Abstract
Background
Spinal cord injury (SCI) results in impaired function and ankle joint spasticity is a common secondary complication. Different interventions have been trialed with variable results.
Objective
We investigated the effects of pharmacological and physical (locomotor training) interventions on function in people living with incomplete motor function loss due to SCI, and used different analytical techniques to understand whether functional levels affect recovery with different interventions.
Methods
Participants with an incomplete SCI were assigned to three groups; no intervention, Lokomat or tizanidine. Outcome measures were the 10-meter walk test (10MWT), 6-minute walk test (6MWT) and the Timed-up and go (TUG). Participants were classified in two ways; i) based on achieving an improvement above the minimally important difference (MID) and; ii) using Growth Mixture Modeling (GMM). Functional levels of participants that achieved the MID were compared and Random Coefficient Regression (RCR) was used to assess recovery in GMM classes.
Results
Overall, walking speed and endurance improved, with no difference between interventions. Only a small number of participants achieved the MID. Both MID and GMM-RCR analyses revealed that tizanidine improved endurance in high functioning participants. GMM-RCR classification also showed that speed and mobility improved after locomotor training.
Conclusions
Improvements in function were achieved in a limited number of people with SCI. Using the MID and GMM techniques, differences in responses to interventions between high and low functioning participants could be identified. These techniques may therefore have potential to be used for characterizing therapeutic effects due to different interventions.
Introduction
Rehabilitation for people that are living with spinal cord injury (SCI) is focused on optimizing function. One secondary consequence of SCI is neuromuscular abnormalities, resulting in hypertonia or spasticity of muscle groups, and has been noted as the main self-reported secondary complication after SCI 1. Spasticity commonly affects the muscles surrounding the ankle joint, which have important roles during functional tasks 2. During gait for example, the gastrocnemius, an ankle extensor, is important for propulsion during the stance phase, and the tibialis anterior for foot clearance during the swing phase. In people living with SCI, there is controversy regarding the relationship between hypertonia and gait function. Severe extensor spasticity of gastrocnemius, blocking flexion movements, can impede locomotor ability, and some studies have demonstrated that increased hypertonia relates to impaired function 3-5. Others however, have demonstrated no functional improvements with reduced hypertonia, based on clinical observations 6,7.
Tizanidine, an antispasticity medication, has been shown to reduce hypertonia in SCI individuals 8-11. As an α2 noradrenergic agonist, it is thought to reduce hypertonia through depression of dorsal horn interneuron excitability 12. While studies commonly report the effects of such antispasticity medications on muscle spasticity, assessed by clinical scores 8,13 or electromyographic activity 13,14, they rarely report effects on the patient’s functional ability 15. The few studies that did report more functional measures demonstrated that Tizanidine substantially reduced reflex mechanical responses in SCI individuals 16, and facilitated locomotor capacity in spinalized cats 17, whereas one study reported that it had no effect on muscle strength or activities of daily living in SCI individuals 8.
An alternative intervention that became popular in SCI rehabilitation to improve function is Locomotor treadmill training (LTT). LTT, incorporating body-weight supported training and robotic-assistive step training, provides gait assistance and body weight support to a patient on a motorized treadmill, which aims to improve locomotor function 18. LTT is thought to improve function through the responsiveness of central pattern generators to afferent stimuli noted in mammalian quadrupeds 18,19. In people with chronic SCI, studies have reported that LTT training improves overground walking speed 20-24 and endurance 22, muscle strength 23,25,26, corticospinal tract function 25, postural alignment 20, coordination of electromyographic activity 20 and subjective well-being 27 23. It has also been reported to reduce abnormal neuromuscular activity (spasticity), measured by clinical scores 28 or electromyographic activity 29, although these changes did not correlate with functional improvements 28. Some authors however believe that the evidence for LTT is limited 30 since studies often omit control and alternative intervention groups, and those that do include alternative interventions have found similar improvements from conventional physical therapy and overgound walking training 23,24,31. Similar findings have been reported from randomized trials in people with acute SCI 32.
Overall, the observed extents and rates of improvement in functional performance from interventions after chronic incomplete SCI tend to be variable 30. In addition, the population of people living long-term with incomplete SCI is heterogeneous in terms of their functional capacities. The combination of these factors results in commonly employed group-averaging techniques showing only small overall improvements 30, which may mask important data. Some SCI individuals may respond to specific treatments whereas others do not; identifying those that do respond and understanding how they differ from non-responders is key. For example, one study reported that a greater proportion of more impaired participants with SCI, classified by Lower Extremity Motor Scores (LEMS), improved walking speed after locomotor training, when compared with less impaired participants 24. Whereas other studies have reported greater improvements in walking speed with LTT in participants with greater baseline muscle strength33 or in those that have the ability to ambulate prior to the intervention34. Determining whether individuals living with SCI respond to interventions differently, according to their baseline functional level, may allow us to recommend optimal interventions on a case-by-case basis.
In order to assess the subjects that did or did not respond to the intervention, it is necessary to firstly define the amount of change required for any improvement to be considered real (i.e. greater than change due to variation or measurement error), and secondly to define the amount of change for the improvement to be considered ‘clinically’ meaningful. That is, a small but statistically significant improvement may be irrelevant clinically if it does not impact the individual’s quality of life, particularly if the intervention is substantially costly in terms of time, effort and/or side effects for the individual. Previously, Beckerman et al.35 proposed the small real difference (SRD) approach, which refers to the smallest possible value required to be considered a true difference; thus the minimally important clinical difference must always be greater than the SRD for a measurement technique to be valid35. Subsequently, Lam et al. 36 proposed SRDs for a number of outcome measures commonly used in the population of individuals living with SCI. While these values may be useful in assessing those people that responded to treatment, it should be acknowledged that they were based on a small number of heterogeneous individuals with incomplete SCI, and only account for the variability in the measurement technique rather than a change that may be considered clinically relevant or regarded as a minimal important difference (MID) to the individual.
An alternative method of classifying responders from non-responders is growth mixture modelling (GMM). GMM is used widely in psychological and educational research to capture heterogeneity in developmental pathways, and has recently been applied to recovery patterns in rehabilitation research. The technique attempts to classify participants into latent classes according to their baseline scores and recovery trends. This may be useful in rehabilitation by accounting for some of the inherent variability in individuals and individual responses, by considering each individual’s baseline function, as well as their recovery trend in response to the intervention. In the future rehabilitation practitioners may use this information to predict, with a known confidence level, whether or not a new patient will respond positively to an intervention based on their baseline clinical scores. This technique has successfully classed stroke survivors based on their recovery patterns 37.
The aims of the study were to determine and compare the effects of two different interventions, LTT and tizanidine, on gait impairments, using a commonly employed group-averaging technique, MID and GMM techniques. We hypothesized that differences in responses to interventions would not be detected by ANOVA and both MID and GMM techniques would identify different responses to the interventions in high compared with low functioning participants, assessed by Walking Index for Spinal Cord Injury II (WISCI II) scores and baseline outcome measures.
Methodology
Participants
Participants with either cervical or thoracic incomplete spinal cord injury, as a result of trauma, were recruited from the outpatient service at the Rehabilitation Institute of Chicago. All participants provided written informed consent and the study had ethical approval from the Northwestern University Institutional Review Board. Inclusion criteria were aged 18-50 years, motor incomplete SCI (ASIA impairment scale [AIS] classification C or D) with level of injury above T10 and >12 months post injury, ambulatory, medical clearance to participate, evidence of clinical spasticity in the ankle joint (Modified Ashworth Score (MAS) ≥1), and lower-limb passive range of motion within functional limits for ambulation. Exclusion criteria were sitting tolerance <2 hours, existing infection, severe cardiovascular or pulmonary disease, concomitant neurological injury, history of fractures post-SCI, known orthopedic or peripheral nerve injury in the lower extremities. The WISCI II assessment was initially carried out to assess the participants walking ability (level of functional impairment). Participants were then randomized into one of three intervention groups; no intervention (control; n=29), LTT (Lok; n=27) and Tizanidine (Tiz; n=27). Subject characteristics are provided in Table 1.
Table 1.
Mean (SD) characteristics of participants in the three groups. F=Female; M=Male; WISCI II= Walking Index for Spinal Cord Injury II; MAS=Modified Ashworth Score.
| Control | Lokomat | Tizanidine | ||
|---|---|---|---|---|
| Gender | 10 F; 19 M | 8 F; 19 M | 8 F; 19 M | |
| Age (years) | 47.8 (13.1) | 46.6 (12.6) | 47.4 (11.6) | |
| Time since injury (years) | 8.1 (8.1) | 9.3 (8.9) | 10.9 (10.8) | |
| WISCI II Score | 13.8 (5.8) | 14.7 (5.2) | 14.9 (4.6) | |
| Ankle MAS |
Left | 1.9 (0.8) | 1.6 (0.9) | 1.5 (0.8) |
| Right | 1.9 (0.8) | 1.9 (1.2) | 1.6 (0.9) | |
Interventions
Interventions were given for 4 weeks in the Lok and Tiz groups. Control participants received no intervention. Participants taking muscle relaxant medications prior to enrolling on the study were tapered from their medication prior to the start of the study. Participants were requested not to alter their current medications otherwise, where possible, and to inform us of any changes in their medications during the intervention.
For the Lok group, locomotor training was provided using a robot-assisted locomotor training device (Lokomat, Hocoma AG, Switzerland). This device provides body-weight supported gait assistance; the individual is suspended in a harness over a motorized treadmill while the frame of the robot, attached by straps to the outside of the lower limbs, moves the limbs in a natural walking pattern.
Training was provided three times per week; each session lasted ≤1 hour, with 30-45 minutes of training. Treadmill speed, body-weight support and robotic guidance force was determined by the physical therapist, based on tolerance and comfort of the subject. Generally however, reducing guidance force was prioritized to promote voluntary drive to muscles, and to minimize passive training. Body-weight support was configured to maximize lower-extremity loading without producing excessive knee flexion during the stance phase, or allowing toe-drag during the swing phase. Participants were instructed to “walk with the robot” to ensure that the lower-extremity movements were consistent with the Lokomat stepping pattern. Participants were also instructed to pay attention to their ankle movements during the gait cycle i.e. to focus on planting the heel of their foot at heel-strike and to “lift their toes” during the swing phase. A mirror placed in front of the participants provided visual feedback.
For the Tiz group, .03 mg/kg of Tizanidine was administered four times a day for four weeks. This dosage represents a useful compromise, in that it usually shows efficacy16, but does not cause overwhelming side effects. In the first week, administration of the drug was progressively increased until the full dosage was received on day 7, and the full dosage was then administered for a subsequent 4-week period.
Outcome measures
Outcomes were measured at four time points: baseline and 1, 2 and 4 weeks into the intervention (or after baseline for controls). For the Tiz group, outcome measures were taken at baseline and 1, 2 and 4 weeks after the participants Tizanidine had been regulated.
Functional measures included; i) the 10-meter walk test (10MWT) whereby participants are instructed to walk 10 meters as quickly and safely as possible, and time is measured 38-40; ii) the 6-minute walk test (6MWT) whereby participants are instructed to walk for 6 minutes and the distance covered is measured40 and; iii) the Timed up and go (TUG) whereby participants are instructed to stand up from an armed chair, walk 3 meters, turn, return to the chair and sit down, and time is measured40.
Data Analysis
Statistical tests were carried out using SPSS (SPPS v21, IBM Corp, USA), and p<0.05 was considered significant. One way analysis of variance (ANOVA) was used to assess differences in subject’s age, time since injury and functional level between the three intervention groups.
Two way mixed design ANOVAs were used to identify significant changes due to time-point (within-subject) and group (between-subject) for the three groups.
Data were classified for each task using two methods:
According to whether or not they achieved the MID for each test. The MID was defined using control group data; this was calculated as proposed by Beckerman et al 35 (1.96*√2*SEM). Participants that achieved a change from baseline equal to or greater than the MID for that test, after 4 weeks of training, were classified as ‘MID achieved’, and those that had a change from baseline less than the MID for that test were classified as ‘MID not achieved’. Differences in MAS and WISCI II scores among the three intervention groups for those that did achieve the MID for each test were assessed using one way ANOVAs. Within each group, differences in WISCI II scores between those that did and did not achieve the MID for each test were assessed using independent sample T-Tests.
-
Growth mixture modelling (GMM) can be used to identify sub-populations within a group that demonstrate similar longitudinal change or growth (for a basic review see41). The model assumes that the population can be separated into a finite number of classes that are similar, in a post-hoc manner (i.e. does not include a priori knowledge of how the data should be classed). The model estimates the probability that each individual should belong to each class, and then classifies that individual into the class that they are most likely to belong.
Using R software (R v2.15, R Systems, India) a given number of classes (2, 3 or 4) were generated by the model according to baseline data and recovery slope trends. For the recovery trend, we forced the model to define the pattern linearly, which can be represented by a single slope, as this was adequate to describe the relationship. The model with the best fit was accepted for the final classification. This was carried out separately for each outcome measure and group. Subsequently, Random Coefficient Regression (RCR) was used to assess whether or not each GMM class improved significantly with time, as a result of the intervention. The RCR method is intended to be a substitute for repeated-measures ANOVA, with the presence of a random effect indexed on subject number for the time factor. The RCR is also more capable to model nonlinearities in the trend.
Results
Participants
There were no significant differences between the participants in each intervention group in terms of age, time since injury, WISCI II scores and MAS (Table 1; p>0.05 for all variables). Two participants in the control group did not complete the study and one participant in the Tiz group was not able to complete the clinical measures, therefore analysis in the Lok and Tiz groups were carried out on n=27 and n=26, respectively. Participants did not report any changes to their medications during the intervention.
Analysis of Variance
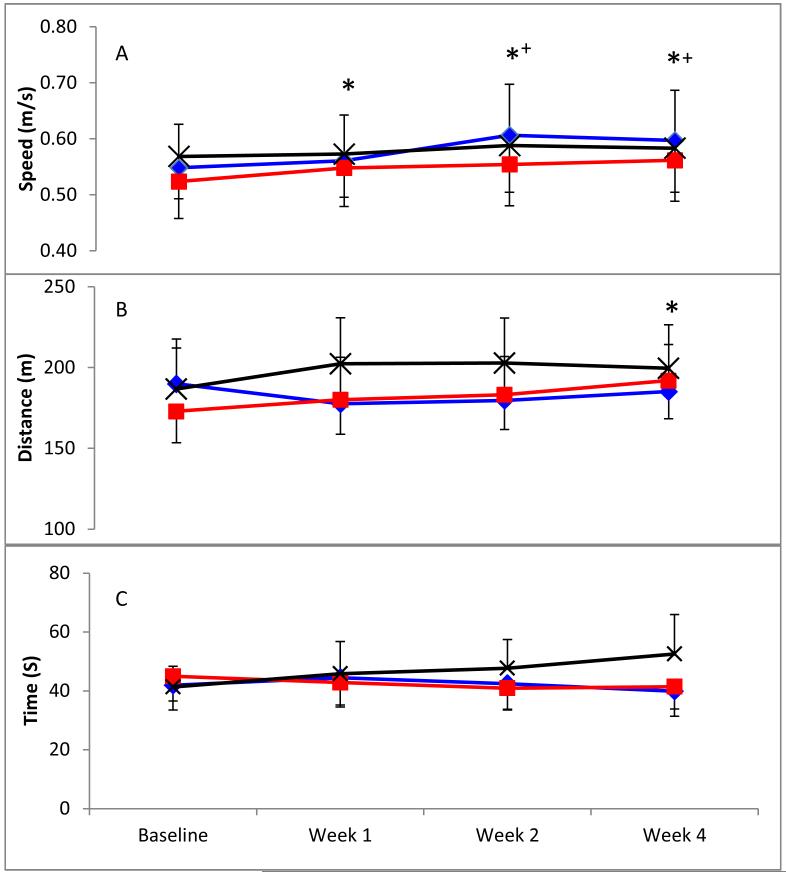
Mixed model ANOVAs revealed significant improvements with time for both walking speed in the 10MWT (p<0.001; Fig 1A) and walking distance in the 6MWT (p=0.002; Fig 1B), with no group effects or interactions. For TUG, there were no significant effects of either time-point or group (Fig 1C).
Figure 1.
Mean (SEM) speed in the 10-meter walk test (A), distance in the 6-minute walk test (B) and time in the Timed up & Go test (C) for Control (black crosses), Lok (blue diamonds) and Tiz (red squares) groups at each timepoint (significantly different from *baseline or +Week 1 (p<0.05)).
Minimal Important Differences
Based on our control data, MID values were 0.11m/s, 37.1m and −14.5s for the 10MWT, 6MWT and TUG, respectively. Participants were classified according to whether or not they achieved the MID for each test. Individuals were excluded if either baseline or week 4 data was missing; final N values for each group are provided in Table 2. For the 10MWT, the number (proportion) of participants that achieved the MID, after 4 weeks of training, were 2 (8%), 4 (15%) and 6 (23%) for Control, Lok and Tiz groups, respectively. Equivalent values for the 6MWT were 3 (13%), 2 (8%) and 5 (20%), and 2 (8%), 2 (8%) and 3 (12%) for the TUG (Table 2). Of those that did attain the MID within each test, there was no significant differences in MAS or WISCI II scores between the three intervention groups. However, those that attained the MID for the 6MWT had significantly higher WISCI II scores than those that did not attain the MID within the Tiz and control groups (p<0.001; Table 2). This was also true for the 10MWT within the Tiz group only (p<0.001; Table 2), with no significant differences observed for the TUG.
Table 2.
Number (N) of participants and mean (SD) baseline scores, mean (SD) Walking Index for Spinal Cord Injury II (WISCI II) scores and Modified Ashworth Scale (MAS) scores for participants that did and did not attain the minimal important difference (MID) for the 10 meter walk test (10MWT), 6 minute walk test (6MWT) and the Timed Up and Go (TUG) in each group (**p<0.001 and *p<0.05 compared with MID not attained within group).
| Score | Clinical outcome |
Control | Lokomat (Lok) | Tizanidine (Tiz) | |||
|---|---|---|---|---|---|---|---|
| MID not attained |
MID attained |
MID not attained |
MID attained |
MID not attained |
MID attained |
||
| N | 10MWT | 23 | 2 | 22 | 4 | 20 | 6 |
| 6MWT | 20 | 3 | 22 | 2 | 20 | 5 | |
| TUG | 24 | 2 | 22 | 2 | 23 | 3 | |
| Baseline score |
10MWT | 0.55 (0.39) |
0.78 (0.07)* |
0.47 (0.34) |
0.99 (0.43) |
0.43 (0.32) |
0.82 (0.16)** |
| 6MWT | 191.6 (101.1) |
313.9 (171.2) |
171.6 (117.0) |
391.9 (217.3) |
145.5 (87.8) |
282.5 (40.0)** |
|
| TUG | 33.4 (28.3) |
71.5 (11.5)* |
35.4 (36.1) |
113.0 (18.4)* |
35.1 (28.6) |
120.6 (64.0) |
|
| WISCI II |
10MWT | 15.1 (4.4) | 18.0 (2.8) | 14.3 (5.2) | 17.0 (5.3) | 14.1 (3.0) | 19.7 (0.5)** |
| 6MWT | 15.3 (4.0) | 20.0 (0.0)** | 14.7 (6.0) | 14.5 (7.8) | 14.5 (3.3) | 19.8 (0.5)** | |
| TUG | 15.4 (4.5) | 12.5 (5.0) | 16.3 (3.9) | 11.0 (2.8) | 16.0 (3.4) | 11.7 (2.3) | |
| MAS | 10MWT | 2.0 (0.7) | 2.0 (1.4) | 2.0 (1.0) | 2.0 (0.8) | 1.8 (0.8) | 1.7 (1.2) |
| 6MWT | 2.2 (0.6) | 1.7 (0.6) | 2.0 (1.1) | 2.5 (0.7) | 1.8 (0.9) | 1.4 (0.9) | |
| TUG | 2.2 (0.6) | 2.5 (0.7) | 1.9 (1.0) | 2.0 (0.0) | 1.8 (0.9) | 1.3 (0.6) | |
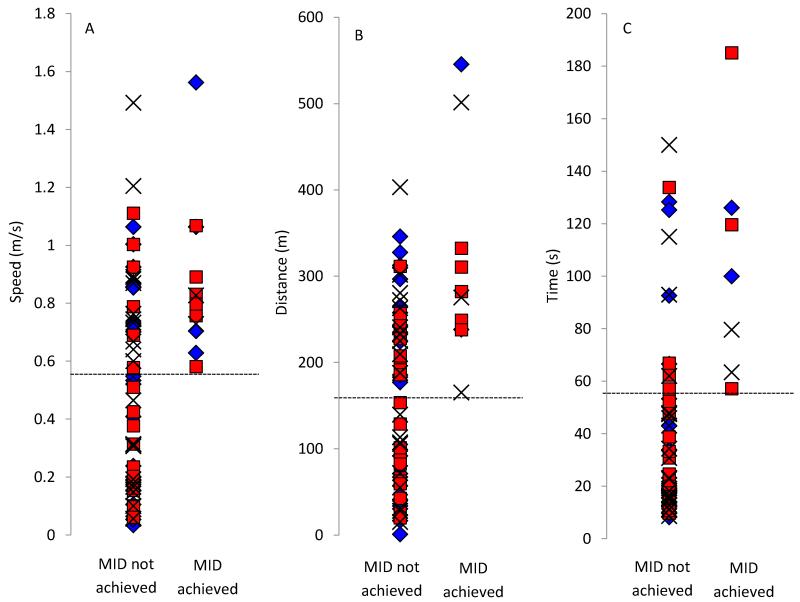
In addition, those participants that achieved the MID for the 10MWT had significantly higher baseline walking speeds in both Tiz (p<0.001) and control (p=0.04) groups, and those that achieved the MID for the 6MWT had significantly higher baseline walking distance in the Tiz group (p<0.001). Finally, those that achieved the MID for the TUG had significantly longer times in the control (p=0.04) and Lok (p=0.04) groups. Overall therefore, for the 10MWT and 6MWT, the participants that attained the MID tended to be higher functioning (Table 2 & Fig 2A-B), and for the TUG, those that achieved the MID tended to be lower functioning (Table 2 & Fig 2C). There were no significant difference in MAS between participants that did and did not attain the MID for all interventions and outcome measures (Table 2).
Figure 2.
Baseline speed in the 10-meter walk test (A), distance in the 6-minute walk test (B) and time in the Timed up & Go test (C) for participants in control (black crosses), Lok (blue diamonds) and Tiz (red squares) groups. Dashed lines indicate the lowest baseline score for individuals that achieved the minimal important difference (MID) for each outcome measure.
Growth Mixture Modelling
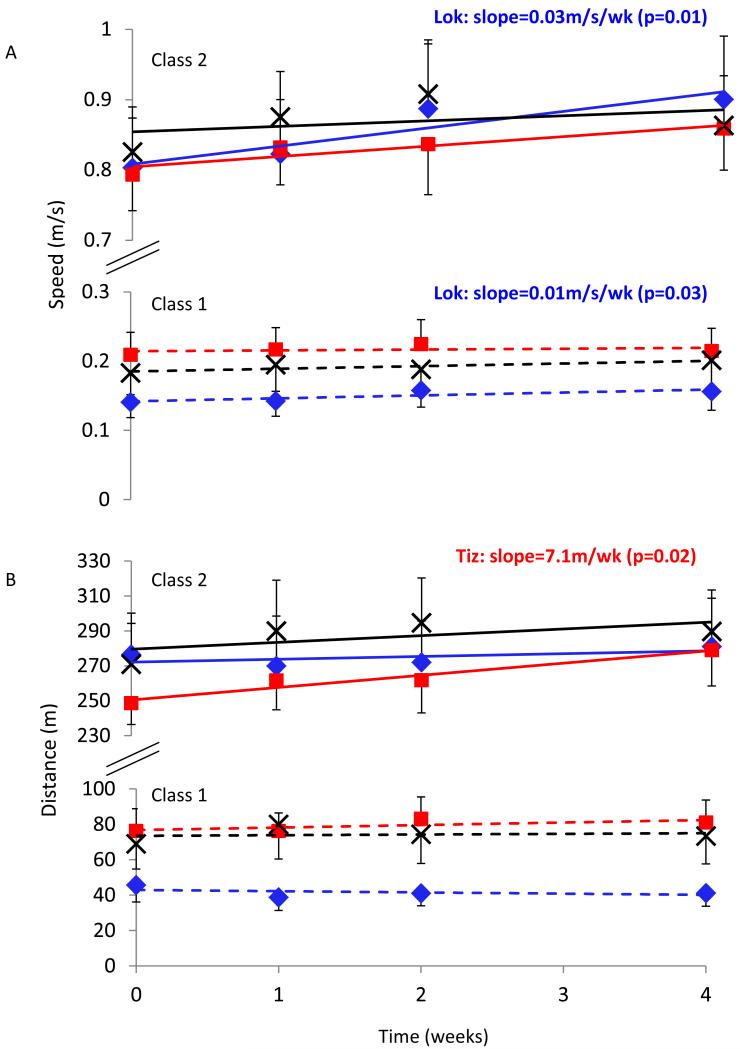
Participants were excluded from the analysis if they did not complete any of the tests for a given outcome measure; this was only the case for the 6MWT where some individuals were not able to walk for 6 minutes duration throughout the study. Final N values for each group are provided in Table 3. The best fit GMM categorized participants for each outcome measure into two classes for all groups and outcome measures. Participants in Class 2 had significantly higher WISCI II scores and significantly improved baseline scores compared with Class 1, for all outcome measures and intervention groups (Table 3). Therefore these classes were considered as high (Class 2) and low (Class 1) functioning. There were no significant difference in MAS between participants in Class 1 compared with Class 2 for all interventions and outcome measures (Table 3). For the 10MWT, RCR revealed significant improvements with time for the Lok group in both the higher (Class 2; p<0.01) and lower (Class 1; p<0.05) functioning classes, with no significant changes for Tiz or control participants (Fig. 3A). For the 6MWT, significant improvements with time were found for the Tiz group in the higher functioning class only (Class 2, p<0.05; Fig. 3B). For the TUG, significant improvements with time were also found for the Lok group in the higher functioning class only (Class 2, p<0.05).
Table 3.
Number (N) of participants and mean (SD) baseline scores, Walking Index for Spinal Cord Injury II (WISCI II) scores and Modified Ashworth Scale (MAS) scores for participants in Class 1 and 2 for the 10 meter walk test (10MWT), 6 minute walk test (6MWT) and the Timed Up and Go (TUG) in each group (**p<0.001 and *p<0.05 compared with Class 1 within group).
| Score | Clinical outcome |
Control | Lokomat (Lok) | Tizanidine (Tiz) | |||
|---|---|---|---|---|---|---|---|
| Class 1 | Class 2 | Class 1 | Class 2 | Class 1 | Class 2 | ||
| N | 10MWT | 12 | 15 | 11 | 16 | 12 | 14 |
| 6MWT | 11 | 14 | 10 | 15 | 11 | 14 | |
| TUG | 6 | 20 | 6 | 20 | 9 | 15 | |
| Baseline score |
10MWT | 0.15 (0.12) |
0.83 (0.25)** |
0.13 (0.08) |
0.80 (0.28)** |
0.21 (0.11) |
0.79 (0.19)** |
| 6MWT | 77.3 (45.8) |
270.9 (87.4)** |
45.7 (28.8) |
276.5 (91.4)** |
76.3 (41.4) |
248.8 (46.2)** |
|
| TUG | 104.2 (51.0) |
27.2 (16.6)* |
85.5 (37.4) |
15.8 (4.1)** |
82.4 (22.4) |
24.5 (15.5)* |
|
| WISCI II |
10MWT | 11.8 (3.3) | 17.3 (3.7)** |
9.6 (5.1) | 17.4 (3.9)** |
12.9 (2.0) | 17.7 (3.0)** |
| 6MWT | 12.4 (3.2) | 17.6 (3.7)** |
10.6 (6.1) | 17.3 (4.0)* |
12.8 (2.2) | 17.7 (3.0)** |
|
| TUG | 12.8 (2.2) | 16.3 (3.6)* | 12.3 (1.6) | 17.1 (3.9)** |
11.0 (2.2) | 16.5 (4.2)** |
|
| MAS | 10MWT | 2.2 (0.8) | 2.1 (0.7) | 2.4 (1.1) | 1.8 (0.8) | 1.9 (0.8) | 1.6 (0.9) |
| 6MWT | 2.2 (0.9) | 2.0 (0.7) | 2.3 (1.2) | 1.8 (0.9) | 1.9 (0.8) | 1.6 (0.9) | |
| TUG | 1.8 (0.8) | 1.7 (0.9) | 2.1 (1.2) | 1.8 (0.9) | 2.0 (1.1) | 2.1 (0.6) | |
Figure 3.
Mean (SEM) speed in the 10-meter walk test (A) and distance in the 6-minute walk test (B) for Control (black crosses), Lok (blue diamonds) and Tiz (red squares) groups and slope for Class 1 (dashed lines) and Class 2 (solid lines) generated by GMM-RCR. For the classes that showed a significant improvement with time, determined by RCR, p-values and recovery slope values are provided.
Discussion
This study compared the effects of two different interventions, LTT and tizanidine, on gait impairments and used different analytical techniques to understand whether functional levels of people with chronic incomplete spinal cord injury affect functional recovery with different interventions. Mixed model ANOVAs revealed small improvements in walking speed and endurance with no significant differences between the interventions (locomotor treadmill training, anti-spasticity medication and control). Using MID analysis, the number of participants that achieved the MID was small. Both MID and GMM-RCR analyses revealed improved endurance after Tizanidine for high functioning participants only. GMM-RCR analysis also revealed significant improvements in walking speed after Lokomat training for both high and low functioning participants, and improved mobility for high functioning participants only.
Using group averaging techniques, we found small improvements in walking speed and endurance, with no significant difference between intervention groups. These findings are consistent with previous reports, which conclude that small functional changes do occur with LTT, however these changes are similar compared with other physical interventions 23,24,31. The effects of anti-spasticity medications on functional measures have seldom been reported, and studies that have reported the effects of other pharmacological interventions, found small to negative effects on walking speed 42, and concluded that locomotor training resulted in better outcomes than any of the pharmacological interventions studied 42. In the present study we found small effects from Tizanidine, which were similar compared to the effects of LTT. There was large variability in the clinical outcome data for each group (Fig 1), which also agrees with previous work 30, and may relate to the wide variability in functional levels among individuals with chronic incomplete SCI.
Therefore, we considered two alternative analysis techniques; the MID for clinical outcomes taken, in order to classify the participants that did or did not attain a real (clinical) change due to each intervention, and growth mixture modeling (GMM). From the MID analysis, we noted that 2-6 participants achieved the MID, depending on the outcome measure and intervention used. When comparing the classification of subjects between the two techniques, it was notable that all participants classified as achieving the MID for the 10MWT and 6MWT, were also classified as Class 2 (high functioning) by the GMM; this was true across all intervention groups. For the TUG however, all participants that achieved the MID were classed as low functioning (Class 1).
Tizanidine improved both walking speed and endurance for the highest number of participants attaining the MID across all groups (5-6 participants). These participants were found to have significantly higher functional levels than those that did not attain the MID, evidenced by significantly higher WISCI II scores (p<0.001), and significantly higher baseline scores (p<0.001) for both speed and endurance (Table 2). In agreement, GMM-RCR analysis revealed significant improvements in walking endurance due to Tizanidine in Class 2 only; Class 2 were higher functioning subjects evidenced by significantly higher WISCI II (p<0.001) and baseline (p<0.001) scores (Table 3), similar to MID analysis. However, significant improvements in walking speed were not noted due to Tizanidine in GMM-RCR analysis, which was in contrast to MID results. Tizanidine may have reduced spasticity in the gastrocnemius muscle, which allowed improved (more co-ordinated) functioning of the tibialis anterior muscle, resulting in improved walking function. Further research is required to corroborate this speculation.
GMM-RCR analysis also revealed improved walking speed in the LTT group only, which was irrespective of functional level (a significant improvement was noted for both classes (Fig. 3)). MID analysis revealed improved walking speed due to LTT in 4/27 participants, with no difference in functional levels between those that did and did not achieve the MID (no difference in WISCI II scores or baseline walking speed; Table 2). Improved walking speed with LTT has been reported previously 20-24, and may be the result of improved muscle strength 23. The fact that the improvement in walking speed was unrelated to functional level, evidenced in both MID and GMM-RCR analyses, is in contrast with a previous study that noted a greater proportion of more impaired participants with SCI improved walking speed after locomotor training compared with less impaired participants 24. In that study, the lower extremity motor score (LEMS) was used to assess impairment level, which assesses lower limb muscle strength, as opposed to walking ability, as assessed by WISCI II scores used in the present study. In addition, participants trained for a longer period (12 weeks) in the study by Field-Fote & Roach 24, compared with only 4 weeks in our study. Longer duration LTT may have elicited greater functional adaptations and may have allowed distinction between functional levels.
GMM-RCR analysis also revealed a significant improvement in walking mobility (TUG) for higher functioning participants only after LTT; however this result was not evident from MID analysis. MID analysis showed that Tizanidine improved mobility in only 3/27 participants, and those that achieved the MID tended to be lower functioning (lower WISCI II score and higher baseline time to complete the TUG), however these differences were not significantly significant, which may be due to the low number of participants that did achieve the MID. As stated previously, all subjects that achieved the MID for the TUG were classed as low functioning by the GMM (Class 1), therefore the significant improvement in TUG for high functioning participants only (Class 2) by GMM-RCR analysis is an unexpected result that opposes the results of MID analysis. Such differences between MID and GMM analyses may relate to the fact that MID uses only the baseline and final data points, rather than the overall trend as was used in GMM analysis; thus the MID approach may be more susceptible to day-to-day measurement variations. This does however highlight the importance of the analysis method chosen. Overall, both GMM and MID techniques could identify some different outcomes for high vs low functioning participants that could not be detected using analysis of variance (ANOVA) alone.
Spasticity
Spasticity may both assist and impair gait function. Thus, baseline levels of spasticity at the ankle joint may be important in determining which subjects will respond to different interventions as opposed to (or as well as) functional levels. We therefore compared ankle joint MAS between those subjects that did and did not attain the MID. Our data suggests that spasticity level does not impact whether or not each individual will attain the MID. However, it has previously been reported that no significant correlation between quantitative measures of muscular and reflex torque/stiffness associated with spasticity and the MAS magnitude 43. Thus, while MAS is sufficient to assess the presence of clinical spasticity as measured by overall stiffness (inclusion criteria), it cannot determine the contribution of neuromuscular properties to the overall stiffness and thus it may be unreliable as an indicator of spasticity level.
Knee joint spasticity may also affect gait impairment; in some individuals, particularly those with muscle weakness, knee spasticity may assist walking, thus anti-spastic medication may not benefit these people, whereas in others it may reduce knee joint spasticity without reducing stability at the knee. We cannot speculate from our data whether changes in knee joint spasticity influenced the outcomes.
Clinical Significance
An unexpected observation from this study was that, overall, the participants that achieved the MID for the 10MWT and the 6MWT, tended to be higher functioning for that measure at baseline. That is, their baseline walking speed and distances were >0.58m/s and >165m, respectively (Fig. 2A-B). In contrast however, it was the lower functioning participants that tended to attain the MID for the TUG outcome measure i.e. those with a baseline time of >57s (Fig. 2C; note that an increase in time represents lower function with this measure). This was not however significant across all groups, which may have been due to the fact that a number of participants that did not achieve the MID had similar baseline scores to those that did (Fig. 2A-C), as well as due to low participant numbers that did attain the MID.
The TUG assesses mobility, walking ability, balance and risk of falling, as opposed to walking speed or endurance, as with the other measures taken. For lower functioning participants it may be that an improvement in mobility and balance is required before changes in speed and/or endurance take place. Thus the TUG may be a more suitable assessment to detect this change in low functioning participants, whereas the 10MWT and 6MWT were more suitable tests for higher functioning participants. This is in agreement with a previous study, which showed the 10MWT and 6MWT to be more sensitive to change in participants with SCI with higher walking proficiency compared with an alternative measure of functional walking tasks (the SCI Functional Ambulation Profile, which includes the TUG)44.
These finding may also be clinically informative. For example, based on our data, it is indicated that individuals with a baseline walking speed of >0.58m/s have a 55% chance of achieving improved walking speed after a 4-week Tizanidine intervention (i.e. 6/11 participants that had a baseline speed >0.58m/s achieved the MID in that group), and have a 33% chance of achieving improved walking speed with 12 sessions of LTT. Whereas those with a baseline walking speed of <0.58m/s have little or no chance of showing an improvement with either intervention.
For the GMM analysis, subjects were classified using both their baseline scores and recovery trends. Therefore, in the groups that showed significant improvements with RCR, the majority, but not necessarily all individuals, showed improvements. GMM-RCR results therefore similarly allow us to predict, from baseline scores, the likelihood of an individual belonging to a class, and thus the probability that the individual will have improved gait function with a given intervention. Both techniques may therefore provide useful knowledge to clinicians and patients when deciding on the most appropriate intervention for each individual, and provide more information than using group averaging techniques such as ANOVAs.
Limitations
While GMM-RCR appears to be a useful technique, it should be noted that the significant improvements found by RCR were small, and considerably less than our MID values, thus the changes due to the interventions may still not be clinically relevant (i.e. may not significantly impact the individuals quality of life). Similarly, for the MID analysis, only a relatively small number of subjects (2-6 participants per group) achieved the MID, and this was also the case in the control group (i.e. some control participants did attain the MID with no intervention). Thus the outcome measures alone may have been sufficient to induce clinically relevant changes in some individuals. In a review article, it has previously been shown that the total number of training sessions required to improve walking outcomes >MID was 10-130 sessions45. In this study we provided only 12 sessions, which is toward the shorter end of that range. Therefore, providing a greater number of training sessions may have resulted in greater improvements, or increased the number of participants that achieved the MID.
This study was sufficiently powered for the group comparisons using ANOVA’s and for the comparisons made between classes for the GMM analysis (>0.80). However, due to the low number of subjects achieving the MID, the study was underpowered for some statistical comparisons in those groups. In addition, the number of participants within each group that did not attain the MID (21-25 individuals) was substantially different to the number that did, which also impacts the validity of the statistical tests used. Therefore these results should be interpreted with caution, and it is recommended that future studies incorporate a greater number of training sessions, which may result in a greater number of participants achieving the MID. A final limitation is that the participants in this study were also not followed up; therefore we cannot speculate on whether noted changes were long-standing in these patients. Such measures would also be beneficial in future studies.
In conclusion, using group averaging (ANOVA) techniques, small improvements in walking speed and endurance occur with interventions in people with chronic SCI; however similar results were observed between interventions. Both MID and GMM-RCR analysis revealed improvements in walking endurance with Tizanidine in high function participants only; a finding that typical group averaging technique could not detect. GMM-RCR analysis additionally revealed improvements in walking speed and mobility with LTT. MID revealed that the TUG may be a more suitable assessment for low functioning subjects, and the 10MWT and 6MWT may be more suitable for higher functioning subjects. Overall, results of MID and GMM-RCR analysis were in agreement for the 10MWT and 6MWT, but disagreed regarding the TUG. These findings demonstrate that both GMM and MID analyses have the potential to characterize the therapeutic effects of various types of interventions on clinical outcome measures.
Acknowledgements
This work was supported by the National Institutes of Health and the Craig H. Neilsen Foundation awards to MMM. We also wish to thank L. Ness, D. Varoqui, M. Kindig and D. Kotsapouikis for their contributions.
References
- 1.Walter JS, Sacks J, Othman R, et al. A database of self-reported secondary medical problems among VA spinal cord injury patients: its role in clinical care and management. Journal of rehabilitation research and development. 2002 Jan-Feb;39(1):53–61. [PubMed] [Google Scholar]
- 2.Winter DA. Overall principle of lower limb support during stance phase of gait. Journal of biomechanics. 1980;13(11):923–927. doi: 10.1016/0021-9290(80)90162-1. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb GL, Myklebust BM. Hyper-reflexia and disordered voluntary movement. In: Thilman AF, Burke DJ, Rymer WZ, editors. Spasticity: Mechanisms and Managment. Springer-Verlag; 1993. pp. 155–169. [Google Scholar]
- 4.Corcos DM, Gottlieb GL, Penn RD, Myklebust B, Agarwal GC. Movement deficits caused by hyperexcitable stretch reflexes in spastic humans. Brain. 1986;109(5):1043–1058. doi: 10.1093/brain/109.5.1043. [DOI] [PubMed] [Google Scholar]
- 5.Eyre J, Miller S. Disturbance of Voluntary Movement. In: Thilman AF, Burke DJ, Rymer WZ, editors. Spasticity: Mechanisms and Management. Berling Heidelberg; Springer-Verlag: 1993. pp. 1970–1973. [Google Scholar]
- 6.Norman K, Pepin A, Barbeau H. Effects of drugs on walking after spinal cord injury. Spinal Cord. 1998;36(10):699–715. doi: 10.1038/sj.sc.3100674. [DOI] [PubMed] [Google Scholar]
- 7.Little JW, Powers RK, Michelson P, Moore D, Robinson LR, Goldstein B. Electrodiagnosis of upper limb weakness in acute quadriplegia. Am J Phys Med Rehabil. 1994;73:15–22. [PubMed] [Google Scholar]
- 8.Nance PW, Bugaresti J, Shellenberger K, Sheremata W, Martinez-rizala A. Efficacy and safety of tizanidine in the treatment of spasticity in patients with spinal cord injury. North American Tizanidine Study Group. Neurology. 1994 Nov;44(11 Suppl 9):S44–51. discussion S51-42. [PubMed] [Google Scholar]
- 9.Knutsson E, Martensson A, Gransberg L. Antiparetic and antispastic effects induced by tizanidine in patients with spastic paresis. Journal of the neurological sciences. 1982 Feb;53(2):187–204. doi: 10.1016/0022-510x(82)90005-3. [DOI] [PubMed] [Google Scholar]
- 10.Lataste X, Emre M, Davis C, Groves L. Comparative profile of tizanidine in the management of spasticity. Neurology. 1994 Nov;44(11 Suppl 9):S53–59. [PubMed] [Google Scholar]
- 11.Mathias CJ, Luckitt J, Desai P, Baker H, el Masri W, Frankel HL. Pharmacodynamics and pharmacokinetics of the oral antispastic agent tizanidine in patients with spinal cord injury. Journal of rehabilitation research and development. 1989 Fall;26(4):9–16. [PubMed] [Google Scholar]
- 12.Jankowska E, Lackberg ZS, Dyrehag LE. Effects of monoamines on transmission from group II muscle afferents in sacral segments in the cat. The European journal of neuroscience. 1994 Jun;6(6):1058–1061. doi: 10.1111/j.1460-9568.1994.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 13.Penn RD, Savoy SM, Corcos D, et al. Intrathecal baclofen for severe spinal spasticity. The New England journal of medicine. 1989 Jun;320(23):1517–1521. doi: 10.1056/NEJM198906083202303. [DOI] [PubMed] [Google Scholar]
- 14.Kravitz HM, Corcos DM, Hansen G, Penn RD, Cartwright RD, Gianino J. Intrathecal baclofen. Effects on nocturnal leg muscle spasticity. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 1992 Feb;71(1):48–52. [PubMed] [Google Scholar]
- 15.Taricco M, Pagliacci MC, Telaro E, Adone R. Pharmacological interventions for spasticity following spinal cord injury: results of a Cochrane systematic review. Europa medicophysica. 2006 Mar;42(1):5–15. [PubMed] [Google Scholar]
- 16.Mirbagheri MM, Chen D, Rymer WZ. Quantification of the effects of an alpha-2 adrenergic agonist on reflex properties in spinal cord injury using a system identification technique. Journal of neuroengineering and rehabilitation. 2010;7:29. doi: 10.1186/1743-0003-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chau C, Barbeau H, Rossignol S. Effects of intrathecal alpha1- and alpha2-noradrenergic agonists and norepinephrine on locomotion in chronic spinal cats. Journal of neurophysiology. 1998 Jun;79(6):2941–2963. doi: 10.1152/jn.1998.79.6.2941. [DOI] [PubMed] [Google Scholar]
- 18.Grillner S. Neurobiological bases of rhythmic motor acts in vertebrates. Science. 1985 Apr;228(4696):143–149. doi: 10.1126/science.3975635. [DOI] [PubMed] [Google Scholar]
- 19.Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain research. 1987 May;412(1):84–95. doi: 10.1016/0006-8993(87)91442-9. [DOI] [PubMed] [Google Scholar]
- 20.Visintin M, Barbeau H. The effects of body weight support on the locomotor pattern of spastic paretic patients. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 1989 Aug;16(3):315–325. doi: 10.1017/s0317167100029152. [DOI] [PubMed] [Google Scholar]
- 21.Wernig A, Muller S, Nanassy A, Cagol E. Laufband therapy based on ‘rules of spinal locomotion’ is effective in spinal cord injured persons. The European journal of neuroscience. 1995 Apr 1;7(4):823–829. doi: 10.1111/j.1460-9568.1995.tb00686.x. [DOI] [PubMed] [Google Scholar]
- 22.Wirz M, Zemon DH, Rupp R, et al. Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: a multicenter trial. Archives of physical medicine and rehabilitation. 2005 Apr;86(4):672–680. doi: 10.1016/j.apmr.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Alexeeva N, Sames C, Jacobs PL, et al. Comparison of training methods to improve walking in persons with chronic spinal cord injury: a randomized clinical trial. The journal of spinal cord medicine. 2011;34(4):362–379. doi: 10.1179/2045772311Y.0000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Field-Fote EC, Roach KE. Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: a randomized clinical trial. Physical therapy. 2011 Jan;91(1):48–60. doi: 10.2522/ptj.20090359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas SL, Gorassini MA. Increases in corticospinal tract function by treadmill training after incomplete spinal cord injury. Journal of neurophysiology. 2005 Oct;94(4):2844–2855. doi: 10.1152/jn.00532.2005. [DOI] [PubMed] [Google Scholar]
- 26.Jayaraman A, Shah P, Gregory C, et al. Locomotor training and muscle function after incomplete spinal cord injury: case series. The journal of spinal cord medicine. 2008;31(2):185–193. doi: 10.1080/10790268.2008.11760710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hicks AL, Adams MM, Martin Ginis K, et al. Long-term body-weight-supported treadmill training and subsequent follow-up in persons with chronic SCI: effects on functional walking ability and measures of subjective well-being. Spinal cord. 2005 May;43(5):291–298. doi: 10.1038/sj.sc.3101710. [DOI] [PubMed] [Google Scholar]
- 28.Wirz M, van Hedel HJ, Rupp R, Curt A, Dietz V. Muscle force and gait performance: relationships after spinal cord injury. Archives of physical medicine and rehabilitation. 2006 Sep;87(9):1218–1222. doi: 10.1016/j.apmr.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 29.Wernig A, Muller S. Laufband locomotion with body weight support improved walking in persons with severe spinal cord injuries. Paraplegia. 1992 Apr;30(4):229–238. doi: 10.1038/sc.1992.61. [DOI] [PubMed] [Google Scholar]
- 30.Morawietz C, Moffat F. Effects of locomotor training after incomplete spinal cord injury: a systematic review. Archives of physical medicine and rehabilitation. 2013 Nov;94(11):2297–2308. doi: 10.1016/j.apmr.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 31.Dobkin BH, Duncan PW. Should body weight-supported treadmill training and robotic-assistive steppers for locomotor training trot back to the starting gate? Neurorehabilitation and neural repair. 2012 May;26(4):308–317. doi: 10.1177/1545968312439687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dobkin B, Apple D, Barbeau H, et al. Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology. 2006 Feb;66(4):484–493. doi: 10.1212/01.wnl.0000202600.72018.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang JF, Norton J, Nevett-Duchcherer J, Roy FD, Gross DP, Gorassini MA. Volitional muscle strength in the legs predicts changes in walking speed following locomotor training in people with chronic spinal cord injury. Physical therapy. 2011 Jun;91(6):931–943. doi: 10.2522/ptj.20100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winchester P, Smith P, Foreman N, et al. A prediction model for determining over ground walking speed after locomotor training in persons with motor incomplete spinal cord injury. The journal of spinal cord medicine. 2009;32(1):63–71. doi: 10.1080/10790268.2009.11760754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beckerman H, Roebroeck ME, Lankhorst GJ, Becher JG, Bezemer PD, Verbeek AL. Smallest real difference, a link between reproducibility and responsiveness. Quality of life research: an international journal of quality of life aspects of treatment, care and rehabilitation. 2001;10(7):571–578. doi: 10.1023/a:1013138911638. [DOI] [PubMed] [Google Scholar]
- 36.Lam T, Noonan VK, Eng JJ. A systematic review of functional ambulation outcome measures in spinal cord injury. Spinal cord. 2008 Apr;46(4):246–254. doi: 10.1038/sj.sc.3102134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mirbagheri MM, Lilaonitkul T, Rymer WZ. Prediction of natural history of neuromuscular properties after stroke using Fugl-Meyer scores at 1 month. Neurorehabilitation and neural repair. 2011 Jun;25(5):458–468. doi: 10.1177/1545968310390222. [DOI] [PubMed] [Google Scholar]
- 38.Jackson AB, Carnel CT, Ditunno JF, et al. Outcome measures for gait and ambulation in the spinal cord injury population. The journal of spinal cord medicine. 2008;31(5):487–499. doi: 10.1080/10790268.2008.11753644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Hedel HJ, Wirz M, Dietz V. Standardized assessment of walking capacity after spinal cord injury: the European network approach. Neurological research. 2008 Feb;30(1):61–73. doi: 10.1179/016164107X230775. [DOI] [PubMed] [Google Scholar]
- 40.van Hedel HJ, Wirz M, Dietz V. Assessing walking ability in subjects with spinal cord injury: validity and reliability of 3 walking tests. Archives of physical medicine and rehabilitation. 2005 Feb;86(2):190–196. doi: 10.1016/j.apmr.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 41.Ram N, Grimm KJ. Growth Mixture Modeling: A Method for Identifying Differences in Longitudinal Change Among Unobserved Groups. International journal of behavioral development. 2009;33(6):565–576. doi: 10.1177/0165025409343765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Domingo A, Al-Yahya AA, Asiri Y, Eng JJ, Lam T. A systematic review of the effects of pharmacological agents on walking function in people with spinal cord injury. Journal of neurotrauma. 2012 Mar;29(5):865–879. doi: 10.1089/neu.2011.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alibiglou L, Rymer WZ, Harvey RL, Mirbagheri MM. The relation between Ashworth scores and neuromechanical measurements of spasticity following stroke. Journal of neuroengineering and rehabilitation. 2008;5:18. doi: 10.1186/1743-0003-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Musselman KE, Yang JF. Spinal Cord Injury Functional Ambulation Profile: a preliminary look at responsiveness. Physical therapy. 2014 Feb;94(2):240–250. doi: 10.2522/ptj.20130071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang JF, Musselman KE. Training to achieve over ground walking after spinal cord injury: a review of who, what, when, and how. The journal of spinal cord medicine. 2012 Sep;35(5):293–304. doi: 10.1179/2045772312Y.0000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]