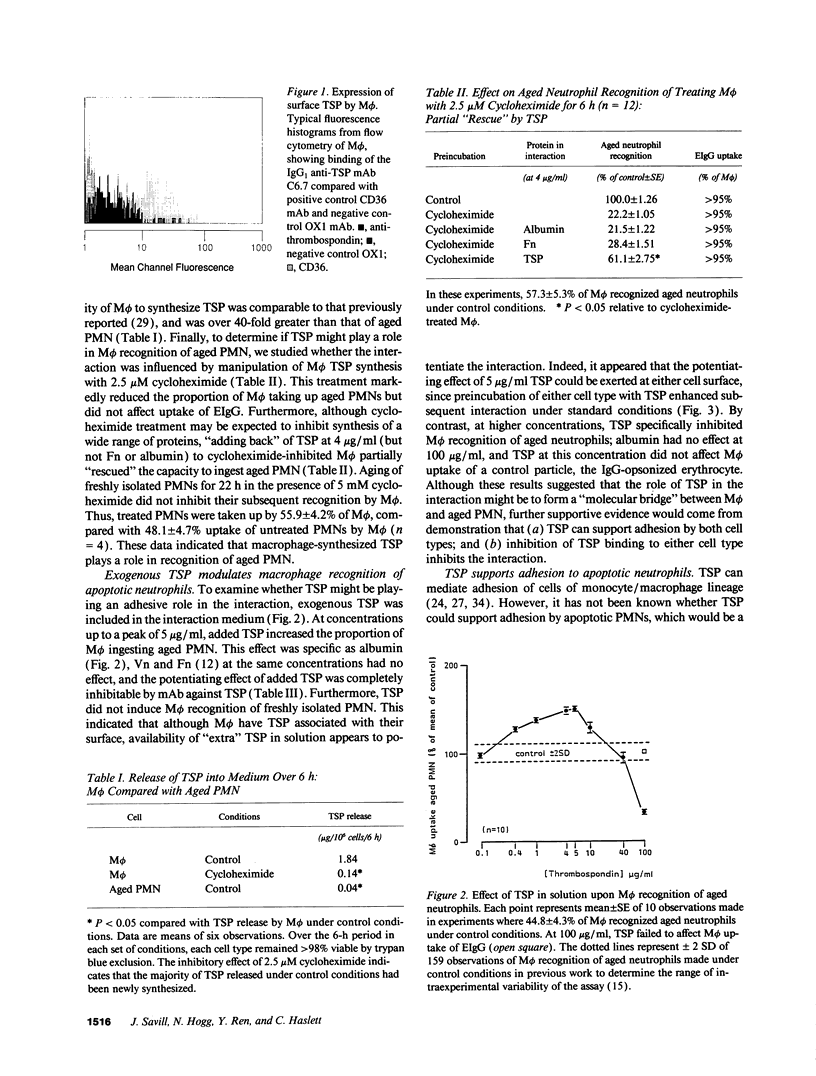
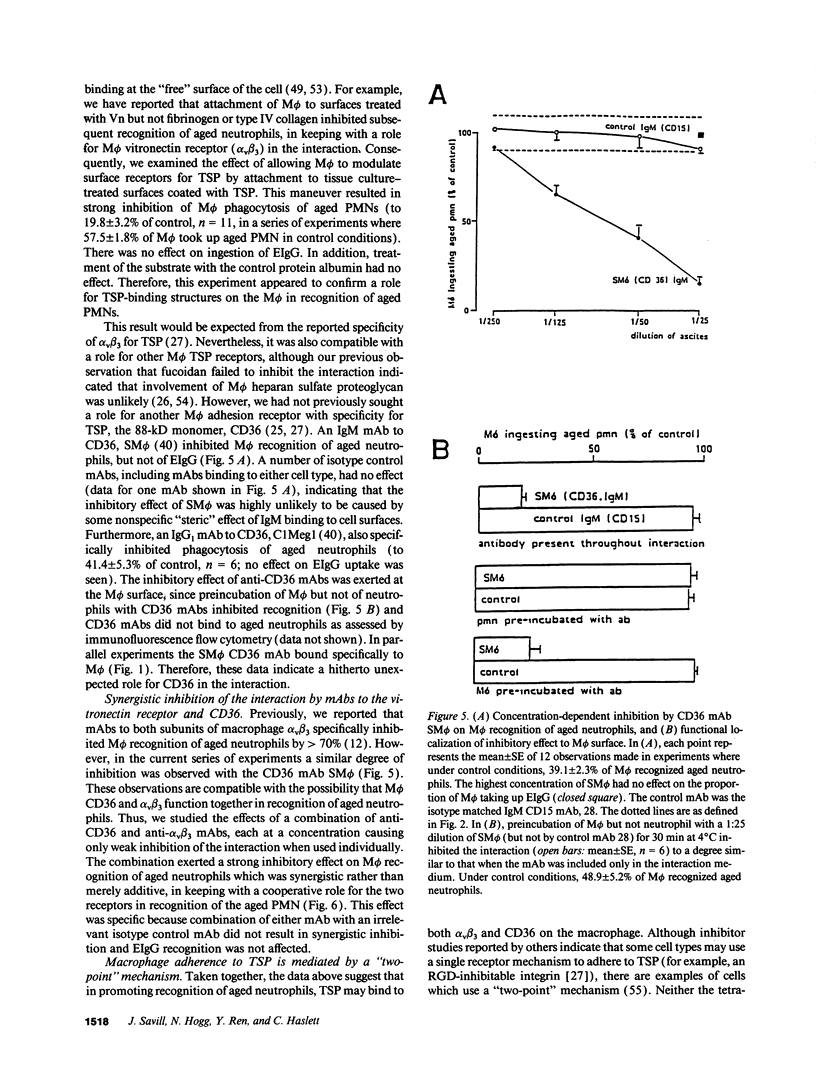
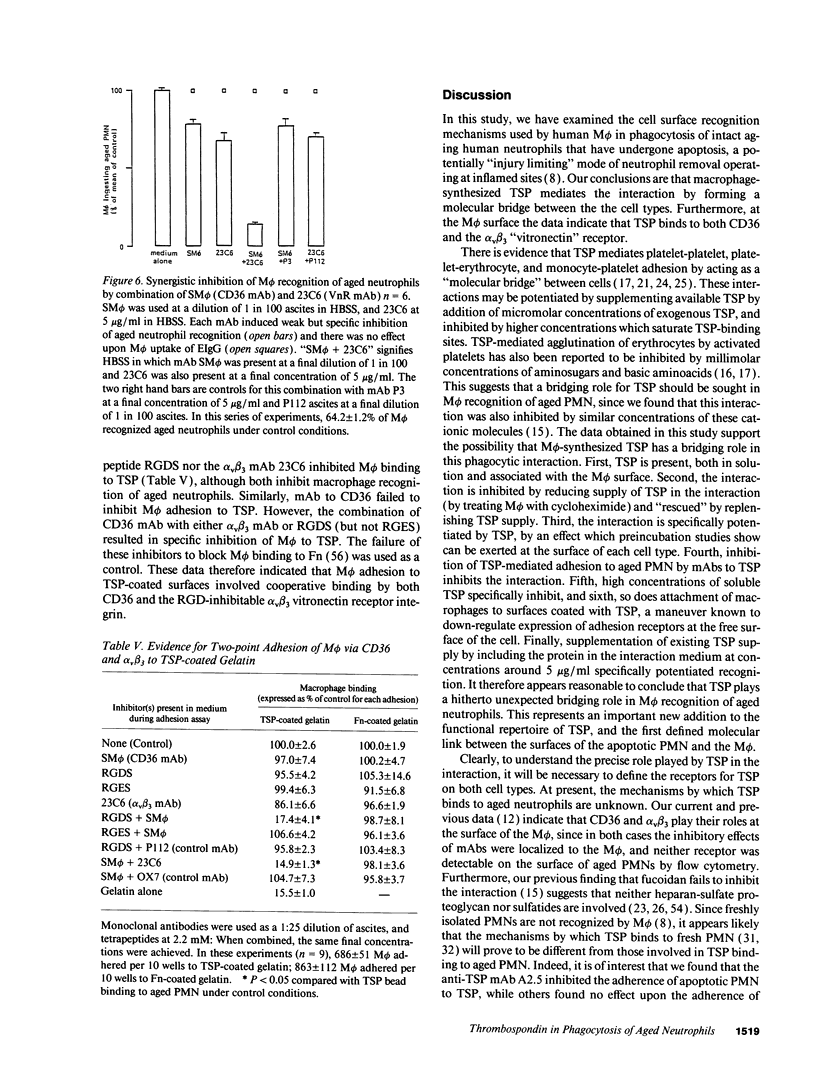
Abstract
We have investigated the cell surface recognition mechanisms used by human monocyte-derived macrophages (M phi) in phagocytosis of intact aging human neutrophils (PMNs) undergoing apoptosis. This study shows that the adhesive protein thrombospondin (TSP) was present in the interaction, both associated with the M phi surface and in solution at a mean concentration of 0.59 micrograms/ml. The interaction was inhibited by treatment of M phi (but not aged PMN) with cycloheximide, but could be "rescued" by replenishment with exogenous TSP. Under control conditions, M phi recognition of aged PMNs was specifically potentiated by purified platelet TSP at 5 micrograms/ml, present either in the interaction or if preincubated with either cell type, suggesting that TSP might act as a "molecular bridge" between the two cell types. In support, both aged PMN and M phi were found to adhere to TSP, and phagocytosis of aged PMN was specifically inhibited by (a) excess soluble TSP; (b) antibodies to TSP that also inhibit TSP-mediated adhesion to aged PMN; and (c) down-regulation of M phi receptors for TSP by plating M phi on TSP-coated surfaces. Furthermore, inhibition with mAbs/Arg-Gly-Asp-Ser peptide of the candidate M phi receptors for TSP, CD36, and alpha v beta 3 exerted synergistic effects on both M phi recognition of aged PMN and M phi adhesion to TSP, indicating that "two point" adhesion of TSP to these M phi structures is involved in phagocytosis of aged PMN. Our findings indicate newly defined roles for TSP and CD36 in phagocytic clearance of senescent neutrophils, which may limit inflammatory tissue injury and promote resolution.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asch A. S., Barnwell J., Silverstein R. L., Nachman R. L. Isolation of the thrombospondin membrane receptor. J Clin Invest. 1987 Apr;79(4):1054–1061. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch A. S., Tepler J., Silbiger S., Nachman R. L. Cellular attachment to thrombospondin. Cooperative interactions between receptor systems. J Biol Chem. 1991 Jan 25;266(3):1740–1745. [PubMed] [Google Scholar]
- Bai Y., Durbin H., Hogg N. Monoclonal antibodies specific for platelet glycoproteins react with human monocytes. Blood. 1984 Jul;64(1):139–146. [PubMed] [Google Scholar]
- Bevilacqua M. P., Amrani D., Mosesson M. W., Bianco C. Receptors for cold-insoluble globulin (plasma fibronectin) on human monocytes. J Exp Med. 1981 Jan 1;153(1):42–60. doi: 10.1084/jem.153.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapes S. K., Haskill S. Evidence for granulocyte-mediated macrophage activation after C. parvum immunization. Cell Immunol. 1983 Feb 1;75(2):367–377. doi: 10.1016/0008-8749(83)90334-9. [DOI] [PubMed] [Google Scholar]
- Charo I. F., Bekeart L. S., Phillips D. R. Platelet glycoprotein IIb-IIIa-like proteins mediate endothelial cell attachment to adhesive proteins and the extracellular matrix. J Biol Chem. 1987 Jul 25;262(21):9935–9938. [PubMed] [Google Scholar]
- Charo I. F., Nannizzi L., Smith J. W., Cheresh D. A. The vitronectin receptor alpha v beta 3 binds fibronectin and acts in concert with alpha 5 beta 1 in promoting cellular attachment and spreading on fibronectin. J Cell Biol. 1990 Dec;111(6 Pt 1):2795–2800. doi: 10.1083/jcb.111.6.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J. Programmed cell death in the immune system. Adv Immunol. 1991;50:55–85. doi: 10.1016/s0065-2776(08)60822-6. [DOI] [PubMed] [Google Scholar]
- Conforti G., Zanetti A., Pasquali-Ronchetti I., Quaglino D., Jr, Neyroz P., Dejana E. Modulation of vitronectin receptor binding by membrane lipid composition. J Biol Chem. 1990 Mar 5;265(7):4011–4019. [PubMed] [Google Scholar]
- Davies J., Warwick J., Totty N., Philp R., Helfrich M., Horton M. The osteoclast functional antigen, implicated in the regulation of bone resorption, is biochemically related to the vitronectin receptor. J Cell Biol. 1989 Oct;109(4 Pt 1):1817–1826. doi: 10.1083/jcb.109.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit V. M., Galvin N. J., O'Rourke K. M., Frazier W. A. Monoclonal antibodies that recognize calcium-dependent structures of human thrombospondin. Characterization and mapping of their epitopes. J Biol Chem. 1986 Feb 5;261(4):1962–1968. [PubMed] [Google Scholar]
- Dixit V. M., Haverstick D. M., O'Rourke K. M., Hennessy S. W., Grant G. A., Santoro S. A., Frazier W. A. A monoclonal antibody against human thrombospondin inhibits platelet aggregation. Proc Natl Acad Sci U S A. 1985 May;82(10):3472–3476. doi: 10.1073/pnas.82.10.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty D. E., Haslett C., Tonnesen M. G., Henson P. M. Human monocyte adherence: a primary effect of chemotactic factors on the monocyte to stimulate adherence to human endothelium. J Immunol. 1987 Mar 15;138(6):1762–1771. [PubMed] [Google Scholar]
- Frazier W. A. Thrombospondin: a modular adhesive glycoprotein of platelets and nucleated cells. J Cell Biol. 1987 Aug;105(2):625–632. doi: 10.1083/jcb.105.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner T. K., Williams D. C., Minion F. C., Phillips D. R. Thrombin-induced platelet aggregation is mediated by a platelet plasma membrane-bound lectin. Science. 1978 Jun 16;200(4347):1281–1283. doi: 10.1126/science.663608. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast receptor for cell-substratum adhesion: studies on the interaction of baby hamster kidney cells with latex beads coated by cold insoluble globulin (plasma fibronectin). J Cell Biol. 1980 Jul;86(1):104–112. doi: 10.1083/jcb.86.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslett C., Guthrie L. A., Kopaniak M. M., Johnston R. B., Jr, Henson P. M. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985 Apr;119(1):101–110. [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Johnston R. B., Jr Tissue injury in inflammation. Oxidants, proteinases, and cationic proteins. J Clin Invest. 1987 Mar;79(3):669–674. doi: 10.1172/JCI112869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg N., MacDonald S., Slusarenko M., Beverley P. C. Monoclonal antibodies specific for human monocytes, granulocytes and endothelium. Immunology. 1984 Dec;53(4):753–767. [PMC free article] [PubMed] [Google Scholar]
- Idell S., Maunder R., Fein A. M., Switalska H. I., Tuszynski G. P., McLarty J., Niewiarowski S. Platelet-specific alpha-granule proteins and thrombospondin in bronchoalveolar lavage in the adult respiratory distress syndrome. Chest. 1989 Nov;96(5):1125–1132. doi: 10.1378/chest.96.5.1125. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Leung L. L., Nachman R. L., Levin R. I., Mosher D. F. Thrombospondin is the endogenous lectin of human platelets. Nature. 1982 Jan 21;295(5846):246–248. doi: 10.1038/295246a0. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Ruggiero J. T., Falcone D. J. Monocytes and macrophages synthesize and secrete thrombospondin. Blood. 1985 Jan;65(1):79–84. [PubMed] [Google Scholar]
- Kreis C., La Fleur M., Ménard C., Paquin R., Beaulieu A. D. Thrombospondin and fibronectin are synthesized by neutrophils in human inflammatory joint disease and in a rabbit model of in vivo neutrophil activation. J Immunol. 1989 Sep 15;143(6):1961–1968. [PubMed] [Google Scholar]
- Krissansen G. W., Elliott M. J., Lucas C. M., Stomski F. C., Berndt M. C., Cheresh D. A., Lopez A. F., Burns G. F. Identification of a novel integrin beta subunit expressed on cultured monocytes (macrophages). Evidence that one alpha subunit can associate with multiple beta subunits. J Biol Chem. 1990 Jan 15;265(2):823–830. [PubMed] [Google Scholar]
- Lawler J. W., Slayter H. S., Coligan J. E. Isolation and characterization of a high molecular weight glycoprotein from human blood platelets. J Biol Chem. 1978 Dec 10;253(23):8609–8616. [PubMed] [Google Scholar]
- Lawler J., Hynes R. O. The structure of human thrombospondin, an adhesive glycoprotein with multiple calcium-binding sites and homologies with several different proteins. J Cell Biol. 1986 Nov;103(5):1635–1648. doi: 10.1083/jcb.103.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J. The structural and functional properties of thrombospondin. Blood. 1986 May;67(5):1197–1209. [PubMed] [Google Scholar]
- Lawler J., Weinstein R., Hynes R. O. Cell attachment to thrombospondin: the role of ARG-GLY-ASP, calcium, and integrin receptors. J Cell Biol. 1988 Dec;107(6 Pt 1):2351–2361. doi: 10.1083/jcb.107.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack R. A., Goodman L. V., Dixit V. M. Cell surface thrombospondin is functionally essential for vascular smooth muscle cell proliferation. J Cell Biol. 1988 Feb;106(2):415–422. doi: 10.1083/jcb.106.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malech H. L., Gallin J. I. Current concepts: immunology. Neutrophils in human diseases. N Engl J Med. 1987 Sep 10;317(11):687–694. doi: 10.1056/NEJM198709103171107. [DOI] [PubMed] [Google Scholar]
- Mansfield P. J., Boxer L. A., Suchard S. J. Thrombospondin stimulates motility of human neutrophils. J Cell Biol. 1990 Dec;111(6 Pt 2):3077–3086. doi: 10.1083/jcb.111.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor J. L., Catimel B., Parmentier S., Clezardin P., Dechavanne M., Leung L. L. Rapid purification and partial characterization of human platelet glycoprotein IIIb. Interaction with thrombospondin and its role in platelet aggregation. J Biol Chem. 1989 Jan 5;264(1):501–506. [PubMed] [Google Scholar]
- Michl J., Pieczonka M. M., Unkeless J. C., Silverstein S. C. Effects of immobilized immune complexes on Fc- and complement-receptor function in resident and thioglycollate-elicited mouse peritoneal macrophages. J Exp Med. 1979 Sep 19;150(3):607–621. doi: 10.1084/jem.150.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganelli P. M., Guyre P. M. IFN-gamma plus glucocorticoids stimulate the expression of a newly identified human mononuclear phagocyte-specific antigen. J Immunol. 1988 Apr 1;140(7):2296–2304. [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Mosher D. F. Interactions of thrombospondin with endothelial cells: receptor-mediated binding and degradation. J Cell Biol. 1987 Oct;105(4):1603–1611. doi: 10.1083/jcb.105.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musson R. A. Human serum induces maturation of human monocytes in vitro. Changes in cytolytic activity, intracellular lysosomal enzymes, and nonspecific esterase activity. Am J Pathol. 1983 Jun;111(3):331–340. [PMC free article] [PubMed] [Google Scholar]
- Newman S. L., Henson J. E., Henson P. M. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J Exp Med. 1982 Aug 1;156(2):430–442. doi: 10.1084/jem.156.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo P., Hundt E., Lawler J., Seed B. CD36 directly mediates cytoadherence of Plasmodium falciparum parasitized erythrocytes. Cell. 1989 Jul 14;58(1):95–101. doi: 10.1016/0092-8674(89)90406-6. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Hayman E. G., Ruoslahti E. Location of the cell-attachment site in fibronectin with monoclonal antibodies and proteolytic fragments of the molecule. Cell. 1981 Oct;26(2 Pt 2):259–267. doi: 10.1016/0092-8674(81)90308-1. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ginsberg M. H., Plow E. F., Ruoslahti E. Platelet membrane glycoprotein IIb/IIIa: member of a family of Arg-Gly-Asp--specific adhesion receptors. Science. 1986 Mar 28;231(4745):1559–1562. doi: 10.1126/science.2420006. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. A 125/115-kDa cell surface receptor specific for vitronectin interacts with the arginine-glycine-aspartic acid adhesion sequence derived from fibronectin. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5766–5770. doi: 10.1073/pnas.82.17.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raugi G. J., Olerud J. E., Gown A. M. Thrombospondin in early human wound tissue. J Invest Dermatol. 1987 Dec;89(6):551–554. doi: 10.1111/1523-1747.ep12461198. [DOI] [PubMed] [Google Scholar]
- Riser B. L., Mitra R., Perry D., Dixit V., Varani J. Monocyte killing of human squamous epithelial cells: role for thrombospondin. Cancer Res. 1989 Nov 1;49(21):6123–6129. [PubMed] [Google Scholar]
- Roberts D. D., Haverstick D. M., Dixit V. M., Frazier W. A., Santoro S. A., Ginsburg V. The platelet glycoprotein thrombospondin binds specifically to sulfated glycolipids. J Biol Chem. 1985 Aug 5;260(16):9405–9411. [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Sanui H., Yoshida S., Nomoto K., Ohhara R., Adachi Y. Peritoneal macrophages which phagocytose autologous polymorphonuclear leucocytes in guinea-pigs. I: induction by irritants and microorgansisms and inhibition by colchicine. Br J Exp Pathol. 1982 Jun;63(3):278–284. [PMC free article] [PubMed] [Google Scholar]
- Savill J. S., Henson P. M., Haslett C. Phagocytosis of aged human neutrophils by macrophages is mediated by a novel "charge-sensitive" recognition mechanism. J Clin Invest. 1989 Nov;84(5):1518–1527. doi: 10.1172/JCI114328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J. S., Wyllie A. H., Henson J. E., Walport M. J., Henson P. M., Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989 Mar;83(3):865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J., Dransfield I., Hogg N., Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990 Jan 11;343(6254):170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- Silverstein R. L., Asch A. S., Nachman R. L. Glycoprotein IV mediates thrombospondin-dependent platelet-monocyte and platelet-U937 cell adhesion. J Clin Invest. 1989 Aug;84(2):546–552. doi: 10.1172/JCI114197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein R. L., Leung L. L., Nachman R. L. Thrombospondin: a versatile multifunctional glycoprotein. Arteriosclerosis. 1986 May-Jun;6(3):245–253. doi: 10.1161/01.atv.6.3.245. [DOI] [PubMed] [Google Scholar]
- Silverstein R. L., Nachman R. L. Thrombospondin binds to monocytes-macrophages and mediates platelet-monocyte adhesion. J Clin Invest. 1987 Mar;79(3):867–874. doi: 10.1172/JCI112896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchard S. J., Burton M. J., Dixit V. M., Boxer L. A. Human neutrophil adherence to thrombospondin occurs through a CD11/CD18-independent mechanism. J Immunol. 1991 Jun 1;146(11):3945–3952. [PubMed] [Google Scholar]
- Sun X., Mosher D. F. Ca2(+)-sensitive binding of thrombospondin to U937 cells is due to the formation of calcium precipitate in the binding medium. J Clin Invest. 1991 Jan;87(1):171–176. doi: 10.1172/JCI114967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Mosher D. F., Rapraeger A. Heparan sulfate-mediated binding of epithelial cell surface proteoglycan to thrombospondin. J Biol Chem. 1989 Feb 15;264(5):2885–2889. [PubMed] [Google Scholar]
- Varani J., Stoolman L., Wang T., Schuger L., Flippen C., Dame M., Johnson K. J., Todd R. F., 3rd, Ryan U. S., Ward P. A. Thrombospondin production and thrombospondin-mediated adhesion in U937 cells. Exp Cell Res. 1991 Jul;195(1):177–182. doi: 10.1016/0014-4827(91)90514-u. [DOI] [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Wright S. D. Methods for the study of receptor-mediated phagocytosis. Methods Enzymol. 1986;132:204–221. doi: 10.1016/s0076-6879(86)32009-3. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Meyer B. C. Fibronectin receptor of human macrophages recognizes the sequence Arg-Gly-Asp-Ser. J Exp Med. 1985 Aug 1;162(2):762–767. doi: 10.1084/jem.162.2.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. The major cell surface glycoprotein of chick embryo fibroblasts is an agglutinin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3158–3162. doi: 10.1073/pnas.72.8.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]




