Abstract
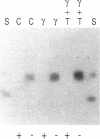
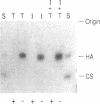
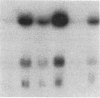
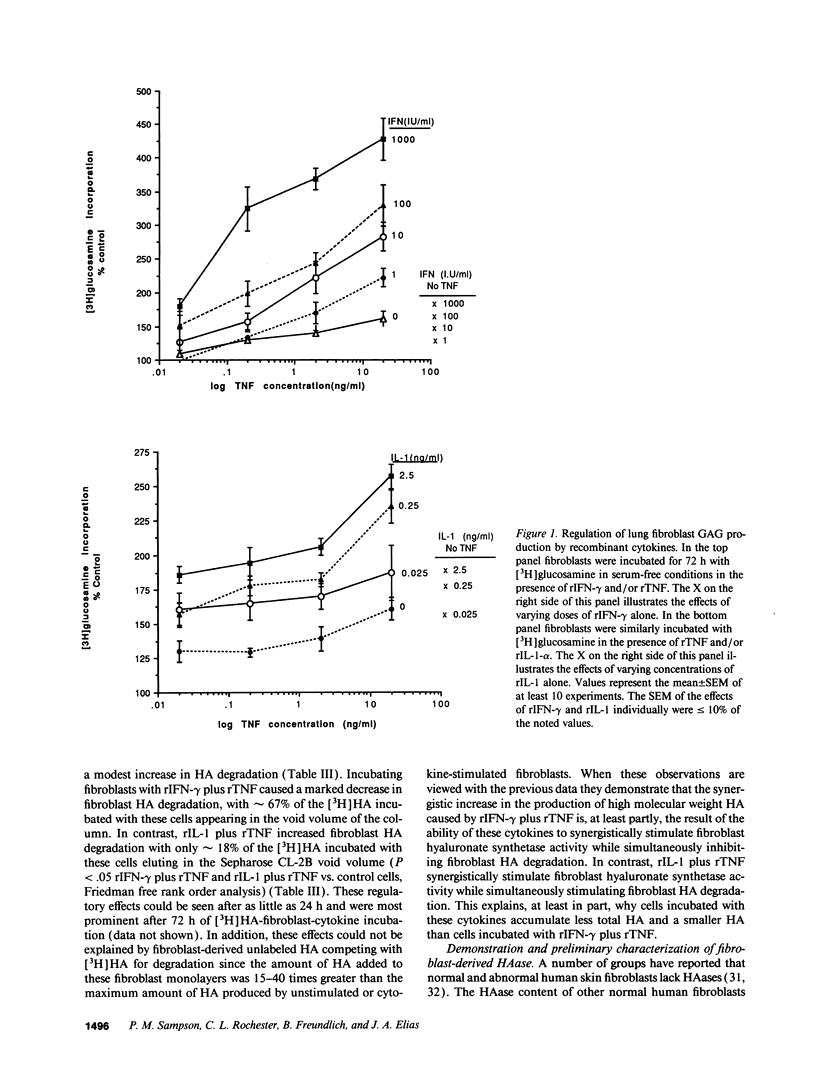
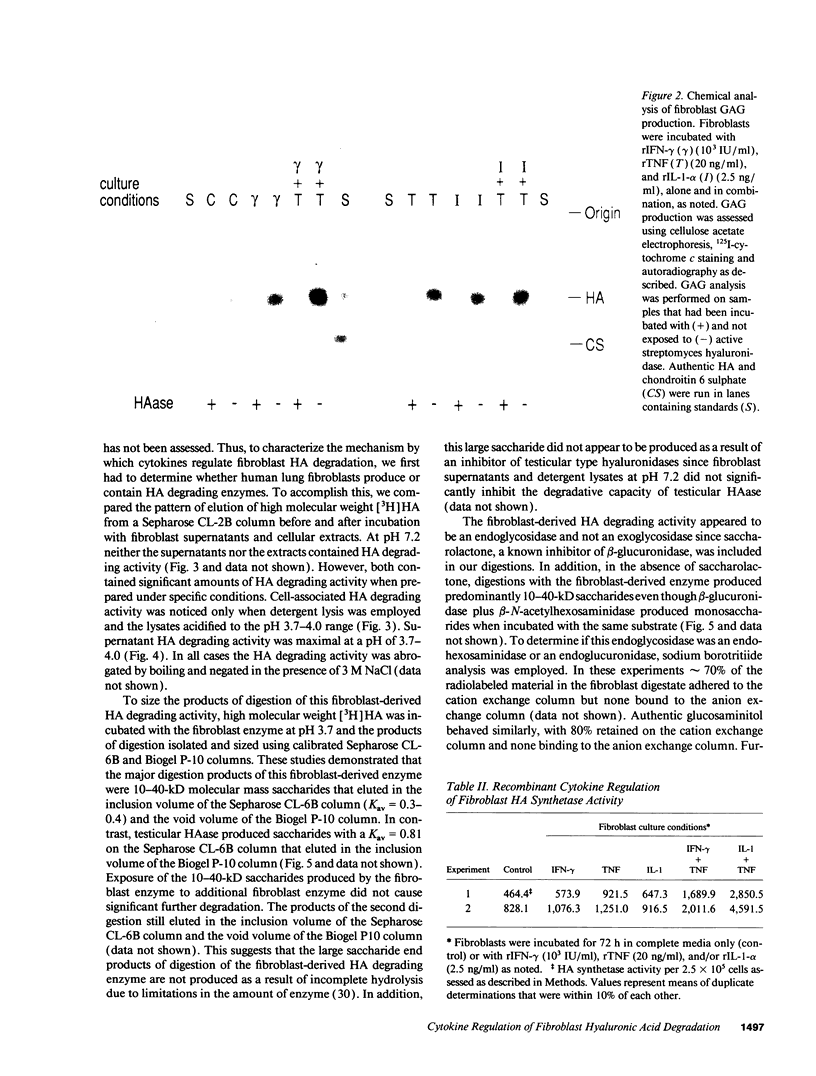
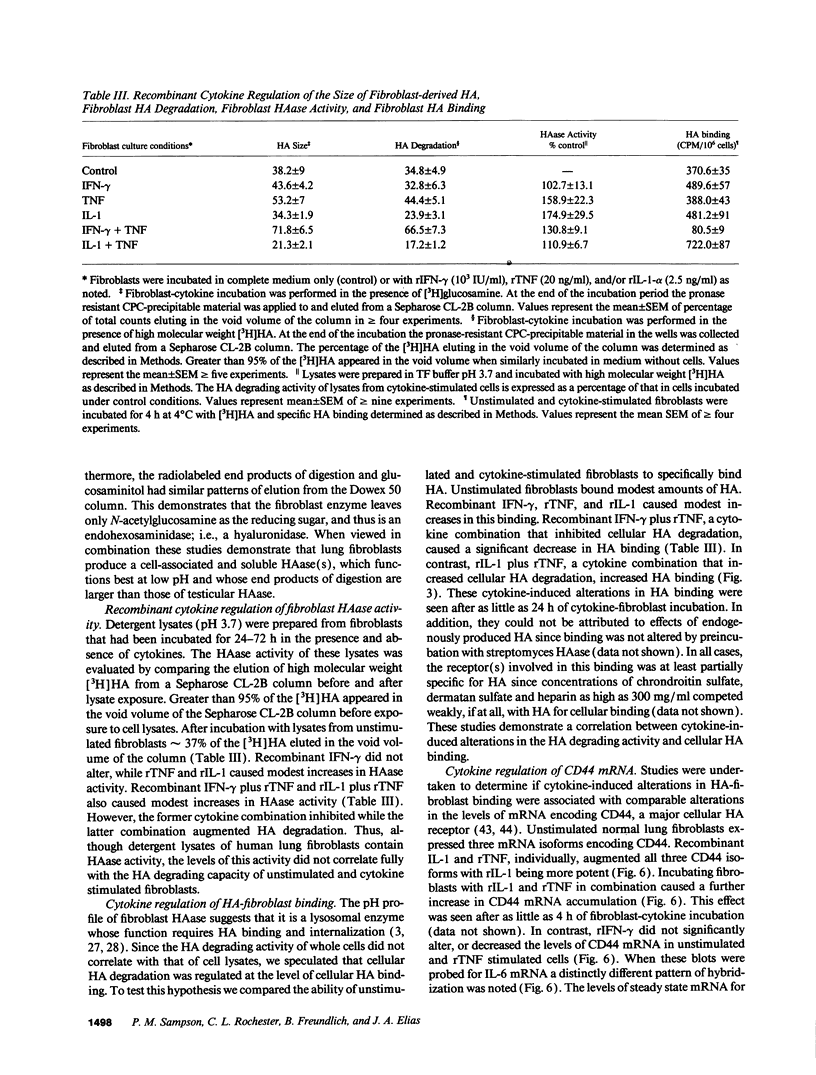
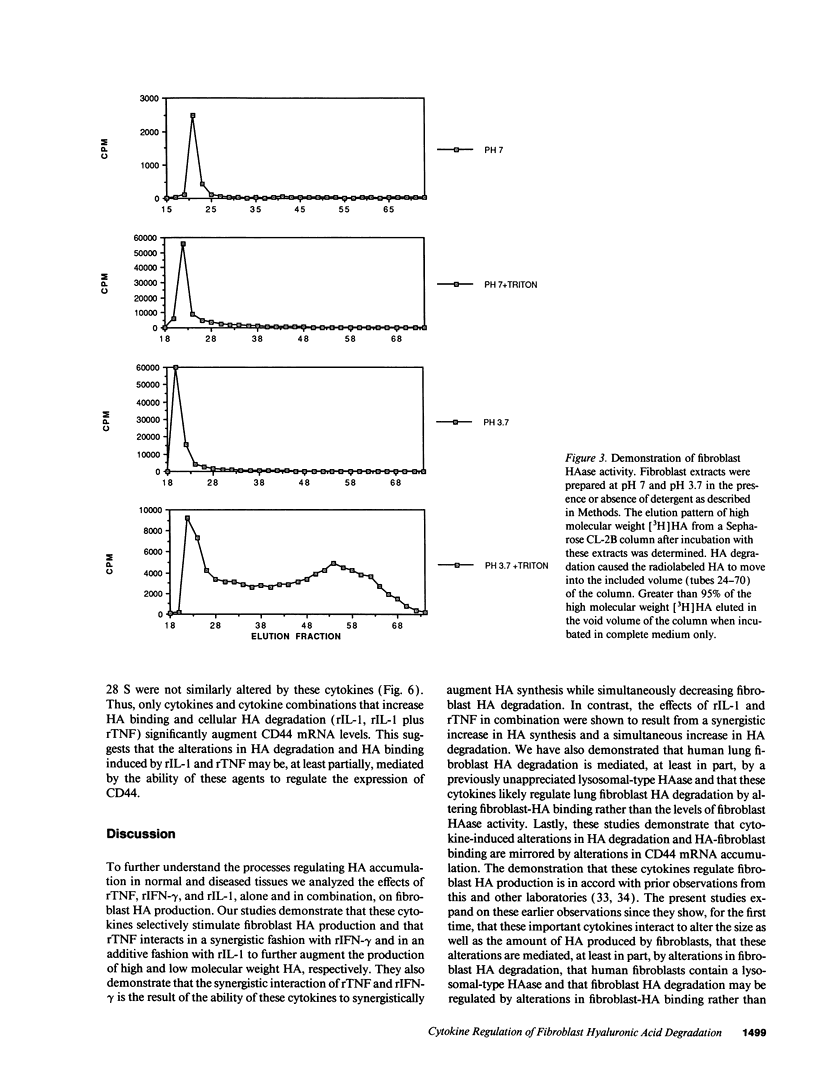
We characterized the mechanisms by which recombinant (r) tumor necrosis factor (TNF), IFN-gamma, and IL-1, alone and in combination, regulate human lung fibroblast hyaluronic acid (HA) production. Each cytokine stimulated fibroblast HA production. The combination of rTNF and rIFN-gamma resulted in a synergistic increase in the production of high molecular weight HA. This was due to a synergistic increase in hyaluronate synthetase activity and a simultaneous decrease in HA degradation. In contrast, when rTNF and rIL-1 were combined, an additive increase in low molecular weight HA was noted. This was due to a synergistic increase in hyaluronate synthetase activity and a simultaneous increase in HA degradation. Human lung fibroblasts contained a hyaluronidase that, at pH 3.7, depolymerized high molecular weight HA to 10-40 kD end products of digestion. However, hyaluronidase activity did not correlate with fibroblast HA degradation. Instead, HA degradation correlated with fibroblast-HA binding, which was increased by rIL-1 plus rTNF and decreased by rIFN-gamma plus rTNF. Recombinant IL-1 and rTNF weakly stimulated and rIL-1 and rTNF in combination further augmented the levels of CD44 mRNA in lung fibroblasts. In contrast, rIFN-gamma did not significantly alter the levels of CD44 mRNA in unstimulated or rTNF stimulated cells. These studies demonstrate that rIL-1, rTNF, and rIFN-gamma have complex effects on biosynthesis and degradation which alter the quantity and molecular weight of the HA produced by lung fibroblasts. They also show that fibroblast HA degradation is mediated by a previously unrecognized lysosomal-type hyaluronidase whose function may be regulated by altering fibroblast-HA binding. Lastly, they suggest that the CD44 HA receptor may be involved in this process.
Full text
PDF



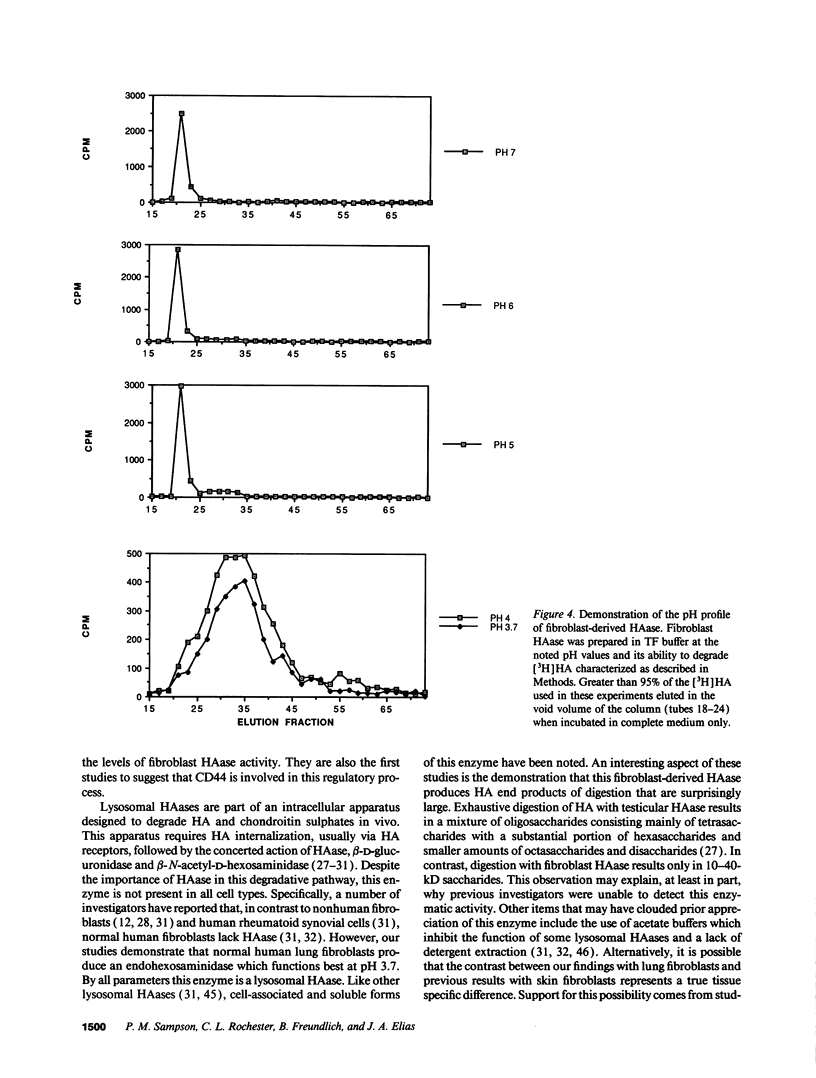
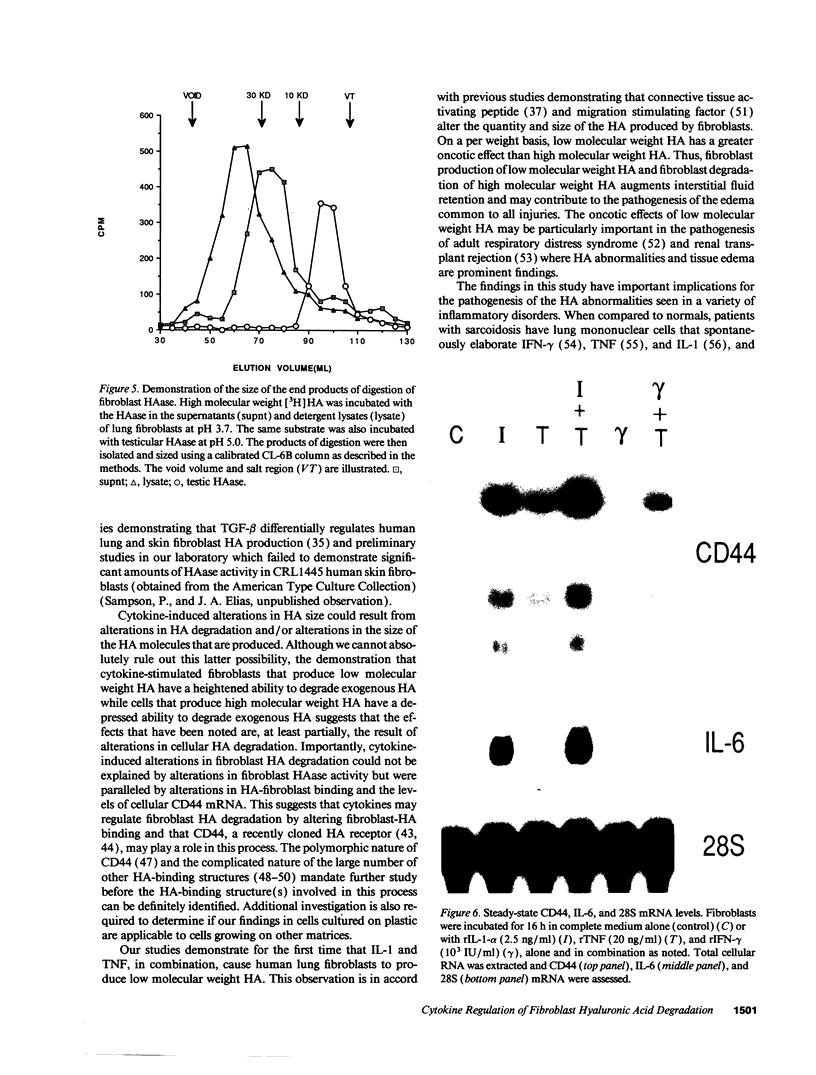


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appel A., Horwitz A. L., Dorfman A. Cell-free synthesis of hyaluronic acid in Marfan syndrome. J Biol Chem. 1979 Dec 10;254(23):12199–12203. [PubMed] [Google Scholar]
- Arbogast B., Hopwood J. J., Dorfman A. Absence of hyaluronidase in cultured human skin fibroblasts. Biochem Biophys Res Commun. 1975 Nov 3;67(1):376–382. doi: 10.1016/0006-291x(75)90326-5. [DOI] [PubMed] [Google Scholar]
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Bashey R. I., Millan A., Jimenez S. A. Increased biosynthesis of glycosaminoglycans by scleroderma fibroblasts in culture. Arthritis Rheum. 1984 Sep;27(9):1040–1045. doi: 10.1002/art.1780270911. [DOI] [PubMed] [Google Scholar]
- Bernanke D. H., Orkin R. W. Hyaluronidase activity in embryonic chick heart muscle and cushion tissue and cells. Dev Biol. 1984 Dec;106(2):351–359. doi: 10.1016/0012-1606(84)90233-1. [DOI] [PubMed] [Google Scholar]
- Bertolami C. N., Donoff R. B. Hyaluronidase activity during open wound healing in rabbits: a preliminary report. J Surg Res. 1978 Sep;25(3):256–259. doi: 10.1016/0022-4804(78)90116-6. [DOI] [PubMed] [Google Scholar]
- Bjermer L., Engström-Laurent A., Lundgren R., Rosenhall L., Hällgren R. Hyaluronate and type III procollagen peptide concentrations in bronchoalveolar lavage fluid as markers of disease activity in farmer's lung. Br Med J (Clin Res Ed) 1987 Oct 3;295(6602):803–806. doi: 10.1136/bmj.295.6602.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray B. A., Sampson P. M., Osman M., Giandomenico A., Turino G. M. Early changes in lung tissue hyaluronan (hyaluronic acid) and hyaluronidase in bleomycin-induced alveolitis in hamsters. Am Rev Respir Dis. 1991 Feb;143(2):284–288. doi: 10.1164/ajrccm/143.2.284. [DOI] [PubMed] [Google Scholar]
- Brecht M., Mayer U., Schlosser E., Prehm P. Increased hyaluronate synthesis is required for fibroblast detachment and mitosis. Biochem J. 1986 Oct 15;239(2):445–450. doi: 10.1042/bj2390445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castor C. W. Connective tissue activation. 3. Observations on the mechanism of action of connective tissue activating peptide. J Lab Clin Med. 1972 Feb;79(2):285–301. [PubMed] [Google Scholar]
- Comper W. D., Laurent T. C. Physiological function of connective tissue polysaccharides. Physiol Rev. 1978 Jan;58(1):255–315. doi: 10.1152/physrev.1978.58.1.255. [DOI] [PubMed] [Google Scholar]
- Dahl I. M., Husby G. Hyaluronic acid production in vitro by synovial lining cells from normal and rheumatoid joints. Ann Rheum Dis. 1985 Oct;44(10):647–657. doi: 10.1136/ard.44.10.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Demczuk S. Cytokines and other mediators in rheumatoid arthritis. Springer Semin Immunopathol. 1984;7(4):387–413. doi: 10.1007/BF00201968. [DOI] [PubMed] [Google Scholar]
- Dougherty G. J., Landorp P. M., Cooper D. L., Humphries R. K. Molecular cloning of CD44R1 and CD44R2, two novel isoforms of the human CD44 lymphocyte "homing" receptor expressed by hemopoietic cells. J Exp Med. 1991 Jul 1;174(1):1–5. doi: 10.1084/jem.174.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias J. A., Krol R. C., Freundlich B., Sampson P. M. Regulation of human lung fibroblast glycosaminoglycan production by recombinant interferons, tumor necrosis factor, and lymphotoxin. J Clin Invest. 1988 Feb;81(2):325–333. doi: 10.1172/JCI113324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias J. A., Lentz V. IL-1 and tumor necrosis factor synergistically stimulate fibroblast IL-6 production and stabilize IL-6 messenger RNA. J Immunol. 1990 Jul 1;145(1):161–166. [PubMed] [Google Scholar]
- Engström-Laurent A. Changes in hyaluronan concentration in tissues and body fluids in disease states. Ciba Found Symp. 1989;143:233-40; discussion 240-7, 281-5. doi: 10.1002/9780470513774.ch14. [DOI] [PubMed] [Google Scholar]
- Engström-Laurent A., Feltelius N., Hällgren R., Wasteson A. Raised serum hyaluronate levels in scleroderma: an effect of growth factor induced activation of connective tissue cells? Ann Rheum Dis. 1985 Sep;44(9):614–620. doi: 10.1136/ard.44.9.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström-Laurent A., Hällgren R. Circulating hyaluronate in rheumatoid arthritis: relationship to inflammatory activity and the effect of corticosteroid therapy. Ann Rheum Dis. 1985 Feb;44(2):83–88. doi: 10.1136/ard.44.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig J. J., Ward-Bailey P. F. Sulfated glycosaminoglycans inhibit hyaluronic acid synthesizing activity in mouse cumuli oophori. Exp Cell Res. 1984 Feb;150(2):459–465. doi: 10.1016/0014-4827(84)90590-1. [DOI] [PubMed] [Google Scholar]
- Goggins J. F., Lazarus G. S., Fullmer H. M. Hyaluronidase activity of alveolar macrophages. J Histochem Cytochem. 1968 Nov;16(11):688–692. doi: 10.1177/16.11.688. [DOI] [PubMed] [Google Scholar]
- Goldstein L. A., Zhou D. F., Picker L. J., Minty C. N., Bargatze R. F., Ding J. F., Butcher E. C. A human lymphocyte homing receptor, the hermes antigen, is related to cartilage proteoglycan core and link proteins. Cell. 1989 Mar 24;56(6):1063–1072. doi: 10.1016/0092-8674(89)90639-9. [DOI] [PubMed] [Google Scholar]
- Havez R., Degand P., Boersma A., Richet C. Caractérisation et dosage de l'acide hyaluronique dans le liquide pleural de mésothéliome. Clin Chim Acta. 1971 Jul;33(2):443–454. doi: 10.1016/0009-8981(71)90505-5. [DOI] [PubMed] [Google Scholar]
- Hiro D., Ito A., Matsuta K., Mori Y. Hyaluronic acid is an endogenous inducer of interleukin-1 production by human monocytes and rabbit macrophages. Biochem Biophys Res Commun. 1986 Oct 30;140(2):715–722. doi: 10.1016/0006-291x(86)90790-4. [DOI] [PubMed] [Google Scholar]
- Hopkins S. J., Meager A. Cytokines in synovial fluid: II. The presence of tumour necrosis factor and interferon. Clin Exp Immunol. 1988 Jul;73(1):88–92. [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W. Release of interleukin-1 by alveolar macrophages of patients with active pulmonary sarcoidosis. Am Rev Respir Dis. 1984 Apr;129(4):569–572. [PubMed] [Google Scholar]
- Hällgren R., Eklund A., Engström-Laurent A., Schmekel B. Hyaluronate in bronchoalveolar lavage fluid: a new marker in sarcoidosis reflecting pulmonary disease. Br Med J (Clin Res Ed) 1985 Jun 15;290(6484):1778–1781. doi: 10.1136/bmj.290.6484.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hällgren R., Samuelsson T., Laurent T. C., Modig J. Accumulation of hyaluronan (hyaluronic acid) in the lung in adult respiratory distress syndrome. Am Rev Respir Dis. 1989 Mar;139(3):682–687. doi: 10.1164/ajrccm/139.3.682. [DOI] [PubMed] [Google Scholar]
- Klein U., von Figura K. Characterization of dermatan sulfate in mucopolysaccharidosis VI. Evidence for the absence of hyaluronidase-like enzymes in human skin fibroblasts. Biochim Biophys Acta. 1980 Jun 5;630(1):10–14. doi: 10.1016/0304-4165(80)90131-2. [DOI] [PubMed] [Google Scholar]
- Knudson W., Biswas C., Toole B. P. Interactions between human tumor cells and fibroblasts stimulate hyaluronate synthesis. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6767–6771. doi: 10.1073/pnas.81.21.6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberg S. I., Stoolmiller A. C. Glycosaminoglycans. A biochemical and clinical review. J Invest Dermatol. 1974 Dec;63(6):433–449. doi: 10.1111/1523-1747.ep12680346. [DOI] [PubMed] [Google Scholar]
- Laurent T. C., Dahl I. M., Dahl L. B., Engström-Laurent A., Eriksson S., Fraser J. R., Granath K. A., Laurent C., Laurent U. B., Lilja K. The catabolic fate of hyaluronic acid. Connect Tissue Res. 1986;15(1-2):33–41. doi: 10.3109/03008208609001971. [DOI] [PubMed] [Google Scholar]
- Love S. H., Shannon B. T., Myrvik Q. N., Lynn W. S. Characterization of macrophage agglutinating factor as a hyaluronic acid-protein complex. J Reticuloendothel Soc. 1979 Mar;25(3):269–282. [PubMed] [Google Scholar]
- McGuire P. G., Castellot J. J., Jr, Orkin R. W. Size-dependent hyaluronate degradation by cultured cells. J Cell Physiol. 1987 Nov;133(2):267–276. doi: 10.1002/jcp.1041330210. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Takagaki K., Kubo K., Morikawa A., Tamura S., Endo M. Extracellular depolymerization of hyaluronic acid in cultured human skin fibroblasts. Biochem Biophys Res Commun. 1990 Oct 15;172(1):70–76. doi: 10.1016/s0006-291x(05)80174-3. [DOI] [PubMed] [Google Scholar]
- Ohya T., Kaneko Y. Novel hyaluronidase from streptomyces. Biochim Biophys Acta. 1970 Mar 18;198(3):607–609. doi: 10.1016/0005-2744(70)90139-7. [DOI] [PubMed] [Google Scholar]
- Orkin R. W., Toole B. P. Chick embryo fibroblasts produce two forms of hyaluronidase. J Cell Biol. 1980 May;85(2):248–257. doi: 10.1083/jcb.85.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin R. W., Toole B. P. Isolation and characterization of hyaluronidase from cultures of chick embryo skin- and muscle-derived fibroblasts. J Biol Chem. 1980 Feb 10;255(3):1036–1042. [PubMed] [Google Scholar]
- Orkin R. W., Underhill C. B., Toole B. P. Hyaluronate degradation in 3T3 and simian virus-transformed 3T3 cells. J Biol Chem. 1982 May 25;257(10):5821–5826. [PubMed] [Google Scholar]
- Postlethwaite A. E., Smith G. N., Jr, Lachman L. B., Endres R. O., Poppleton H. M., Hasty K. A., Seyer J. M., Kang A. H. Stimulation of glycosaminoglycan synthesis in cultured human dermal fibroblasts by interleukin 1. Induction of hyaluronic acid synthesis by natural and recombinant interleukin 1s and synthetic interleukin 1 beta peptide 163-171. J Clin Invest. 1989 Feb;83(2):629–636. doi: 10.1172/JCI113927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson B. W., McLemore T. L., Crystal R. G. Gamma interferon is spontaneously released by alveolar macrophages and lung T lymphocytes in patients with pulmonary sarcoidosis. J Clin Invest. 1985 May;75(5):1488–1495. doi: 10.1172/JCI111852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero S. L., Bertolami C. N., Bronson R. E., Damiani P. J. Hyaluronidase activity of rabbit skin wound granulation tissue fibroblasts. J Dent Res. 1987 Jul;66(7):1283–1287. doi: 10.1177/00220345870660071301. [DOI] [PubMed] [Google Scholar]
- Sahu S. C. Hyaluronic acid. An indicator of pathological conditions of human lungs? Inflammation. 1980 Mar;4(1):107–112. doi: 10.1007/BF00914107. [DOI] [PubMed] [Google Scholar]
- Sahu S., Lynn W. S. Hyaluronic acid in the pulmonary secretions of patients with alveolar proteinosis. Inflammation. 1978 Jun;3(2):149–158. doi: 10.1007/BF00910736. [DOI] [PubMed] [Google Scholar]
- Sahu S., Lynn W. S. Hyaluronic acid in the pulmonary secretions of patients with asthma. Biochem J. 1978 Aug 1;173(2):565–568. doi: 10.1042/bj1730565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor S. L., Schor A. M., Grey A. M., Chen J., Rushton G., Grant M. E., Ellis I. Mechanism of action of the migration stimulating factor produced by fetal and cancer patient fibroblasts: effect on hyaluronic and synthesis. In Vitro Cell Dev Biol. 1989 Aug;25(8):737–746. doi: 10.1007/BF02623727. [DOI] [PubMed] [Google Scholar]
- Stephens R. W., Sutherland J., Ghosh P., Taylor T. K. Human serum and synovial fluid hyaluronidase--bovine testicular hyaluronidase is not a valid substitute in drug evaluation studies. Biochem Pharmacol. 1976 Jul 1;25(13):1507–1511. doi: 10.1016/0006-2952(76)90069-1. [DOI] [PubMed] [Google Scholar]
- Turino G. M. The lung parenchyma--a dynamic matrix. J. Burns Amberson lecture. Am Rev Respir Dis. 1985 Dec;132(6):1324–1334. doi: 10.1164/arrd.1985.132.6.1324. [DOI] [PubMed] [Google Scholar]
- Turley E. A. The role of a cell-associated hyaluronan-binding protein in fibroblast behaviour. Ciba Found Symp. 1989;143:121-33; discussion 133-7, 281-5. doi: 10.1002/9780470513774.ch8. [DOI] [PubMed] [Google Scholar]
- Uitto J., Helin G., Helin P., Lorenzen I. Connective tissue in scleroderma. A biochemical study on the correlation of fractionated glycosaminoglycans and collagen in human skin. Acta Derm Venereol. 1971;51(6):401–406. [PubMed] [Google Scholar]
- Wells A. F., Larsson E., Tengblad A., Fellström B., Tufveson G., Klareskog L., Laurent T. C. The localization of hyaluronan in normal and rejected human kidneys. Transplantation. 1990 Aug;50(2):240–243. doi: 10.1097/00007890-199008000-00014. [DOI] [PubMed] [Google Scholar]
- West D. C., Hampson I. N., Arnold F., Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985 Jun 14;228(4705):1324–1326. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
- Westergren-Thorsson G., Särnstrand B., Fransson L. A., Malmström A. TGF-beta enhances the production of hyaluronan in human lung but not in skin fibroblasts. Exp Cell Res. 1990 Jan;186(1):192–195. doi: 10.1016/0014-4827(90)90227-2. [DOI] [PubMed] [Google Scholar]