.04) and HDRS (p = 0.04). Significant drug effects (all p < 0.001) suggested both anxious and non-anxious groups had an antidepressant response to ketamine. The drug by anxiety interactions were not significant (all p > 0.28).
Conclusions
Both anxious and non-anxious patients with bipolar depression had significant antidepressant responses to ketamine, though the anxious depressed group did not show a clear antidepressant response disadvantage over the non-anxious group. Given that anxiety has been shown to be a predictor of poor treatment response in bipolar depression when traditional treatments are used, our findings suggest the need for further investigations into ketamine’s novel role in the treatment of anxious bipolar depression.
Keywords: anxious depression, bipolar disorder, ketamine, N-methyl-d-aspartate antagonist, predictors of response
Patients with both bipolar disorder and comorbid anxiety disorders/anxiety symptoms have worse clinical outcomes compared to non-anxious bipolar disorder (1, 2). This anxious subtype of bipolar disorder is common, with a prevalence of approximately 25–50% in some studies (3, 4). Furthermore, anxiety’s influence in bipolar disorder is becoming increasingly recognized, as reflected by the addition of an anxious distress specifier to the diagnosis of bipolar disorder in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) (1). Regarding patients with primary bipolar illness accompanied by symptoms of anxiety, one study examined a sample of patients with bipolar disorder (type I and II) and found that high anxiety symptoms (≥ 16 on anxiety items from the Schedule for Affective Disorders-Lifetime version) were significantly associated with alcohol abuse, cyclothymia, and more suicide attempts (4)—all factors that increase morbidity. Also in this sample, high anxiety within bipolar disorder predicted a trend toward non-responsiveness to lithium. Similarly, another study found non-remission in bipolar disorder to be associated with current or past anxiety symptoms, as well as a history of panic attacks, more severe depression, and a greater number of past depressive or manic episodes (3). Patients with bipolar disorder with anxiety report significantly more side effects despite no difference in blood lithium levels or dosing compared to patients without anxiety, and require a larger number of medication trials in order to achieve remission, primarily due to the need for more antidepressant trials (3). Regarding randomized controlled trials (RCTs), risperidone monotherapy was not an effective anxiolytic in patients with bipolar disorder with anxious symptoms and comorbid panic disorder or generalized anxiety disorder (GAD) (5). Similarly, ziprasidone monotherapy did not significantly improve anxiety symptoms in patients with bipolar disorder with comorbid panic disorder or GAD; instead, it was associated with more negative side effects versus placebo (6).
Despite the clinical challenge that anxious bipolar depression presents, some positive findings exist in the literature. A review of 11 RCTs found that quetiapine (monotherapy or in combination) was associated with significant improvements in anxiety [measured by the Hamilton Anxiety Rating Scale (HAM-A)] in bipolar depression (7). Similarly, patients with bipolar depression and high anxiety (≥ 18 on the HAM-A) experienced significant reductions in symptoms of both depression and anxiety following treatment with olanzapine or olanzapine-fluoxetine combination versus placebo; however, weight gain and increased cholesterol levels were also significant (8). Recently, lurasidone monotherapy (9) and in combination with lithium or valproate (10) was found to significantly reduce anxiety symptoms in patients with bipolar depression.
Despite a few positive studies, anxious bipolar disorder is generally a more severe subtype that is clinically challenging to treat, especially during a depressive episode. Although of urgent clinical need, novel psychopharmacological interventions for anxious bipolar depression have been understudied (11). Ketamine is of particular interest for difficult-to-treat patients with depression because it differs from many currently available antidepressants in that it is an N-methyl-D-aspartate (NMDA) receptor antagonist, though it’s precise mechanism of antidepressant action remains largely unknown. A single intravenous infusion of ketamine (0.5 mg/kg over 40 minutes) has been shown to have a rapid (within 40 minutes) and robust antidepressant effect when given to moderately-to-severely depressed patients with bipolar disorder (despite having failed multiple medications trials) maintained on therapeutic levels of lithium or valproate; this effect lasted through Day 3 post-infusion (12, 13). In addition, ketamine rapidly improved suicidal ideation in these patients (12).
Indeed, ketamine has been shown to rapidly and robustly reduce symptoms of depression in a sample of patients with treatment-resistant bipolar disorder (with and without anxiety) (13, 14). What remains unknown is whether stratifying patients with bipolar depression based on baseline anxious status predicts a poorer treatment response to ketamine, since anxiety generally predicts poorer outcome for standard treatments for bipolar disorder (3, 4). We sought to determine whether baseline anxiety status reduced ketamine’s ability to effectively treat depression. In order to answer this question, we examined ketamine’s effect on depression scores over 14 days post-infusion in patients with both anxious and non-anxious bipolar depression.
Patients and methods
In this post-hoc analysis, data were combined from two separate, but identical, randomized crossover trials investigating ketamine for the treatment of bipolar depression (types I and II) (ClinicalTrials.gov identifier: NCT00088699) (12, 13). Pooled data were available for 36 patients: 18 from the first report (12) and 15 from the second report (13). Three additional patients completed the study since the publication of these initial reports. All studies were approved by the National Institutes of Health (NIH) Institutional Review Board in accordance with the Declaration of Helsinki.
Patient selection
Descriptions of patient procedures, inclusion/exclusion criteria, and study design have previously been published (12, 13). Briefly, all inpatients (ages 18–65 years) were admitted to the Mood and Anxiety Disorder Research Unit at the NIH (Bethesda, MD, USA). Bipolar disorder (type I or II; currently depressed) was diagnosed by clinician interview and confirmed with the Structured Clinical Interview for DSM-IV Disorders-Patient version (SCID-P) (15) and collateral information, if necessary. Anxiety disorders were allowed insofar as bipolar depression remained the primary diagnosis. A score ≥ 20 on the Montgomery–Åsberg Depression Rating Scale (MADRS) (16) at screening and at baseline (60 minutes prior to infusion) was required despite being maintained on a therapeutic level of an open-label mood stabilizer for at least four weeks prior to study (lithium with serum levels 0.6–1.2 mEg/L or valproic acid with serum levels 50–125 μg/mL). Otherwise, patients were free of other psychopharmacological agents/structured psychotherapy for at least two weeks prior to and throughout the entire duration of the entire study.
Study design and sample size
All patients received a single open-label intravenous infusion of ketamine hydrochloride (0.5 mg/kg) over 40 minutes as part of larger randomized, double-blind, placebo-controlled crossover studies assessing the efficacy and safety of ketamine for the treatment of treatment-resistant bipolar depression combined with lithium or valproate monotherapy.
Anxious depression definition
Patients were delineated into anxious (n = 21) and non-anxious (n = 15) bipolar depression. Anxious depression was defined as a baseline Hamilton Depression Rating Scale (HDRS) Anxiety/Somatization (A/S) Factor Score of ≥ 7 (17); derived from a factor analysis of the HDRS (18), plus a current DSM-IV (19) diagnosis of bipolar disorder (type I/II; currently depressed). The HDRS A/S Factor Score consists of the following six items: psychic and somatic anxiety, general and gastrointestinal somatic symptoms, hypochondriasis, and insight (17). Although no reports have utilized this factor score for the definition of anxious bipolar depression, it is frequently used to define anxious unipolar depression (20), and has discriminant ability in identifying depressed patients with anxious features and is translatable between clinical and research domains (21).
Main outcome measures
Patients were rated at baseline (60 minutes prior to ketamine infusion); at 40, 80, 110, and 230 minutes post-infusion; and at Days 1, 2, 3, 7, 10, and 14 post-infusion. The primary outcome measures for depression were MADRS and HDRS. The Clinician Administered Dissociative States Scale (CADSS) was used as a secondary outcome measure for side effects (22). The HAM-A was used as a secondary measure of anxiety (23).
Statistical analysis
Demographic variables were compared between anxious and non-anxious groups using χ2 tests for categorical and t-tests for continuous variables. Factorial linear mixed models were used to compare the groups over time. These models used a compound symmetry covariance structure with restricted maximum likelihood estimation. The baseline value was used as a covariate. Bonferroni simple effects tests were used post hoc to examine omnibus effects. Significance was evaluated at p < 0.05, two-tailed.
Results
Anxious bipolar depression accounted for 58% of the total sample. Table 1 shows demographic data. As expected, anxious patients with bipolar disorder with depression had significantly higher baseline HDRS A/S scores compared to non-anxious patients with bipolar depression (7.9 ± 0.8 versus 5.3 ± 0.9, respectively; p < 0.001), in addition to higher baseline scores on the MADRS (35.4 ± 4.5 versus 31.9 ± 5.1, p = 0.036), HDRS (24 ± 3.1 versus 19.4 ± 3.5, p < 0.001), and HAM-A (24.8 ± 3.1 versus 18.3 ± 3.9; p < 0.001). As such, both groups exhibited moderate-to-severe depression at baseline.
Table 1.
Demographic and illness characteristics
| Anxious depression (n = 21) |
Non-anxious depression (n = 15) |
||
|---|---|---|---|
|
|
|||
| n (%) | n (%) | p-valuea | |
|
| |||
| Gender, female | 12 (57) | 9 (60) | 0.86 |
|
| |||
| Unemployed | 19 (95) | 13 (87) | 0.57 |
|
| |||
| Education, college | 10 (48) | 7 (47) | 0.96 |
|
| |||
| Lifetime diagnosis of anxiety disorder |
11 (52) | 8 (53) | 0.95 |
|
| |||
| Family history mood disorder | 18 (86) | 14 (93) | 0.63 |
|
| |||
| Mean (SD) | Mean (SD) | p-value a | |
|
| |||
| BMI | 29.4 (6.4) | 31.1 (5.5) | 0.42 |
|
| |||
| Age, years | 45.4 (11.5) | 48.5 (10.6) | 0.41 |
|
| |||
| Age at onset, years | 16.8 (7.3) | 19.5 (7.3) | 0.28 |
|
| |||
| Length of illness, years | 28.5 (9.0) | 29.2 (12.8) | 0.84 |
|
| |||
| Length of current depressive episode, months |
18.4 (23.7) | 29.2 (12.8) | 0.63 |
|
| |||
| Hospital admissions | 5.2 (8.3) | 4.3 (2.8) | 0.69 |
|
| |||
| Suicide attempts | 1.0 (1.8) | 0.9 (1.0) | 0.88 |
|
| |||
| MADRS (baseline score) | 35.4 (4.5) | 31.9 (5.1) | 0.036b |
|
| |||
| HDRS (baseline score) | 24.0 (3.1) | 19.4 (3.5) | < 0.001b |
|
| |||
| HDRS A/S (baseline score) | 7.9 (0.8) | 5.3 (0.9) | < 0.001b |
|
| |||
| HAM-A (baseline score) | 24.8 (3.1) | 18.3 (3.9) | < 0.001b |
|
| |||
| YMRS score | 5.4 (2.9) | 4.3 (2.5) | 0.25 |
|
| |||
| SSI (baseline score) | 3.7 (4.9) | 2.6 (3.7) | 0.49 |
SD = standard deviation; BMI = body mass index; MADRS = Montgomery–Åsberg Depression Rating Scale; HDRS = Hamilton Depression Rating Scale; A/S = Anxiety Somatization Factor Score; HAM-A = Hamilton Anxiety Rating Scale; YMRS = Young Mania Rating Scale; SSI = Scale of Suicidal Ideation.
Two-tailed.
p < 0.05.
The linear mixed models controlling for baseline revealed a significant effect of anxiety group on MADRS (p = 0.04) and HDRS (p = 0.04), but there was no significant interaction with drug (MADRS: p = 0.51; HDRS: p = 0.29) or drug by time [MADRS: p = 0.83 (Fig. 1); HDRS: p = 0.62 (Fig. 2)]. The non-anxious group had less depression in general, but group status did not influence drug effects. Significant drug main effects (all p < 0.001) suggest anxious and non-anxious groups improved on ketamine—thus, ketamine worked just as well for anxious as non-anxious patients. There was no group difference with the HAM-A (Anxious group: p = 0.097; Group × Drug: p = 0.30; Group × Drug × Time: p = 0.23) or CADSS (Anxious group: p = 0.35; Group × Drug: p = 0.47; Group × Drug × Time: p = 0.99).
Fig. 1.
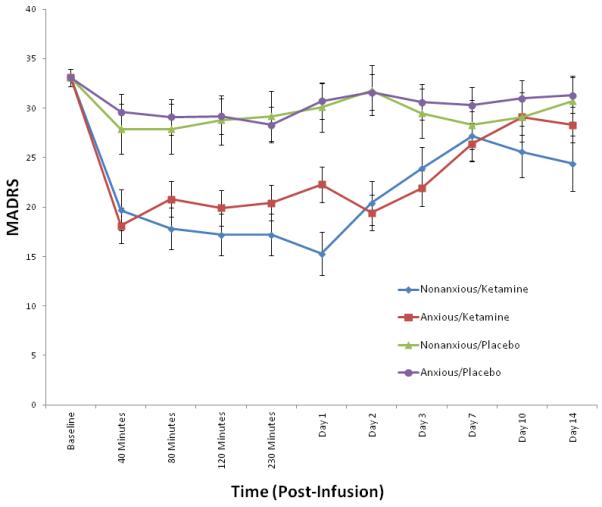
Mean Montgomery–Åsberg Depression Rating Scale (MADRS) scores in anxious versus non-anxious depressed patients at baseline through Day 14 post-infusion (ketamine versus placebo). Values co-varied for baseline MADRS.
Fig. 2.
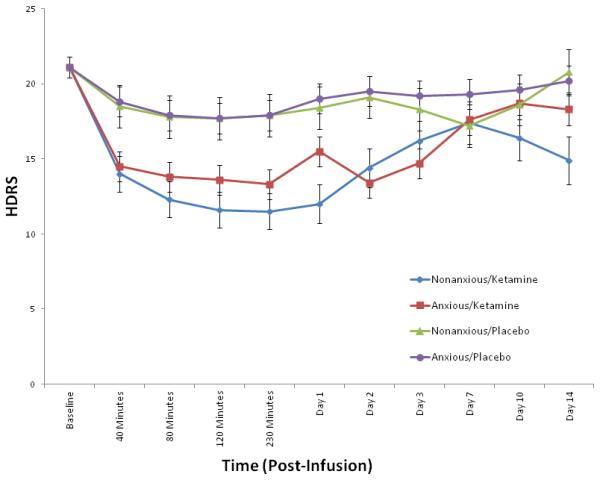
Mean Hamilton Depression Rating Scale (HDRS) scores in anxious versus non-anxious depressed patients at baseline through Day 14 post-infusion (ketamine versus placebo). Values co-varied for baseline HDRS.
Discussion
In this study, we have shown that a single intravenous infusion of sub-anesthetic ketamine rapidly reduces symptoms of depression in patients with anxious bipolar depression to the same extent as those without anxiety. Specifically, our sample consisted of treatment-resistant patients that did not previously respond to more traditional treatments. In addition, anxious patients did not differ significantly from non-anxious patients with regard to reported dissociative side effects due to ketamine.
Although previous research suggests that anxious bipolar disorder may represent a more severe subtype compared to non-anxious bipolar disorder (2-4), our results found no differences in treatment response or side effects to ketamine between the two groups. This result may have several implications from both research and clinical standpoints. For one example, understanding the mechanism by which ketamine is able to reduce depressive symptoms in anxious patients with severe, treatment-resistant bipolar depression in a way that currently approved therapies cannot remains a priority. Since anxious bipolar disorder may represent a clinically meaningful subtype of bipolar illness, with more alcohol abuse, cyclothymia, and suicide attempts (4), all contributing to increased morbidity, the discovery of novel treatments for this devastating disease are imperative as a means of preventing further suffering. Further research is necessary in both basic and clinical research, as elucidating the mechanism by which ketamine exerts its antidepressant effects may lead to the discovery of clinically meaningful treatment targets for drug development.
Note, we recently found that the anxious depression subtype of unipolar illness predicts a significantly better response to ketamine compared to non-anxious depressed patients, with significantly longer time-to-relapse (24). Although anxious bipolar depression does not appear to be a predictor of ‘better’ antidepressant treatment response to ketamine, the fact that anxious patients with bipolar disorder experience the same rapid antidepressant response to ketamine as non-anxious patients is of significant interest. Following ketamine treatment, anxious bipolar depression does not appear to be a predictor of worse treatment response as is for traditional therapies. This is noteworthy, given the previous challenges found in the treatment of anxious bipolar depression with currently available therapies.
Several notable strengths of this study should be considered. All patients were analyzed as part of a randomized, double blind, placebo-controlled, crossover study. Patients were well-characterized and free of psychopharmacological interventions except for therapeutic lithium or valproate, which were stable for at least four weeks prior to the study. Still, several limitations exist. First, the HDRS A/S Factor Score has not been previously used to define anxious bipolar disorder. However, the scale has been used successfully for research examining anxious unipolar depression [reviewed in (20)] and previous studies have used similar methods for defining anxious bipolar disorder (3, 4) with meaningful clinical results.
This underscores the dearth in the literature on this dimensional definition of anxious bipolar depression. Second, although patients had been maintained on either lithium or valproate for an average of six weeks prior to the study without response, we cannot rule out the influence these medications may have had on the reduction of depressive symptoms. Third, the post-hoc nature of this study makes it susceptible to type I error, rendering the need for more a priori studies. Finally, although ketamine’s antidepressant properties as a research tool are promising, the dissociative risks, abuse potential, and unknown long-term effects complicate its current use in clinical practice (25). Future investigations, such as repeat-dosing and active-comparator trials, are necessary to determine ketamine’s utility and safety in clinical practice for bipolar depression.
In conclusion, ketamine’s ability to significantly reduce symptoms of depression in patients with treatment-resistant anxious bipolar depression is novel, considering the treatment challenge that typically defines this subtype of bipolar illness. Future a priori research should examine other methods of defining anxious bipolar disorder, including use of the DSM-5 anxious-bipolar specifier (1, 26). Indeed, explorations into the mechanism by which ketamine exerts its rapid antidepressant effects will be critical for the discovery of more targeted treatments for this traditionally difficult-to-treat subtype of bipolar disorder.
Acknowledgements
Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH), by a NARSAD Independent Investigator to CAZ, and by the Brain & Behavior Mood Disorders Research Award to CAZ.
Footnotes
Clinical Trials Registration: ClinicalTrials.gov identifier: NCT00088699.
Disclosures
CAZ is listed as a co-inventor on a patent application for the use of ketamine and its metabolites in major depression; he has assigned his rights in the patent to the U.S. government but will share a percentage of any royalties that may be received by the government. DFI, DAL, MJN, and EMR have no conflict of interest to disclose, financial or otherwise.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. APA; Washington, DC: 2013. [Google Scholar]
- 2.El-Mallakh RS, Hollifield M. Comorbid anxiety in bipolar disorder alters treatment and prognosis. Psychiatr Q. 2008;79:139–150. doi: 10.1007/s11126-008-9071-5. [DOI] [PubMed] [Google Scholar]
- 3.Feske U, Frank E, Mallinger AG, et al. Anxiety as a correlate of response to the acute treatment of bipolar I disorder. Am J Psychiatry. 2000;157:956–962. doi: 10.1176/appi.ajp.157.6.956. [DOI] [PubMed] [Google Scholar]
- 4.Young LT, Cooke RG, Robb JC, Levitt AJ, Joffe RT. Anxious and non-anxious bipolar disorder. J Affect Disord. 1993;29:49–52. doi: 10.1016/0165-0327(93)90118-4. [DOI] [PubMed] [Google Scholar]
- 5.Sheehan DV, McElroy SL, Harnett-Sheehan K, et al. Randomized, placebo-controlled trial of risperidone for acute treatment of bipolar anxiety. J Affect Disord. 2009;115:376–385. doi: 10.1016/j.jad.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Suppes T, McElroy SL, Sheehan DV, et al. A randomized, double-blind, placebo-controlled study of ziprasidone monotherapy in bipolar disorder with co-occurring lifetime panic or generalized anxiety disorder. J Clin Psychiatry. 2014;75:77–84. doi: 10.4088/JCP.12m08297. [DOI] [PubMed] [Google Scholar]
- 7.Suttajit S, Srisurapanont M, Maneeton N, Maneeton B. Quetiapine for acute bipolar depression: a systematic review and meta-analysis. Drug Design Develop Ther. 2014:827–838. doi: 10.2147/DDDT.S63779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tohen M, Calabrese J, Vieta E, et al. Effect of comorbid anxiety on treatment response in bipolar depression. J Affect Disord. 2007;104:137–146. doi: 10.1016/j.jad.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171:160–168. doi: 10.1176/appi.ajp.2013.13070984. [DOI] [PubMed] [Google Scholar]
- 10.Loebel A, Cucchiaro J, Silva R, et al. Lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171:169–177. doi: 10.1176/appi.ajp.2013.13070985. [DOI] [PubMed] [Google Scholar]
- 11.Provencher MD, Guimond AJ, Hawke LD. Comorbid anxiety in bipolar spectrum disorders: a neglected research and treatment issue? J Affect Disord. 2012;137:161–164. doi: 10.1016/j.jad.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Archiv Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarate CA, Jr, Brutsche NE, Ibrahim L, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiazGranados N, Ibrahim LA, Brutsche NE, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I disorders (SCID I) Biometrics Research, New York State Psychiatric Institute; New York: 1997. [Google Scholar]
- 16.Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 17.Cleary P, Guy W. Factor analysis of Hamilton Depression Scale. Drugs Exp Clin Res. 1977:115–120. [Google Scholar]
- 18.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. 4th ed. APA; Washington, DC: 2000. Task Force on DSM-IV. [Google Scholar]
- 20.Ionescu DF, Niciu MJ, Henter ID, Zarate CA. Defining anxious depression: a review of the literature. CNS Spectr. 2013;18:252–260. doi: 10.1017/S1092852913000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClintock SM, Husain MM, Bernstein IH, et al. Assessing anxious features in depressed outpatients. Int J Methods Psychiatr Res. 2011;20:e69–e82. doi: 10.1002/mpr.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bremner JD, Krystal JH, Putnam FW, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) J Traumat Stress. 1998;11:125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 24.Ionescu DF, Luckenbaugh DA, Niciu MJ, et al. Effect of baseline anxious depression on initial and sustained antidepressant response to ketamine. J Clin Psychiatry. 2014;75:e932–e938. doi: 10.4088/JCP.14m09049. [DOI] [PubMed] [Google Scholar]
- 25.Schatzberg AF. A word to the wise about ketamine. Am J Psychiatry. 2014;171:262–264. doi: 10.1176/appi.ajp.2014.13101434. [DOI] [PubMed] [Google Scholar]
- 26.Zimmerman M, Chelminski I, Young D, Dalrymple K, Walsh E, Rosenstein L. A clinically useful self-report measure of the DSM-5 anxious distress specifier for major depressive disorder. J Clin Psychiatry. 2014;75:601–607. doi: 10.4088/JCP.13m08961. [DOI] [PubMed] [Google Scholar]


