Abstract
Background
Relatively little is known about the environmental and genetic contributions to gambling frequency and disordered gambling (DG), the full continuum of gambling-related problems that includes pathological gambling (PG).
Method
A web-based sample (n=43 799 including both members of 609 twin and 303 sibling pairs) completed assessments of number of lifetime gambling episodes, DSM-IV criteria for PG, alcohol, nicotine and caffeine intake, and nicotine dependence (ND) and DSM-III-R criteria for lifetime major depression (MD). Twin modeling was performed using Mx.
Results
In the entire cohort, symptoms of DG indexed a single dimension of liability. Symptoms of DG were weakly related to caffeine intake and moderately related to MD, consumption of cigarettes and alcohol, and ND. In twin and sibling pairs, familial resemblance for number of times gambled resulted from both familial–environmental (c2=42%) and genetic factors (a2=32%). For symptoms of DG, resemblance resulted solely from genetic factors (a2=83%). Bivariate analyses indicated a low genetic correlation between symptoms of DG and MD (ra=+0.14) whereas genetic correlations with DG symptoms were substantially higher with use of alcohol, caffeine and nicotine, and ND (ranging from +0.29 to +0.80). The results were invariant across genders.
Conclusions
Whereas gambling participation is determined by shared environmental and genetic factors, DG constitutes a single latent dimension that is largely genetically determined and more closely related to externalizing than internalizing behaviors. Because these findings are invariant across genders, they suggest that the etiological factors of DG are likely to be similar in men and women.
Keywords: Alcohol, depression, nicotine, pathological gambling, twin
Introduction
Pathological gambling (PG) is a persistent and recurrent maladaptive pattern of gambling behavior characterized by increased preoccupation with gambling activities, loss of control and continued gambling despite problems in social or occupational functioning. It is associated with significant financial losses, legal problems (Ledgerwood et al. 2007) and disrupted interpersonal and familial relationships (Shaw et al. 2007). Individuals with PG have an increased prevalence of and risk for incident psychiatric disorders (Petry et al. 2005; Kessler et al. 2008), suicide attempts (Phillips et al. 1997) and several medical conditions (Morasco et al. 2006). Previous studies have found that PG is part of a broader continuum often called disordered gambling (DG), which includes less severe forms of the disorder (Slutske et al. 2000; Strong & Kahler, 2007). A better understanding of the environmental and genetic influences on gambling behavior and DG could help in the development of treatment and preventive interventions.
Most of what is known about genetic and environmental influences on DG comes from analyses of the Vietnam Era Twin (VET) Registry. Results from the VET Registry, which was restricted to men, indicate that most of the familial aggregation of DG is due to genetic influences (Slutske et al. 2000), and that there is a substantial overlap of the genes predisposing to DG and those associated with major depression (Potenza et al. 2005), antisocial personality disorder (Slutske et al. 2001) and alcohol dependence (Slutske et al. 2000), suggesting that genetic influences on DG may be related to genetic liabilities to both internalizing and externalizing disorders. In the recent Australian Twin Study of Gambling (OZ-GAM), Slutske et al. (2010) further established that genetic influences play a similar role in men and women on the familial aggregation of DG.
Important questions remain regarding the role of genetic and environmental influences on the etiology of gambling behavior and DG. For example, prior research has shown that most individuals who gamble do not develop DG (Petry et al. 2005; Kessler et al. 2008), suggesting that the determinants of gambling initiation differ from those of progression to DG. However, whether genetic and environmental factors play different roles in the initiation and frequency of gambling versus the progression to DG is unknown. Similarly, although the prevalence of DG is higher in men than women (Petry et al. 2005; Blanco et al. 2006; Kessler et al. 2008), only the one study, the OZ-GAM, has examined whether genetic and environmental influences are similar across genders (Slutske et al. 2010). Although it found that they were, this finding needs replication, particularly given that the prevalence of DG in Australia is four to five times the prevalence of DG in the USA, and that the genes influencing gambling behavior may be expressed differently in environments that are more permissive to gambling (Slutske et al. 2009). Furthermore, no study has examined simultaneously the role of genes and environment in the association of DG with internalizing and externalizing behaviors.
The current study sought to address these gaps in knowledge by pursuing four major objectives. First, we wanted to clarify, in a large web-based cohort (n=43 799), the phenotypic associations between symptoms of DG on the one hand and lifetime major depression (MD) and psychoactive substance use on the other. Second, using the twins and sibling pairs from the cohort, we sought to clarify (i) whether the symptoms of DG lie on a latent continuum and (ii) the role of familial and genetic factors in the etiology of two key gambling-related variables: total lifetime frequency of gambling and symptoms of DG. In particular, we wanted to determine whether familial– environmental factors might be more important for the former than the latter trait. Third, with the aim of understanding more about the nature of genetic risk factors for DG, we examined, in bivariate twin-sibling models, the correlation between the genetic risk factors for symptoms of DG and lifetime MD, alcohol, caffeine and cigarette use, and nicotine dependence (ND). Fourth, given the paucity of behavioral genetics work on DG, we examined, in all our twin-sibling analyses, whether our results were invariant across genders.
Method
As outlined in detail previously (Kendler et al. 2009), data on participants in this study were collected from the ‘Twins: An Interactive Personality Test!’ (www.outofservice.com/twins/) from 1 July 2005 until 19 April 2010. This survey was designed as an interactive assessment tool for measures of personality, psychopathology, and substance use and dependence. The website permits any two people, regardless of whether they are twins or not, to compare their personalities and behaviors. Participants can take the survey as individuals. All participants were volunteers and were recruited over the internet. Potential respondents found out about the website using internet search engines, direct access to its address (www.outofservice.com/twins/), or through links from other sites.
Data were collected by automated computerized administration, data entry and scoring. All participants received individualized feedback after completing the survey. The data presented in this article were collected using a non-commercial, advertisement-free website (www.outofservice.com) that contains personality measures in addition to several games, quizzes and questionnaires for entertainment purposes. Participants did not provide any identifying information and anonymity was assured. This research obtained exempt ethics approval at Virginia Commonwealth University.
Responses that included information on DG were received from 43 799 individuals with a mean (s.d.) age of 25.4 (11.7) years. This sample was 66.7% female, 70.8% white, with other common self-identified ethnicities including black (6.0%), Indian/Pakistani (3.7%), Latino (3.7%) and Chinese (3%).
We used a variety of quality control measures to assess the amount of duplicate or faked responses, or false twin pairs in the sample (Kendler et al. 2009). This included examining distributions of our personality measures and finding no excess of extreme scores, examining similarity of reported year of birth, height and weight in twin pairs, and asking and following up the small number of positive responses to an item in our questionnaire about duplicate entries. Consistent with other reports of internet samples (Gosling et al. 2004), these analyses indicated fairly low levels of faked or duplicate data.
Responses including information on DG were received from both members of 609 twin and 303 sibling pairs. Zygosity was assessed by responses in both twin pairs to the three items found most discriminating when tested against DNA results in the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (VATSPSUD; Kendler & Prescott, 2006). All sibling pairs were included in these analyses but 36 twin pairs had to be excluded, largely because of missing or uncertain zygosity information. The zygosity distribution for the remaining pairs was 414 monozygotic (MZ) and 159 dizygotic (DZ) pairs.
Assessments
All of the variables used in this report were assessed using the web-based questionnaires. To reduce the chances of respondents learning to say ‘no’ to the stem items, because it allows them to skip over sections of the questionnaire, we asked all the stem questions at the beginning of the questionnaire (e.g. about lifetime use of alcohol and nicotine, gambling and symptoms of depression).
Lifetime MD was measured using a self-report instrument operationalizing DSM-III-R criteria (APA, 1994) that had been used previously and validated in the Swedish Twin Registry (Kendler et al. 1993). In this study we used the narrow diagnosis of MD as outlined previously (Kendler et al. 1993). ND at the time of heaviest cigarette use was assessed by the Fagerstrom Test for Nicotine Dependence (FTND; Heatherton et al. 1991). We also assessed lifetime heaviest cigarette use as in cigarettes per day and lifetime heaviest alcohol use per week using responses to questions about the average number of drinks per day and number of days per month on which alcohol was consumed ‘during the time you used alcohol the most’. Drink was defined as ‘a 12 oz. bottle of beer, a 5 oz. glass of wine, or a 1.5 oz. shot of liquor’. Caffeine consumption per day was estimated by using responses to questions about the average consumption per day when caffeine products were used and the average number of days per month in which caffeine was consumed (Kendler & Prescott, 1999). Our calculation of caffeine consumption used the following estimates of caffeine content: 125 mg/cup of brewed coffee, 90 mg/cup of instant coffee, 60 mg/cup of tea, and 40 mg/can of caffeinated soft drinks (James, 1997).
Subjects were all asked: ‘Please enter the number of times you have gambled in your life. Examples of gambling include: lottery tickets (includes scratch tickets, excluding gifts) casino type games (including machines, internet and casinos) and placing bets (including bingo, sports events, cards, dice, horses, and raffles).’ For those who reported more than 24 lifetime gambling episodes, the DSM-IV criteria for PG were assessed by the Stinchfield questionnaire (Stinchfield, 2003). Of note, we did not assess lifetime manic episodes so we could not implement DSM-IV criterion B for PG (‘gambling not better accounted for by a manic episode’). Fully syndromal DSM-IV PG was too rare in this sample to be analyzed using twin-siblings modeling. Therefore, we created a four-category variable we termed ‘symptoms of DG’. The four categories were: (1) 0–24 lifetime episodes of gambling, (2) >25 lifetime episodes of gambling and zero endorsed PG criteria, (3) >25 lifetime episodes of gambling and one PG criteria endorsed, and (4) >25 lifetime episodes of gambling and two or more PG criteria endorsed. Twin data permit testing, using a multiple threshold model (Reich et al. 1972), the degree to which the categories of a polychotomous scale reflect the same underlying liability. Multiple threshold models were fitted to the ‘symptoms of DG’ variable for all five twin zygosity groups (male–male and female–female MZ and male–male, female–female and male–female DZ) and in each case the fit was good (p>0.05).
As symptoms of DG were polychotomous, statistical analysis by Mx (Neale et al. 2003) required that we polychotomized the other variables. Therefore, for modeling purposes, we created four or five category measures as follows: alcohol (drinks/week): (1) 0, (2) 1–10, (3) 11–25 and (4) >25; caffeine (converted back from mg caffeine to equivalent cups of brewed coffee): (1) 0, (2) 1, (3) 2, (4) 3–4 and (5) >4; cigarettes/ day: (1) 0, (2) 1–10, (3) 11–20 and (4) >20; and FTND score: (1) 0, (2) 1–3, (3) 4–5 and (4) >5. The cut-offs were chosen to represent as full a spread of scores as possible but avoid categories with few members.
Analyses
The aim of our twin analyses was to decompose the covariance in liability to gambling and symptoms of DG into their genetic and environmental components. We assume that twin and sibling resemblance arises from three latent factors: (i) additive genes (A), contributing twice as much to the MZ as to the DZ twin and sibling correlations, (ii) ‘common’ environment (C), which contributes equally to the correlation in MZ and DZ twins and siblings, and (iii) special twin environment (T), which reflects environmental factors that make twins more similar than siblings. In addition, the model also contains individual-specific environment (E), which reflects measurement error and those environmental experiences that make members of a twin or sibling pair different.
Using the software package Mx (Neale et al. 2003), we fit models by the method of maximum likelihood to data from all twin and full-sibling pairs. Our first set of analyses was univariate, examining lifetime frequency of gambling and symptoms of DG respectively. In our second set of twin-sibling models, we used bivariate models to decompose the associations between symptoms of DG, and substance use and ND. In both sets of analyses we began with a full model that included the four parameters: ACET. We first determined whether there was any evidence for qualitative sex effects (i.e. different genetic risk factors operative in males versus females) or quantitative sex effects (i.e. different magnitude of genetic or environmental factors in the two sexes). Then we tried to simplify the model step by step, setting the A, C and T parameters to zero in turn. We did not attempt to set the E parameters to zero because that would assume, unrealistically, that our variables were measured without error. We used Akaike's Information Criterion (AIC; Akaike, 1987; Williams & Holahan, 1994) for model selection: the lower its value, the better is the balance between explanatory power and parsimony.
Results
Descriptive results
The 43 799 individuals, on whom we had data on times gambled, fell into the following broad categories: 13 233 (30.2%) who denied ever gambling in their lives, and 11 070 (25.3%), 7020 (16.0%), 2748 (6.3%), 4517 (10.3%), and 5221 (11.9%) who reported gambling 1–5, 6–15, 16–24, 25–50, and >50 times respectively. The distribution was highly skewed with a mean of 33.4 times gambled and a standard deviation of 99.5.
Among those who reported gambling ≥25 times in their lives (n=9728 and 22.2% of the entire sample and for whom we asked about DG symptoms), 6815 (70.1%) denied any of the DMS-IV criteria for PG. A total of 2052 (21.1% of those who asked and 4.7% of the entire sample) reported one or two PG criteria and 463 reported three or four criteria (4.8% of those who asked and 1.1% of the entire sample). Only 398 individuals (4.1% of those who asked and 0.9% of the entire sample) endorsed five or more PG criteria, so meeting the A criteria for PG.
To examine whether the 10 PG criteria defined a coherent factor in our sample, we performed an exploratory factor analysis in those individuals who had reported gambling ≥25 times in their lives (n=9728). A single-factor solution (eigenvalue=7.53) provided an excellent fit to the data [Comparative Fit Index (CFI)=0.993, Tucker–Lewis Index (TLI)=0.992, root mean square error of approximation (RMSEA)= 0.026], with individual criteria loading on the common factor ranging from +0.79 (DSM criterion A6) to +0.92 (criterion A4).
Prediction of symptoms of DG
In the entire web-based sample (n=43 799), controlling for sex and age, symptoms of DG were highly significantly predicted (all p<0.00001) by a lifetime diagnosis of MD [odds ratio (OR) 1.32, 95% confidence interval (CI) 1.26–1.39], standardized consumption of alcohol (OR 1.52, 95% CI 1.49–1.55), caffeine (OR 1.20, 95% CI 1.18–1.22) and cigarettes (OR 1.41, 95% CI 1.38–1.44), and standardized symptoms of ND (OR 1.41, 95% CI 1.38–1.44). Among the three psychoactive substances examined, alcohol consumption was most strongly related to symptoms of DG, followed by cigarettes and then caffeine.
Twin-sibling analyses
Gambling frequency
We next focused on gambling frequency defined as the number of reported lifetime gambling episodes. The polychoric correlation for number of times gambled was quite high in MZ twins (+0.76±0.03), and moderately lower and similar in DZ twins (+0.57±0.07) and full siblings (+0.61±0.05). Model fitting results are summarized in Table 1. We began with a full ACET model (model I) that included both qualitative and quantitative sex effects. Model II assumed identical genetic risk factors in males and females (i.e. no qualitative sex effects) and this improved the AIC. Model III then set the parameter estimates to equality in males and females (i.e. no quantitative sex effects), further improving the fit index. Model IV dropped the special twin environment and the AIC improved yet further. In models V and VI, we tried to drop the A and C components respectively. In both cases the model fit deteriorated substantially. The parameter estimates of the best-fit model IV (along with 95% CIs) are shown in Fig. 1. Heritability (a2) for gambling frequency was estimated at 32% (with wide 95% CIs 17–50) whereas the shared environment accounted for 42% of the variance in liability (95% CIs 27–56).
Table 1.
Results of model fitting for number of lifetime gambling episodes using twin and sibling pairs
| Sex effects |
||||||
|---|---|---|---|---|---|---|
| Model | Description | Qualitative | Quantitative | Δ χ 2 | Δdf | ΔAIC |
| Ia | ACET | + | + | – | – | – |
| II | ACET | + | – | + 3.369 | +3 | –2.631 |
| III | ACET | – | – | + 3.369 | +5 | –6.631 |
| IVb | ACE | – | – | + 3.900 | +6 | –8.100 |
| V | CE | – | – | + 19.330 | +7 | + 5.330 |
| VI | AE | – | – | + 28.362 | +7 | + 14.362 |
A, Additive genetic effects; C, shared environment; E, individual-specific environment; T, special twin environment; df, degrees of freedom; AIC, Akaike's Information Criterion.
–2 log likelihood = 4615.856, df = 1736, AIC = 1143.856.
Best-fit model.
Fig. 1.
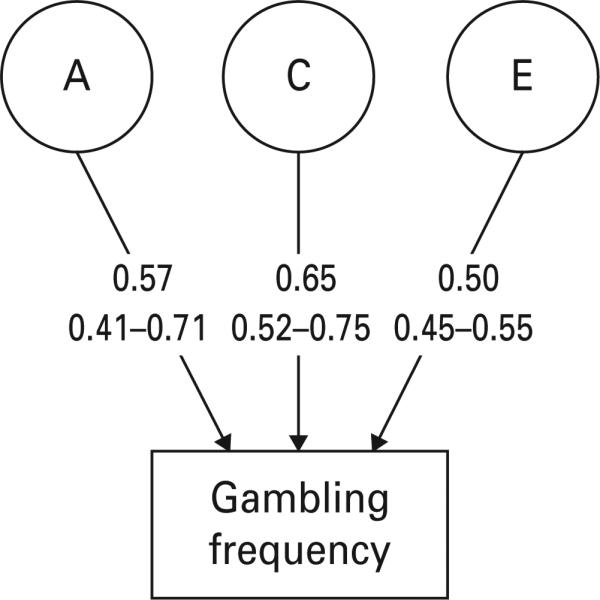
Parameter estimates with 95% confidence intervals for the best-fit model (model IV) for the number of lifetime gambling episodes. These analyses were conducted using both twin and sibling pairs. A, additive genetic effects; C, common or shared environmental effects; E, individual specific environmental effects. The path estimates represent standardized partial regression coefficients.
Symptoms of DG
We next turned to examine symptoms of DG. The polychoric correlation for these symptoms were fairly high in MZ twins (+0.83±0.04) but substantially lower and broadly similar in DZ twins (+0.32±0.17) and full siblings (+0.42±0.10). Model fitting results are summarized in Table 2. We again began with a full ACET model (model I) with sex effects. As with gambling frequency, the results for models II and III indicated that we had no evidence for either qualitative or quantitative sex effects. Model IV dropped the special twin environment and the AIC improved. In models V and VI, we tried to drop the A and C components respectively. Although the fit deteriorated substantially when we dropped A (model V), the AIC improved when C was eliminated, making model VI the best-fit model, the parameter estimates of which (along with 95% CIs) are shown in Fig. 2. The estimate for the heritability (a2) for symptoms of DG is high (83%) with a relatively narrow range for 95% CIs (74– 88), with the remainder (17%) resulting from unique environmental effects.
Table 2.
Results of model fitting for symptoms of disordered gambling (DG) using twin and sibling pairs
| Sex effects |
||||||
|---|---|---|---|---|---|---|
| Model | Description | Qualitative | Quantitative | Δ χ 2 | Δdf | ΔAIC |
| Ia | ACET | + | + | – | – | – |
| II | ACET | + | – | + 0.702 | +3 | –5.298 |
| III | ACET | – | – | + 0.702 | + 5 | –9.298 |
| IV | ACE | – | – | + 0.709 | +6 | –11.291 |
| V | CE | – | – | + 22.929 | +7 | + 8.929 |
| VIb | AE | – | – | + 0.731 | +7 | –13.269 |
A, Additive genetic effects; C, shared environment; E, individual-specific environment; T, special twin environment; df, degrees of freedom; AIC, Akaike's Information Criterion.
–2 log likelihood = 1601.027, df = 1738, AIC =–1874.973.
Best-fit model.
Fig. 2.
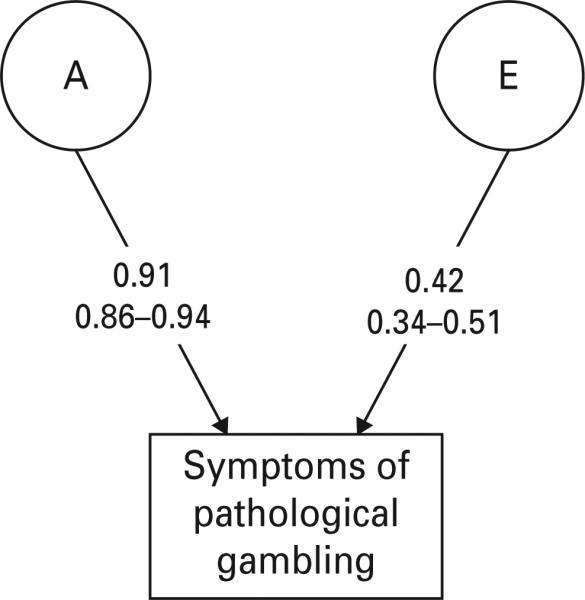
Parameter estimates with 95% confidence intervals for the best-fit model (model VI) for symptoms of disordered gambling (DG). These analyses were conducted using both twin and sibling pairs. A, additive genetic effects; E, individual specific environmental effects. The path estimates represent standardized partial regression coefficients.
Genetic and environmental correlations between symptoms of DG and MD and substance use/misuse
Appendix Table A1 depicts the results of bivariate twin modeling for symptoms of DG and lifetime MD, alcohol, caffeine and cigarette use, and ND. In Table 3, we present the parameter estimates from the best-fit models. For MD and lifetime heaviest cigarette use, the best-fit bivariate model with symptoms of DG included only A and E components for both phenotypes. For past-year caffeine intake, shared environmental effects were required but only for caffeine use. These models are easy to interpret as the shared familial/ genetic effects for the two phenotypes are summarized in a single parameter: the genetic correlation (ra).
Table 3.
Parameter estimates and 95 % confidence intervals (CIs) for the results of the best-fitting (and selected second best-fitting) bivariate twin models for symptoms of disordered gambling (DG) and substance use and depression
| Phenotype | Best-fit model | Parameter (estimate) and 95 % confidence interval (lower, upper) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenotype |
Pathological gambling |
Correlation |
|||||||||
| a | c | e | a | c | e | r a | r c | r e | |||
| Major depression | AE | Estimate | 0.56 | — | 0.44 | 0.83 | — | 0.17 | 0.14 | — | 0.31 |
| Lower CI | 0.45 | — | 0.32 | 0.74 | — | 0.14 | –0.04 | — | –0.01 | ||
| Upper CI | 0.67 | — | 0.55 | 0.88 | — | 0.26 | 0.30 | — | 0.58 | ||
| Alcohol consumption | ACE | Estimate | 0.29 | 0.53 | 0.18 | 0.69 | 0.14 | 0.17 | 0.41 | 1.00 | 0.20 |
| Lower CI | 0.14 | 0.48 | 0.17 | 0.44 | 0.03 | 0.10 | 0.07 | 0.55 | –0.04 | ||
| Upper CI | 0.38 | 0.66 | 0.24 | 0.83 | 0.37 | 0.24 | 0.73 | 1.00 | 0.43 | ||
| Alcohol consumption | ACE-AEa | Estimate | 0.37 | 0.44 | 0.19 | 0.83 | — | 0.17 | 0.80 | — | 0.07 |
| Lower CI | 0.22 | 0.29 | 0.15 | 0.74 | — | 0.12 | 0.61 | — | –0.17 | ||
| Upper CI | 0.53 | 0.56 | 0.24 | 0.88 | — | 0.26 | 1.00 | — | 0.29 | ||
| Caffeine consumption | ACE-AE | Estimate | 0.31 | 0.26 | 0.43 | 0.83 | — | 0.17 | 0.38 | — | –0.09 |
| Lower CI | 0.11 | 0.08 | 0.36 | 0.74 | — | 0.12 | 0.17 | — | –0.30 | ||
| Upper CI | 0.53 | 0.42 | 0.50 | 0.88 | — | 0.27 | 0.72 | — | 0.14 | ||
| Cigarette use | AE | Estimate | 0.88 | — | 0.12 | 0.81 | — | 0.19 | 0.55 | — | –0.06 |
| Lower CI | 0.81 | — | 0.08 | 0.72 | — | 0.13 | 0.43 | — | –0.41 | ||
| Upper CI | 0.52 | — | 0.18 | 0.88 | — | 0.28 | 0.67 | — | 0.31 | ||
| Nicotine dependence | ACE | Estimate | 0.46 | 0.36 | 0.18 | 0.64 | 0.18 | 0.18 | 0.29 | 1.00 | 0.29 |
| Lower CI | 0.16 | 0.08 | 0.12 | 0.37 | 0.01 | 0.12 | –0.16 | 0.45 | –0.10 | ||
| Upper CI | 0.76 | 0.59 | 0.29 | 0.83 | 0.41 | 0.29 | 0.63 | 1.00 | 0.61 | ||
| Nicotine dependence | ACE-AEa | Estimate | 0.64 | 0.18 | 0.18 | 0.81 | — | 0.19 | 0.57 | — | 0.19 |
| Lower CI | 0.32 | 0b | 0.12 | 0.72 | — | 0.12 | 0.40 | — | –0.19 | ||
| Upper CI | 0.88 | 0.43 | 0.28 | 0.88 | — | 0.28 | 0.86 | — | 0.55 | ||
a, Additive genetic effects; c, shared environmental effects; e, individual-specific environmental effects; ra, additive genetic correlation; rc, shared environmental correlation; re, individual-specific environmental correlation.
Second best-fit model included for comparison.
Actual 95 % CI for path was −0.66 to +0.66.
The genetic correlation between symptoms of DG and lifetime MD was modest (+0.14). By contrast, the genetic correlations between symptoms of DG and past-year caffeine use and lifetime heaviest cigarette use were substantially greater: +0.38 and +0.55 respectively.
The results were more complex for lifetime heaviest alcohol consumption and ND as the best-fit model included shared environmental effects for both the drug-related variables and symptoms of DG. In these models, the correlation between the common environmental effects (rc) must also be included thus giving two sources for familial/genetic effects shared between the two phenotypes. In both best-fit models, rc was estimated at +1.00; ra was estimated at +0.41 between DG and alcohol consumption; and +0.29 with DG and ND. For comparison with the other models, we also report results from the second best-fit model, which, as with caffeine consumption, contained no c2 effects for DG. In these models, where the shared familial/genetic effects for the two phenotypes was represented by only a single parameter, the genetic correlations involving symptoms of DG were substantial: +0.80 for heaviest alcohol consumption and +0.57 for ND.
The individual-specific environmental correlation (re) reflects the degree to which environmental experiences unique to the individual that impacted on symptoms of DG also affected the other trait. In general, these correlations were modest, with the strongest correlations (both negative and positive) being in the range from 0.25 to 0.30, suggesting that environmental experiences that increased risk for DG also increased risk for MD and ND and alcohol consumption, and modestly decreased the level of caffeine consumption.
Discussion
These analyses had four major objectives. We review the results in turn. In accord with previous clinical (Strong et al. 2004), general population (Strong & Kahler, 2007) and twin studies (Slutske et al. 2000, 2010), in our sample an exploratory factor analysis and a multiple threshold model indicated that a single latent dimension provided an excellent fit to the symptoms of DG, suggesting that DG exists on a continuum. These findings raise the question of how to determine a clinically useful, public health, sound diagnostic threshold for DG. This determination is crucial to establish the prevalence of DG, which varies from 0.42% when five criteria are required for diagnosis to 5.38% when only one criterion is required (Blanco et al. 2006). This determination is also essential to estimate the societal burden associated with DG, project treatment needs, determine eligibility for insurance reimbursement, and select scientifically valid inclusion criteria for research studies. In DSM-IV, five out of 10 criteria are required to make a diagnosis of PG. However, that cut-off was not determined empirically (Lesieur & Rosenthal, 1998), and leaves undiagnosed a large percentage of individuals with gambling problems. As a result, clinicians and researchers have developed alternative terminology (e.g. problem gambling, subclinical pathological gambling) to describe individuals with gambling problems who fall below the DSM-IV diagnostic threshold for PG, despite evidence of substantial economic losses, job difficulties, legal problems, significant levels of psychosocial impairment, increased co-morbidity rates, and decreased quality of life (Gerstein et al. 1999; Scherrer et al. 2005; Blanco et al. 2006). There is a need to determine empirically the optimal diagnostic threshold for DG.
A second, important finding of our study was that resemblance for the lifetime frequency of gambling was only modestly greater in MZ twins than the DZ and sibling pairs. Model fitting indicated that the familial resemblance for frequency of gambling resulted both from substantial common environmental effects that impacted equally on twin and sibling pairs and, to a slightly lesser degree, from genetic effects. Our results are intermediate between those of the National Merit Scholarship Qualifying Test twin study (Loehlin & Nichols, 1976), which was conducted in the USA in the early 1960s and suggested strong effects of shared environmental factors and absence of genetic influences on gambling participation, and the more recent OZ-GAM, conducted in Australia in the early 2000s (Slutske et al. 2009), which found strong genetic effects on gambling participation but almost no evidence of shared environmental factors. By contrast, when we examined symptoms of DG, resemblance was much greater in MZ twins than in DZ pairs or siblings. Model fitting showed that familial resemblance for DG could be explained by only genetic risk factors with fairly high heritability estimates, consistent with prior studies (Slutske et al. 2000, 2010).
These results for gambling frequency and symptoms of DG have interesting parallels with prior findings for psychoactive substance use and use disorders. That is, in most (van den Bree et al. 1998; Karkowski et al. 2000; Kendler et al. 2000; Hopfer et al. 2003), but not all (Kendler et al. 2006), twin studies, individual variation in substance use typically results from both common environmental and genetic risk factors. By contrast, in many (Kendler & Prescott, 1998; van den Bree et al. 1998; Kendler et al. 1999a, 2000), but not all, twin studies (Tsuang et al. 1996), individual variation in substance use disorders is entirely the result of genetic factors. Although certainly indirect, these results suggest that the pattern of familial influences on the frequency of gambling and gambling problems is similar to that seen with psychoactive substance use and use disorders. That is, familial–environmental influences are important for the frequency of the behavior. However, for the progression from frequency of use to disorder, shared environmental influences fall away and genetic factors become predominant. Differences in dopaminergic and serotonergic genes related to sensitivity to environmental cues, reward and impulsivity may partially mediate this increased vulnerability to DG (Ibanez et al. 2003). Alternatively, the effect of shared familial–environmental influences may decrease over time, and be able to impact exposure to, or initiation of, gambling behavior, which can happen at a very early age, but have much less influence on the onset of DG, which generally occurs in the mid-twenties or later in life (Kessler et al. 2008).
Our bivariate genetic modeling provides insights into the nature of the genetic risk factors for DG. Although consistent with prior studies (Petry et al. 2005; Kessler et al. 2008), we found an association between DG and MD (the one classic internalizing disorder assessed in this cohort); the genetic association between them was modest. Our results differ in part from those of Potenza et al. (2005), who found that DG and MD shared 34% of their genetic variance. However, Potenza et al. (2005) focused on fully syndromal DSM-IV PG rather than on the full range of DG. Inclusion in their analyses of individuals meeting less than five DSM-III-R diagnostic criteria for PG led to a much weaker association between DG and MD, suggesting that the percentage of shared genetic variance between these two disorders may increase with the severity of DG.
In contrast with the results on MD, we found much stronger evidence for genetic links between symptoms of DG, and substance use and ND, the only drug dependence measures in this survey. The results were most clear-cut for cigarette and caffeine use, where we found relatively strong (+0.55) and moderate (+0.38) genetic correlations respectively with DG. The results are somewhat more difficult to interpret for alcohol consumption and ND because of the confounding influences of shared environmental effects. However, the twin correlations for DG show very clearly no evidence for common environmental effects. As predicted by genetic theory, the MZ twin correlations were either equal to, or slightly greater than, twice the correlations observed for siblings and DZ twins respectively. We suspect that the common environmental effects seen for DG in the bivariate analyses with alcohol consumption and ND reflect a ‘bleeding over’ of these influences from the drug variables to DG. This would occur because the DG parameters are estimated with less precision than the drug-related variables because of their much greater rarity. We are therefore inclined to believe the reported genetic correlations from the second best-fitting models (that assumed only additive genetic and unique environmental effects on DG), which reported a high genetic correlation with ND (+0.57) and especially with heaviest lifetime alcohol consumption (+0.80). This interpretation of our results is consistent with the findings of Slutske et al. (2000), who also found that the co-morbidity of DG and alcohol dependence was explained exclusively by shared genetic variance. Regardless of whether the best- or second best-fitting model is preferred, our findings are consistent with the hypothesis that DG is more closely related genetically to externalizing than internalizing behaviors, as hypothesized previously (Blanco et al. 2001; Potenza et al. 2005; Petry, 2006).
The findings that gambling frequency resulted from both common environmental and genetic effects, whereas symptoms of DG could be explained by only genetic risk factors, and the findings of higher shared genetic variance of DG with substance use behaviors than with MD converge with prior findings documenting similarities between DG and substance use disorders. These include similarities in phenomenology, patterns of co-morbidity (Petry et al. 2005), neurocircuitry (Frascella et al. 2010) and treatment approaches (Petry et al. 2006; Wulfert et al. 2006; Grant et al. 2008). By contrast, similarities between DG and other internalizing disorders such as obsessive– compulsive disorder are much more limited (Blanco et al. 2001), suggesting that DG is probably noso-logically closer to the externalizing than to the internalizing behaviors. These findings have nosological, research and policy implications. From the nosological point of view, they support the inclusion of DG in DSM-V in the addictive disorders category, and raise the issue of commonalities and differences between substance use disorders and behavioral addictions. From the research point of view, these findings support the use of DG as a model for the study of addictive disorders without the confounding effects of psychoactive use that are unavoidable in studies of substance dependence. They also suggest that findings from the more mature field of substance use disorders can help guide research on the etiology of DG, and the development of treatment and prevention interventions for it. From the policy point of view, the similarities and classification of DG and substance use disorders under the same category raise the issue of which governmental agencies should be responsible for funding treatment and research on DG. They also raise the issue of how to regulate an activity whose addictive liability is substantially influenced by genetic factors.
A final, important result from our study was that the best-fitting univariate model for DG indicated that the proportion of genetic to individual-specific environmental influence was the same in men and women, in accord with a recent study (Slutske et al. 2010). We extended those findings by documenting that the best-fitting bivariate models and the models for frequency of gambling did not differ significantly in men and women. Overall, these findings suggest that even if the symptom patterns and course of DG differ across genders, its etiology is likely to be similar in men and women. However, because the lifetime prevalence of DG is two times greater in men than in women (Petry et al. 2005; Blanco et al. 2006; Kessler et al. 2008), the fact that the proportional contribution of genetic and individual-specific environmental factors on the DG is the same across genders implies that both factors increase the absolute risk of DG in men. There is a need to investigate why similar risk factors for DG have different effects on men and women.
Limitations
These results need to be interpreted in the context of six potential methodological limitations. First, although diverse, the sample of subjects completing the web-based assessment is not entirely representative of the general population. However, a recent review of the psychological literature suggests that fears about the unrepresentativeness of web-based samples are exaggerated (Gosling et al. 2004). Second, because the endorsement rate of symptoms of DG was relatively low, it was infeasible to conduct twin analyses of fully syndromal DG. Much of the power of our twin-sibling analyses therefore comes from subjects with subsyndromal levels of DG symptomatology, as in previous twin studies of DG (Slutske et al. 2000, 2010). However, also as in those twin studies, our multiple threshold models fitted the twin data well, suggesting that the four categories in our symptoms of DG measure reflected varying levels of severity of a single continuum of liability. Low endorsement rates also made it impossible to fit a twin model that would jointly examine frequency of lifetime gambling episodes and the developing of DG conditional upon high-frequency gambling (Kendler et al. 1999b). Third, we could not verify the identity of the twin pairs, so it is possible that subjects who were not twins could have participated. Several checks of the data did not suggest high rates of faking. Most convincingly, we included in our survey questions about height and weight and obtained the following correlations: height MZ +0.90, DZ +0.51; weight MZ +0.87, DZ +0.47. These results are comparable with those found in prior twin studies, as exemplified by the results from an epidemiological study in 2008 of more than 3300 Swedish twin pairs: height MZ +0.93, DZ +0.53; weight MZ +0.87, DZ +0.44 (Silventoinen et al. 2008).
Fourth, twin and sibling pairs had to cooperate actively to participate in this survey, each giving the same identifying code. This method could result in a bias in the twin pairs selected, with twins who were particularly close or similar more likely to participate. However, as noted earlier, review of twin and sibling resemblance for key traits such as height, weight and personality produced levels of resemblance similar to that seen previously in the literature (Kendler et al. 2009).
Fifth, the genetic correlations between DG symptoms and caffeine use may have been attenuated relative to that seen with alcohol and cigarette use because, for caffeine, we only assessed past year intake whereas for alcohol and cigarettes we assessed heaviest lifetime intake.
Sixth, our multiple threshold tests suggest that the liability to gamble frequently and the liability to develop gambling problems were on a single continuum. However, when we analyzed each of the traits separately, gambling frequency was influenced by both familial environmental and genetic factors whereas symptoms of DG were influenced only by genetic factors. With a very large twin sample, these results would be inconsistent. However, with the moderate sample available for our study and the relatively low power of the multiple threshold test, both results could be approximately true: that familial factors underlying gambling frequency and problems are closely related but familial–environmental factors are more important for the former than the latter.
Conclusions
Despite these limitations, this study contributes important information to our knowledge on DG. Our study found that whereas gambling participation is determined by shared environmental and genetic factors, DG seems to constitute a single latent dimension that is largely genetically determined. The genetic determinants of DG are predominantly shared with externalizing rather than internalizing behaviors and disorders, and converge with prior findings in supporting the consideration of DG as a behavioral addiction. Furthermore, these findings seem to be invariant across genders, suggesting that the etiological factors of DG are likely to be similar in men and women. Future research should continue to investigate the specific genes and environmental influences involved in the etiology of DG.
Acknowledgments
This research was supported in part by grants from the Institute for Research on Pathological Gambling and Related Disorders (K. S. Kendler) and the Borderline Personality Disorder Foundation (K. S. Kendler), National Institutes of Health (NIH) grants DA023200 and MH082773 (C. Blanco) and the New York State Psychiatric Institute (C. Blanco).
Appendix
Table A1.
Twin-sibling model-fitting results for bivariate models for symptoms of disordered gambling (DG) and measures of major depression (MD), alcohol, caffeine and cigarette use and nicotine dependence (ND)
| Sex effects |
||||||
|---|---|---|---|---|---|---|
| Model | Description | Qualitative | Quantitative | Δ-2LL | Δdf | ΔAIC |
| MD and symptoms of DG using twin pairs and non-twin sibling pairs | ||||||
| Ia | ACET | + | + | – | – | – |
| II | ACET | + | – | +19.801 | +10 | –0.199 |
| III | ACET | – | – | +19.827 | +11 | –2.173 |
| IV | ACE | – | – | +21.251 | +14 | –6.749 |
| V | CE | – | – | +47.086 | +17 | +13.086 |
| VI | AE | – | – | +22.132 | +17 | –11.868 |
| Alcohol and symptoms of DG using twin pairs and non-twin sibling pairs | ||||||
| Ib | ACET | + | + | – | – | – |
| II | ACET | + | – | +4.709 | +10 | –15.291 |
| III | ACET | – | – | +4.723 | +11 | –17.277 |
| IV | ACE | – | – | +5.766 | +14 | –22.234 |
| V | CE | – | – | +44.073 | +17 | +10.073 |
| VI | AE | – | – | +47.773 | +17 | +13.773 |
| VII | ACE-AE | – | – | +18.508 | +16 | –13.492 |
| Caffeine and symptoms of DG using twin pairs and non-twin sibling pairs | ||||||
| Ic | ACET | + | + | – | – | – |
| II | ACET | + | – | +21.704 | +10 | +1.704 |
| III | ACET | – | – | +21.708 | +11 | –0.292 |
| IV | ACE | – | – | +21.910 | +14 | –6.090 |
| V | CE | – | – | +51.753 | +17 | +17.753 |
| VI | AE | – | – | +31.488 | +17 | –2.512 |
| VII | ACE-AE | – | – | +23.589 | + 16 | –8.411 |
| Cigarettes and symptoms of DG using twin pairs and non-twin sibling pairs | ||||||
| Id | ACET | + | + | – | – | – |
| II | ACET | + | – | +10.738 | +10 | –9.262 |
| III | ACET | – | – | +12.233 | +11 | –9.767 |
| IV | ACE | – | – | +13.452 | +14 | –14.548 |
| V | CE | – | – | +57.175 | +17 | +23.175 |
| VI | AE | – | – | +18.310 | + 17 | –15.690 |
| ND and symptoms of DG using twin pairs and non-twin sibling pairs | ||||||
| Ie | ACET | + | + | – | – | – |
| II | ACET | + | – | +16.806 | +10 | –3.194 |
| III | ACET | – | – | +17.076 | +11 | –4.924 |
| IV | ACE | – | – | +18.100 | +14 | –9.900 |
| V | CE | – | – | +47.084 | +17 | +13.084 |
| VI | AE | – | – | +25.127 | +17 | –8.873 |
| VII | ACE-AE | – | – | +23.672 | +16 | –8.328 |
A, Additive genetic effects; C, shared environment; E, individual specific environment; T, special twin environment; df, degrees of freedom; AIC, Akaike's Information Criterion; LL, log likelihood.
–2LL =3621.396, df = 3474, AIC =–3326.604.
–2LL = 5476.595, df = 3445, AIC =–1413.405.
–2LL = 6493.133, df = 3469, AIC = –444.867.
–2LL= 3410.396, df = 3471, AIC= –3531.604.
–2LL = 3195.931, df = 3471, AIC = –3746.069.
Best-fit models are shown in bold.
Footnotes
Declaration of Interest
None.
References
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- APA . Diagnostic and Statistical Manual of Mental Disorders. 4th edn. American Psychiatric Association; Washington, DC.: 1994. [Google Scholar]
- Blanco C, Hasin DS, Petry N, Stinson FS, Grant BF. Sex differences in subclinical and DSM-IV pathological gambling: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychological Medicine. 2006;36:943–953. doi: 10.1017/S0033291706007410. [DOI] [PubMed] [Google Scholar]
- Blanco C, Moreyra P, Nunes EV, Saiz-Ruiz J, Ibanez A. Pathological gambling: addiction or compulsion? Seminars in Clinical Neuropsychiatry. 2001;6:167–176. doi: 10.1053/scnp.2001.22921. [DOI] [PubMed] [Google Scholar]
- Frascella J, Potenza MN, Brown LL, Childress AR. Shared brain vulnerabilities open the way for nonsubstance addictions : carving addiction at a new joint? Annals of the New York Academy of Science. 2010;1187:294–315. doi: 10.1111/j.1749-6632.2009.05420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein DR, Volberg RA, Toce MT, Harwood H, Johnson RA, Bule T, Christiansen E, Chuchro L, Cummings W, Engelman L, Hill MA, Hoffmann J, Larison C, Murphy SA, Palmer A, Sinclair S, Tucker A. Gambling Impact and Behavior Study: Report to the National Gambling Impact Study Commission. National Opinion Research Center at the University of Chicago; Chicago, IL.: 1999. [Google Scholar]
- Gosling SD, Vazire S, Srivastava S, John OP. Should we trust web-based studies? A comparative analysis of six preconceptions about Internet questionnaires. American Psychologist. 2004;59:93–104. doi: 10.1037/0003-066X.59.2.93. [DOI] [PubMed] [Google Scholar]
- Grant JE, Kim SW, Hartman BK. A double-blind, placebo-controlled study of the opiate antagonist naltrexone in the treatment of pathological gambling urges. Journal of Clinical Psychiatry. 2008;69:783–789. doi: 10.4088/jcp.v69n0511. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hopfer CJ, Crowley TJ, Hewitt JK. Review of twin and adoption studies of adolescent substance use. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:710–719. doi: 10.1097/01.CHI.0000046848.56865.54. [DOI] [PubMed] [Google Scholar]
- Ibanez A, Blanco C, Perez de Castro I, Fernandez-Piqueras J, Saiz-Ruiz J. Genetics of pathological gambling. Journal of Gambling Studies. 2003;19:11–22. doi: 10.1023/a:1021271029163. [DOI] [PubMed] [Google Scholar]
- James JE. Understanding Caffeine: A Biobehavioral Analysis. Sage Publications; Thousand Oaks, CA.: 1997. [Google Scholar]
- Karkowski LM, Prescott CA, Kendler KS. Multivariate assessment of factors influencing illicit substance use in twins from female-female pairs. American Journal of Medical Genetics. 2000;96:665–670. [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Tambs K, Reichborn-Kjennerud T. Illicit psychoactive substance use, abuse and dependence in a population-based sample of Norwegian twins. Psychological Medicine. 2006;36:955–962. doi: 10.1017/S0033291706007720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Corey LA, Prescott CA, Neale MC. Genetic and environmental risk factors in the aetiology of illicit drug initiation and subsequent misuse in women. British Journal of Psychiatry. 1999a;175:351–356. doi: 10.1192/bjp.175.4.351. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Archives of General Psychiatry. 2000;57:261–269. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers JM, Potter J, Opalesky J. A web-based study of personality, psychopathology and substance use in twin, other relative and relationship pairs. Twin Research and Human Genetics. 2009;12:137–141. doi: 10.1375/twin.12.2.137. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychological Medicine. 1999b;29:299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Pedersen N, Johnson L, Neale MC, Mathe AA. A pilot Swedish twin study of affective illness, including hospital- and population-ascertained subsamples. Archives of General Psychiatry. 1993;50:699–700. doi: 10.1001/archpsyc.1993.01820210033004. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Cannabis use, abuse, and dependence in a population-based sample of female twins. American Journal of Psychiatry. 1998;155:1016–1022. doi: 10.1176/ajp.155.8.1016. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Caffeine intake, tolerance, and withdrawal in women: a population-based twin study. American Journal of Psychiatry. 1999;156:223–228. doi: 10.1176/ajp.156.2.223. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Genes, Environment, and Psychopathology: Understanding the Causes of Psychiatric and Substance Use Disorders. Guilford Press; New York: 2006. [Google Scholar]
- Kessler RC, Hwang I, LaBrie R, Petukhova M, Sampson NA, Winters KC, Shaffer HJ. DSM-IV pathological gambling in the National Comorbidity Survey Replication. Psychological Medicine. 2008;38:1351–1360. doi: 10.1017/S0033291708002900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood DM, Weinstock J, Morasco BJ, Petry NM. Clinical features and treatment prognosis of pathological gamblers with and without recent gambling-related illegal behavior. Journal of the American Academy of Psychiatry and the Law. 2007;35:294–301. [PubMed] [Google Scholar]
- Lesieur HR, Rosenthal RJ. Analysis of pathological gambling. In DSM-IV Sourcebook. In: Widiger RA, Frances AJ, Pincus HA, Ross R, First MB, Davis D, Kline M, editors. Vol. 4. American Psychiatric Association; Washington, DC.: 1998. pp. 393–401. [Google Scholar]
- Loehlin JC, Nichols RC. Heredity, Environment and Personality: A Study of 850 Sets of Twins. University of Texas Press; Austin, TX.: 1976. [Google Scholar]
- Morasco BJ, Pietrzak RH, Blanco C, Grant BF, Hasin D, Petry NM. Health problems and medical utilization associated with gambling disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosomatic Medicine. 2006;68:976–984. doi: 10.1097/01.psy.0000238466.76172.cd. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. Department of Psychiatry, Virginia Commonwealth University Medical School; Box 980126, Richmond, VA.: 2003. [Google Scholar]
- Petry NM. Should the scope of addictive behaviors be broadened to include pathological gambling? Addiction. 2006;101(Suppl. 1):152–160. doi: 10.1111/j.1360-0443.2006.01593.x. [DOI] [PubMed] [Google Scholar]
- Petry NM, Ammerman Y, Bohl J, Doersch A, Gay H, Kadden R, Molina C, Steinberg K. Cognitive-behavioral therapy for pathological gamblers. Journal of Consulting and Clinical Psychology. 2006;74:555–567. doi: 10.1037/0022-006X.74.3.555. [DOI] [PubMed] [Google Scholar]
- Petry NM, Stinson FS, Grant BF. Comorbidity of DSM-IV pathological gambling and other psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry. 2005;66:564–574. doi: 10.4088/jcp.v66n0504. [DOI] [PubMed] [Google Scholar]
- Phillips DP, Welty WR, Smith MM. Elevated suicide levels associated with legalized gambling. Suicide and Life-Threatening Behavior. 1997;27:373–378. [PubMed] [Google Scholar]
- Potenza MN, Xian H, Shah K, Scherrer JF, Eisen SA. Shared genetic contributions to pathological gambling and major depression in men. Archives of General Psychiatry. 2005;62:1015–1021. doi: 10.1001/archpsyc.62.9.1015. [DOI] [PubMed] [Google Scholar]
- Reich T, James JW, Morris CA. The use of multiple thresholds in determining the mode of transmission of semi-continuous traits. Annals of Human Genetics. 1972;36:163–184. doi: 10.1111/j.1469-1809.1972.tb00767.x. [DOI] [PubMed] [Google Scholar]
- Scherrer JF, Xian H, Shah KR, Volberg R, Slutske W, Eisen SA. Effect of genes, environment, and lifetime co-occurring disorders on health-related quality of life in problem and pathological gamblers. Archives of General Psychiatry. 2005;62:677–683. doi: 10.1001/archpsyc.62.6.677. [DOI] [PubMed] [Google Scholar]
- Shaw MC, Forbush KT, Schlinder J, Rosenman E, Black DW. The effect of pathological gambling on families, marriages, and children. CNS Spectrums. 2007;12:615–622. doi: 10.1017/s1092852900021416. [DOI] [PubMed] [Google Scholar]
- Silventoinen K, Magnusson PKE, Tynelius P, Kaprio J, Rasmussen F. Heritability of body size and muscle strength in young adulthood: a study of one million Swedish men. Genetic Epidemiology. 2008;32:341–349. doi: 10.1002/gepi.20308. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Eisen S, True WR, Lyons MJ, Goldberg J, Tsuang M. Common genetic vulnerability for pathological gambling and alcohol dependence in men. Archives of General Psychiatry. 2000;57:666–673. doi: 10.1001/archpsyc.57.7.666. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Eisen S, Xian H, True WR, Lyons MJ, Goldberg J, Tsuang M. A twin study of the association between pathological gambling and antisocial personality disorder. Journal of Abnormal Psychology. 2001;110:297–308. doi: 10.1037//0021-843x.110.2.297. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Meier MH, Zhu G, Statham DJ, Blaszczynski A, Martin NG. The Australian Twin Study of Gambling (OZ-GAM): rationale, sample description, predictors of participation, and a first look at sources of individual differences in gambling involvement. Twin Research and Human Genetics. 2009;12:63–78. doi: 10.1375/twin.12.1.63. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Zhu G, Meier MH, Martin NG. Genetic and environmental influences on disordered gambling in men and women. Archives of General Psychiatry. 2010;67:624–630. doi: 10.1001/archgenpsychiatry.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchfield R. Reliability, validity, and classification accuracy of a measure of DSM-IV diagnostic criteria for pathological gambling. American Journal of Psychiatry. 2003;160:180–182. doi: 10.1176/appi.ajp.160.1.180. [DOI] [PubMed] [Google Scholar]
- Strong DR, Kahler CW. Evaluation of the continuum of gambling problems using the DSM-IV. Addiction. 2007;102:713–721. doi: 10.1111/j.1360-0443.2007.01789.x. [DOI] [PubMed] [Google Scholar]
- Strong DR, Lesieur HR, Breen RB, Stinchfield R, Lejuez CW. Using a Rasch model to examine the utility of the South Oaks Gambling Screen across clinical and community samples. Addictive Behaviors. 2004;29:465–481. doi: 10.1016/j.addbeh.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, Meyer JM, Toomey R, Faraone SV, Eaves L. Genetic influences on DSM-III-R drug abuse and dependence: a study of 3,372 twin pairs. American Journal of Medical Genetics. 1996;67:473–477. doi: 10.1002/(SICI)1096-8628(19960920)67:5<473::AID-AJMG6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- van den Bree MB, Johnson EO, Neale MC, Pickens RW. Genetic and environmental influences on drug use and abuse/dependence in male and female twins. Drug and Alcohol Dependence. 1998;52:231–241. doi: 10.1016/s0376-8716(98)00101-x. [DOI] [PubMed] [Google Scholar]
- Williams L, Holahan P. Parsimony-based fit indices for multiple-indicator models: do they work? Structural Equation Modeling. 1994;1:161–189. [Google Scholar]
- Wulfert E, Blanchard EB, Freidenberg BM, Martell RS. Retaining pathological gamblers in cognitive behavior therapy through motivational enhancement: a pilot study. Behavior Modification. 2006;30:315–340. doi: 10.1177/0145445503262578. [DOI] [PubMed] [Google Scholar]


