Abstract
Theory of mind (ToM) is an aspect of social cognition that refers to the ability to make inferences about the thoughts, feelings, and intentions of other people. It is believed to be related to social functioning. Previous investigations of ToM in schizotypy have yielded mixed results. Using a correlational approach, the present study explored the relationship between schizotypal traits, ToM, neurocognition, depressed mood, and social functioning in a sample of 50 undergraduate students. Schizotypy was related to poor social functioning. Contrary to predictions, schizotypal traits were not associated with impaired ToM. In fact, schizotypal traits were associated with enhanced performance on a ToM task that involved detection of ironic statements. However, strong relationships emerged among schizotypy, depressed mood, and social functioning, highlighting the need to also examine depression when assessing the relations between elevated schizotypy and poor social functioning.
Keywords: Schizotypy, neurocognition, social cognition, social functioning, depression
Social cognition refers to cognitive processes associated with the perception, interpretation, and manipulation of social information (Green et al., 2005; Penn et al., 1997). There is evidence that social cognition mediates the relationship between neuropsychological impairments and poor social and occupational functioning in persons with schizophrenia (e.g., Addington et al., 2006, 2010; Bowie et al., 2008; Sergi et al., 2006). Theory of mind (ToM) is an aspect of social cognition that refers to the ability to make inferences about the thoughts, feelings, and intentions of other people. Individuals with schizophrenia have demonstrated inaccurate ToM in experimental paradigms (e.g., Brüne and Bodenstein, 2005; Corcoran et al., 1995; Craig et al., 2004; Herold et al., 2002; Langdon et al., 2002; Pickup and Frith, 2001; Sarfati et al., 1999). ToM disruptions are hypothesized to play a role in the etiology and maintenance of psychotic symptoms such as disordered speech and delusions (Brüne, 2005). In addition, ToM impairments may have a direct negative impact on the social functioning of individuals with schizophrenia because of misperception of the intentions of others. As with other neurocognitive and social cognitive deficits, ToM impairment may precede the onset of psychotic symptoms and be present in those with schizotypal personality traits. However, relatively few studies have examined ToM in nonclinical schizotypy. Moreover, within these studies, the findings have been mixed, and the methodology has been limited in some ways, including failure to control for potential confounding variables such as general intellectual ability (e.g., Meyer and Shean, 2006), and ceiling effects for ToM tasks (e.g., Pickup, 2006).
Associations between schizotypal traits in nonclinical adults and impaired performance on various ToM tasks have been reported in the literature (Henry et al., 2008; Langdon and Coltheart, 1999, 2004; Meyer and Shean, 2006; Pickup, 2006), although Jahshan and Sergi (2007) found no such association. Versmissen et al. (2008) found impaired ToM in schizophrenia patients and a sample of nonschizophrenic first-degree relatives; however, ToM task performance of a nonclinical psychometrically identified schizotypy group did not differ from that of a nonclinical control group with lower levels of schizotypy. Versmissen et al. (2008) suggest that their findings support a continuum conceptualization of impairment associated with psychosis-proneness, whereby degree of ToM impairment escalates with increased genetic vulnerability.
Previous research suggests that social functioning impairments are present in nonclinical schizotypy (Claridge, 1997; Henry et al., 2008; Jahshan and Sergi, 2007). However, there is mixed evidence regarding whether poor social functioning in schizotypy is related to social cognitive deficits (e.g., ToM impairment). The results of one study suggest that social functioning impairment is not related to ToM in schizotypy (Jahshan and Sergi, 2007). In this particular study, when extremely high and extremely low scorers on a schizotypy scale were compared on several measures, no group differences for ToM were found. However, the high schizotypy group did demonstrate poorer social functioning. A potential limitation of that study was that levels of depression were not assessed in the sample. Considering the frequent co-occurrence of schizotypy and depression (Lewandowski et al., 2006; Verdoux et al., 1999) and the well-established relationship between depression and poor social functioning (Hirschfeld et al., 2000), depressed mood is a potential contributor to the relationship between schizotypy and social functioning. Henry et al. (2008) found that negative schizotypy (e.g., social anhedonia, blunted affect) was associated with impaired social functioning even after controlling for depression. Moreover, ToM partially mediated the relationship between schizotypy and social functioning in the sample. Given these conflicting findings, the relationship between ToM and social functioning in schizotypy is unclear.
The aims of the present study were to examine the validity of the findings of associations between schizotypy and poor social functioning and to investigate whether ToM impairment mediates this relationship beyond any contribution of depressed mood. Specifically, it was hypothesized that the endorsement of schizotypal traits would be associated with poor social functioning, ToM impairments, and depressed mood. Furthermore, it was hypothesized that ToM impairments would partially mediate the relationship between schizotypal traits and social functioning beyond the contribution of depression. Finally, tests of general intellectual ability, executive functions, and working memory were included to allow for consideration of potential neurocognitive correlates of ToM task performance, social functioning, and schizotypal traits.
METHODS
Participants
Fifty undergraduate subjects—30 (60%) women and 20 men-participated in the study in exchange for course credit. Most of the sample were in their first year of college (n = 37; 74%), were white (n = 43; 86%), and had a mean age of 20 years (range, 18 to 26 years; SD, 2 years). All eligible volunteers were enrolled; exclusion criteria were minimal and included a history of head injury resulting in loss of consciousness and seizure disorder.
Procedure
Participants were assessed individually in a single testing session approximately 3 hours in duration. The study was approved by the university institutional review board, and informed consent was obtained from each participant before testing.
Measures
Schizotypy
The Oxford-Liverpool Inventory of Feelings and Experiences (O-LIFE) (Mason et al., 1995) is a 104-item yes/no self-report multidimensional measure of schizotypal traits that was developed in the context of a four-factor (positive, negative, disorganization, impulsivity/ disinhibition) dimensional model of schizotypy. The O-LIFE has been demonstrated to be a reliable and valid measure of schizotypy (Mason et al., 1995; Mason and Claridge, 2006). It consists of four subscales: unusual experiences (UE; positive schizotypy), introvertive anhedonia (IA; negative schizotypy), cognitive disorganization (CD; disorganized schizotypy), and impulsive nonconformity (IN; disinhibition, emotion dysregulation). Items within each subscale are summed to obtain a scale score; higher scores are indicative of higher levels of schizotypy. In the current sample, internal consistency was α = 0.93 for O-LIFE total score, α = 0.96 for CD, α = 0.88 for UE, α = 0.74 for IN, and α = 0.60 for IA.
Depression
Levels of depression during the 2 weeks before testing session were assessed using the Beck Depression Inventory II (BDI-II; Beck et al., 1996), a self-report measure of depressive symptoms. The BDIII contains 21 items that are rated from 0 to 3. Higher total BDI-II score indicates more severe depressive symptoms. For the current sample, internal consistency for the BDI-II was α = 0.89.
Neurocognition
General intellectual ability was estimated using the Shipley Institute of Living Scale (SILS; Shipley, 1940; Zachary, 1986).Working memory was assessed using the Letter-Number Sequencing Task (Weschler, 1997), and executive functioning was assessed using the computerized version of the Wisconsin Card Sort Test–64 card version (WCST-64; Kongs et al., 2000a, 2000b).
Theory of Mind
Because of the mixed findings in previous investigations of ToM in schizotypy, three measures of ToM were used. For the strange stories task (Fletcher et al., 1995), the subjects read short stories and were asked to make inferences about the story characters’ thoughts, feelings, or intentions. ToM stories involve deception, “white lies,” and misunderstandings between story characters, and control stories require the subject to make physical cause-and-effect inferences. Eight stories of each type (i.e., ToM and physical control) were presented to participants, and reading time for each story was recorded. Participants’ responses were digitally recorded for transcription. Responses were scored as 0, 1, or 2 according to criteria supplied by Happé, yielding scores ranging from 0 to 16, with higher scores reflecting better ToM. For the current sample, internal consistency for the strange stories task was α = 0.69.
For the irony perception task (72-item version; Langdon et al., 2002), the participants were asked to decide whether the statements made by characters in short vignettes “make sense.” Statements are literal (n = 12), metaphorical (n = 12), or ironic (n = 12). Nonsense items (n = 36) were created by pairing statements with unrelated stories. Participants simply answer “yes” or “no” after reading each vignette. Responses were subjected to signal detection analysis to calculate sensitivity (A’) and bias (B”) scores for each response type (i.e., literal, metaphor, ironic). For the current sample, internal consistency for the irony perception task was α = 0.85.
For the eyes task (Baron-Cohen et al., 2001), the participants were asked to identify what the individual in each of 36 photographs is thinking or feeling based only on pictures of their eyes. Each photograph has four response options, and the participants are asked to select which response best describes the photographed individual’s mental state. Definitions for each response option were available to participants. For the current sample, internal consistency for the eyes task was α = 0.66. For all ToM measures, higher scores are indicative of better ToM abilities.
Social Functioning
Social functioning was assessed using two self-report measures. The Social Adjustment Scale–self-report (SAS-SR; Weismann and Bothwell, 1976) measures functioning in academic performance and peer and family relationships during the past 2 weeks. Following the method of Jahshan and Sergi (2007), data from items assessing peer social functioning, academic social functioning, and family social functioning were averaged to create three scale scores, with higher scores indicative of worse social functioning. Internal consistency for the SAS-SR peer, academic, and family social functioning items for the current sample was α = 0.64. Life satisfaction was assessed using the Quality of Life Inventory (QOLI; Frisch, 1994), a scale created for use with healthy adults that measures satisfaction across 16 life areas and yields a total weighted satisfaction score. For the current sample, internal consistency for the QOLI total weighted satisfaction score was α = 0.82.
Data Analysis
Correlations were used to examine the relationships among study variables, and linear regressions and mediation analyses were used to test these relationships further. The impact of demographic and cognitive factors was examined using t-tests and correlations, and these variables were considered in subsequent analyses as needed.
RESULTS
Descriptive Analyses and Identification of Covariates
Descriptive data for all measures are presented in Table 1. The full range of possible scores for the O-LIFE and each of its subscales were represented, with the exception of IA, which had a somewhat restricted range. Sex effects were evident for the CD subscale of the O-LIFE (t(48) = −2.86, p = 0.01), with the women attaining higher scores than the men. This difference was of the magnitude reported by Mason et al. (1995) in their normative sample. Sex differences were also evident for the peer relationships subscale of the SAS-SR (xmen = 1.62, SD = 0.37; xwomen = 1.87, SD = 0.34; t(48) = −2.48, p = 0.02), in which the women achieved higher scores than the men. Therefore, data for men and women were analyzed separately in subsequent regression analyses involving SAS-SR peer social functioning.
TABLE 1.
Descriptive Data (N = 50)
| Mean (SD) | Range | |
|---|---|---|
| Schizotypy | ||
| UE | 9.98 (6.30) | 0–28 |
| IA | 3.38 (2.62) | 0–11 |
| CD | 10.66 (6.75) | 0–22 |
| IN | 8.54 (3.91) | 1–18 |
| O-LIFE total score | 32.56 (15.37) | 3–71 |
| Neurocognition | ||
| SILS estimated IQ | 103.94 (7.35) | 87–117 |
| LN (raw score) | 11.72 (2.94) | 5–19 |
| WCST number of categories completed | 3.66 (1.51) | 0–5 |
| WCST perseverative errors, T-score | 52.34 (11.80) | 20–79 |
| Irony perception | ||
| Hit rate | ||
| Irony | 0.78 (0.20) | 0.17–0.96 |
| Metaphors | 0.84 (0.13) | 0.50–0.96 |
| Literal | 0.94 (0.03) | 0.83–0.96 |
| False alarm rate | ||
| Irony | 0.20 (0.16) | 0.04–0.75 |
| Metaphors | 0.23 (0.15) | 0.04–0.58 |
| Literal | 0.22 (0.15) | 0.04–0.75 |
| Sensitivity (A’) | ||
| Irony | 0.87 (0.08) | 0.65–0.97 |
| Metaphors | 0.87 (0.07) | 0.69–0.97 |
| Literal | 0.92 (0.05) | 0.80–0.98 |
| Response bias (B”) | ||
| Irony | 0.02 (0.62) | −0.96 to 0.98 |
| Metaphors | −0.21 (0.53) | −0.94 to 0.92 |
| Literal | −0.50 (0.33) | −0.94 to 0.41 |
| ToM stories | ||
| ToM stories total | 13.66 (1.60) | 10–16 |
| Average time to read ToM stories, secs | 25.25 (5.22) | 15.02–39.07 |
| Physical stories total | 11.88 (2.47) | 6–16 |
| Average time to read physical stories, secs | 30.31 (6.85) | 16.27–48.71 |
| Eyes task total score | 25.68 (4.21) | 15–33 |
| SAS-SR | ||
| SAS-SR: peer relationships | 1.77 (0.37) | 1.00–2.50 |
| SAS-SR: academic functioning | 1.59 (0.39) | 1.00–2.83 |
| SAS-SR: family relationships | 1.70 (0.40) | 1.12–2.88 |
| QOLI: total weighted satisfaction | 46.24 (22.35) | −14.78 to 91.00 |
Maximum possible scores for unusual experiences, 30; introvertive anhedonia, 27; cognitive disorganization, 24; impulsive nonconformity, 23; total score, 104.
UE indicates O-LIFE unusual experiences; IA, O-LIFE introvertive anhedonia; CD, O-LIFE cognitive disorganization; IN, O-LIFE impulsive nonconformity; SILS, Shipley Institute of Living Scale; LN, letter-number sequencing; O-LIFE, Oxford-Liverpool Inventory of Feelings and Experiences; WCST, Wisconsin Card Sorting Test–64; ToM, theory of mind; SAS-SR, Social Adjustment Scale-self-report; QOLI, Quality of Life Inventory.
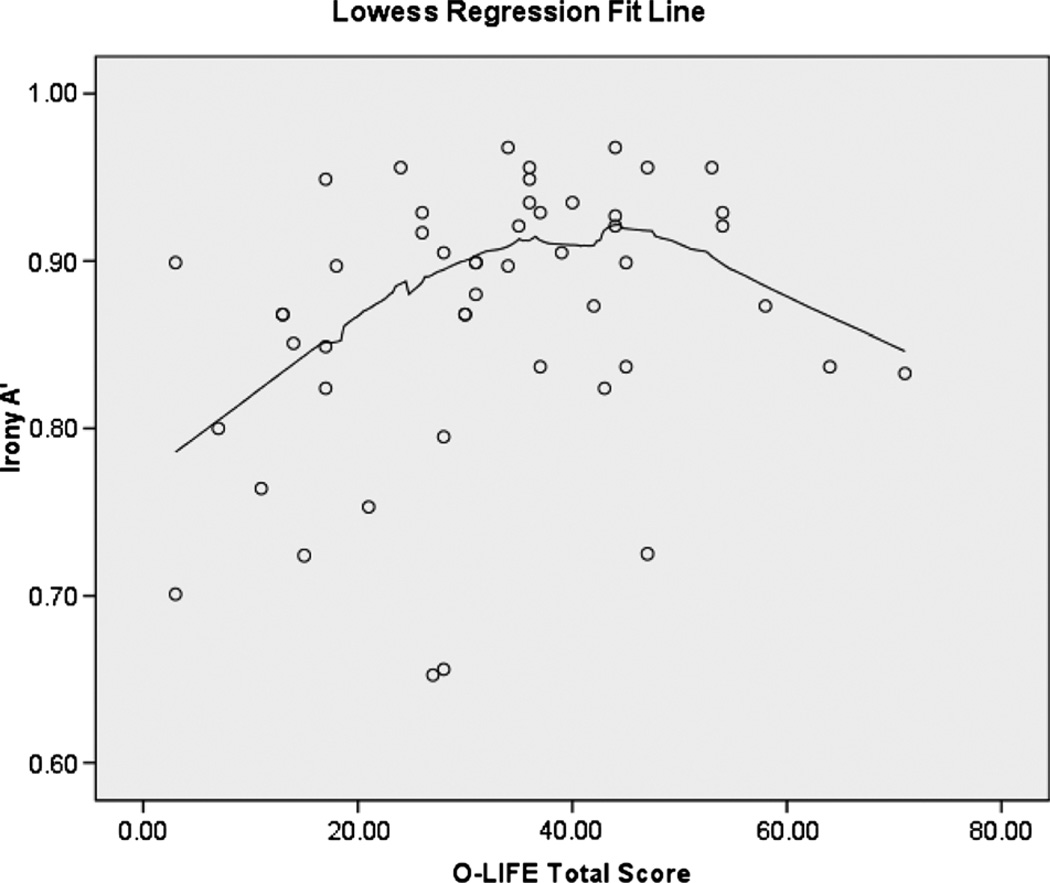
Zero-order correlations of schizotypy with neurocognitive, ToM, and social functioning variables are presented in Table 2. Schizotypy was related to social functioning. However, there were no statistically significant associations between schizotypy and performance in any of the neurocognitive tasks. Total schizotypy score was associated with enhanced irony perception in this sample; however, schizotypy was not associated with any other ToM variables. A scatterplot of the relationship between total schizotypy score and irony sensitivity is presented in Figure 1. A Lowess regression line suggests the possible presence of a curvilinear relationship between total schizotypy score and irony sensitivity. When individual schizotypy scales were examined, only IN was associated with enhanced irony perception (r = 0.34, p = 0.02).1 Surprisingly, the ToM tasks showed minimal association with each other.
TABLE 2.
Zero-Order Correlations Between Schizotypy and Study Variables (N = 50)
| Variable | UE | IA | CD | IN | O-LIFE |
|---|---|---|---|---|---|
| UE | |||||
| IA | 0.10 | ||||
| CD | 0.59** | 0.38** | |||
| IN | 0.62** | 0.24 | 0.50** | ||
| O-LIFE | 0.85** | 0.44** | 0.87** | 0.77** | |
| BDI | 0.71** | 0.21 | 0.67** | 0.62** | 0.78** |
| SILS | 0.04 | −0.08 | −0.04 | 0.13 | 0.02 |
| LN | 0.04 | −0.08 | −0.06 | 0.21 | 0.03 |
| WCST PE | −0.06 | −0.02 | −0.04 | 0.17 | −0.01 |
| WCST CC | −0.06 | −0.08 | −0.08 | 0.09 | −0.05 |
| ToM total | 0.09 | −0.20 | −0.01 | 0.06 | 0.01 |
| ToM time | −0.18 | 0.14 | −0.11 | −0.21 | −0.15 |
| Eyes | 0.17 | −0.18 | 0.06 | 0.07 | 0.08 |
| Irony A’ | 0.20 | 0.04 | 0.27 | 0.34* | 0.29* |
| Irony B” | 0.06 | 0.09 | 0.03 | 0.01 | 0.05 |
| SAS-SR: P | 0.52** | 0.12 | 0.49** | 0.30* | 0.53** |
| SAS-SR: A | 0.40** | 0.16 | 0.49** | 0.53** | 0.54** |
| SAS-SR: F | 0.45** | 0.12 | 0.33* | 0.41** | 0.45** |
| QOLI | −0.45** | −0.21 | −0.41** | −0.45** | −0.52** |
Correlation is significant at the 0.05 level (two-tailed).
Correlation is significant at the 0.01 level (two-tailed).
UE indicates O-LIFE unusual experiences; IA, O-LIFE introvertive anhedonia; CD, O-LIFE cognitive disorganization; IN, O-LIFE impulsive nonconformity; SILS, Shipley Institute of Living Scale IQ; LN, letter-number sequencing; O-LIFE, Oxford-Liverpool Inventory of Feelings and Experiences; WCST, Wisconsin Card Sorting Test–64; PE, perseverative errors; CC, categories completed; ToM, theory of mind; ToM Time, ToM stories average reading time; Irony A’, irony sensitivity; Irony B”, irony response bias; SAS-SR, Social Adjustment ScaleYself-report; QOLI, Quality of Life Inventory; P, peer relationships; A, academic functioning; F, family relationships.
FIGURE 1.
Scatterplot of irony sensitivity (A’) and Oxford-Liverpool Inventory of Feelings and Experiences total score.
Regarding the relationships between neurocognition and ToM, irony sensitivity and eyes task total score were positively associated with estimated IQ score (r = 0.47 and 0.30 respectively, p = 0.001 and 0.03 respectively), whereas average reading time for ToM stories was negatively associated with estimated IQ and working memory (r = −0.31 and −0.35, respectively; p = 0.03 and 0.01, respectively). There was a trend for an association between working memory and irony sensitivity score (r = 0.26, p = 0.07). Executive functioning (WCST-64 categories completed) was positively associated with ToM stories total score (r = 0.38, p = 0.01).2
Contrary to expectations, ToM showed no association with social functioning in this sample. However, depression was strongly associated with poor social functioning. Except for IA, all facets of schizotypy were highly associated with both depression and poor social functioning. Separate analyses were carried out by sex for SAS-SR peer social functioning; for both sexes, schizotypy and depression were strongly associated (rmen = 0.81, p < 0.001; rwomen = 0.74, p < 0.001). For men, impairment in SAS-SR peer social functioning was highly associated with schizotypy (r = 0.69, p = 0.01) and depression (r = 0.76, p < 0.001). For women, impairment in SAS-SR peer social functioning was associated with schizotypy at a trend level (0.34, p = 0.07) but was not significantly associated with depression (r = 0.25, p = 0.18).
Regression Analyses and Tests of Mediation
Because ToM impairment was not related to schizotypy or social functioning in this sample, the planned mediation analysis was unwarranted (Baron and Kenny, 1986). However, the strong relationships between schizotypy, depression, and social functioning are theoretically relevant considering the aforementioned mixed findings in the literature. Therefore, exploratory analyses were carried out to test whether depression mediated the relationship between schizotypal traits and poor social functioning. Separate analyses were carried out by sex for SAS-SR peer social functioning. Regression analyses are presented in Tables 3 and 4.
TABLE 3.
Tests of Mediation
| B | SE | β | t (p) | |
|---|---|---|---|---|
| Schizotypy and depression on peer social functioning for men (n = 20) | ||||
| Step 1. IV (O-LIFE total) on DV (SAS-SR: Peer) | 0.02 | 0.004 | −0.69 | 4.08 (p = 0.001) |
| Step 2. IV (O-LIFE total) on mediator (BDI) | 0.36 | 0.06 | 0.81 | 5.83 (p < 0.001) |
| Step 3. Mediator (BDI) on DV (SAS-SR Peer) | 0.03 | 0.01 | 0.56 | 2.13 (p = 0.05) |
| Step 4. IV (O-LIFE total) on DV (SAS-SR Peer) in presence of mediator (BDI) | 0.01 | 0.01 | 0.24 | 0.90 (p = 0.38) |
| R2 | 0.59 | |||
| F | 12.25 (p = 0.001) | |||
| Sobel | 2.01 (p = 0.04) | |||
| Schizotypy and depression on quality of life for total sample (N = 50) | ||||
| Step 1. IV (O-LIFE total) on DV (QOLI TWS) | −0.75 | 0.18 | −0.52 | −4.19 (p < 0.001) |
| Step 2. IV (O-LIFE total) on mediator (BDI) | 0.35 | 0.04 | 0.78 | 8.64 (p < 0.001) |
| Step 3. Mediator (BDI) on DV (QOLI TWS) | −1.37 | 0.61 | −0.43 | −2.24 (p = 0.03) |
| Step 4. IV (O-LIFE total) on DV (QOLI TWS) in presence of mediator (BDI) | −0.27 | 0.28 | −0.19 | −0.98 (p = 0.33) |
| R2 | 0.34 | |||
| F | 12.03 (p < 0.001) | |||
| Sobel | −2.17 (p = 0.03) | |||
BDI indicates Beck Depression Inventory; DV, dependent variable; IV, independent variable; O-LIFE, Oxford-Liverpool Inventory of Feelings and Experiences; QOLI, Quality of Life Inventory; SAS-SR, Social Adjustment Scale-self-report; TWS, total weighted satisfaction.
TABLE 4.
Regression Analyses
| B | SE | β | t (p) | |
|---|---|---|---|---|
| Schizotypy and depression on peer social functioning for women (n = 30) | ||||
| Schizotypy (O-LIFE total) | 0.01 | 0.01 | 0.34 | 1.27 (p = 0.21) |
| Depression (BDI) | 0.00 | 0.01 | −0.002 | −0.01 (p = 0.99) |
| R2 | 0.12 | |||
| F | 1.76 (p = 0.19) | |||
| Schizotypy and depression on family social functioning for total sample (N = 50) | ||||
| Schizotypy (O-LIFE total) | 0.01 | 0.01 | 0.22 | 1.06 (p = 0.29) |
| Depression (BDI) | 0.02 | 0.01 | 0.30 | 1.50 (p = 0.14) |
| R2 | 0.24 | |||
| F | 7.48 (p = 0.002) | |||
| Schizotypy and depression on academic social functioning for total sample (N = 50) | ||||
| Schizotypy (O-LIFE total) | 0.01 | 0.01 | 0.36 | 1.83 (p = 0.07) |
| Depression (BDI) | 0.01 | 0.01 | 0.24 | 1.21 (p = 0.23) |
| R2 | 0.31 | |||
| F | 10.61 (p < 0.001) | |||
BDI indicates Beck Depression Inventory; O-LIFE, Oxford-Liverpool Inventory of Feelings and Experiences
For the total sample, depression mediated the relationship between schizotypy and QOLI total life satisfaction (Sobel = −2.17, p = 0.03; Table 3). For men, depression mediated the relationship between schizotypal traits and SAS-SR peer social functioning (Sobel = 2.01, p = 0.04; Table 3).
For women, schizotypy and depression were not significantly associated with SAS-SR peer social functioning (r = 0.34, p = 0.07 and r = 0.25, p = 0.18, respectively), so the requirements for mediation were not met (Baron and Kenny, 1986). When depression and schizotypy were considered simultaneously, the model did not fit the data well (Table 4). For the total sample, when depression and schizotypy were considered simultaneously, there was a trend for depression to predict SAS-SR family social functioning, whereas schizotypy did not contribute appreciable predictive power to the model (Table 4). In contrast, when considered simultaneously, there was a trend for schizotypy to predict SAS-SR academic social functioning, whereas depression did not contribute appreciable predictive power to the model (Table 4).
DISCUSSION
Using a dimensional approach, schizotypy was associated with poor social functioning and depression in this sample but not with neurocognitive or social cognitive impairments. Contrary to expectations, individuals endorsing a greater number of schizotypal characteristics were more adept at detecting ironic statements. Moreover, ToM and social functioning were essentially unrelated to each other. However, there were strong associations between schizotypal traits, depression, and social functioning impairment. The level of depression was found to mediate the relationship between schizotypy and some aspects of social functioning.
The relationship between social cognition and social functioning impairment is well-established in the schizophrenia literature (Addington et al., 2006; Couture et al., 2006; Penn et al., 1996; Sergi et al., 2006; Vauth et al., 2004). The current study failed to find robust associations between performance on ToM tasks and social functioning in schizotypy. It is possible that associations may be evident only in individuals with extreme levels of schizotypal traits or, as indicated by the findings of Versmissen et al. (2008), individuals with genetic vulnerability for psychosis (i.e., first-degree relatives of schizophrenia patients). The results of the mediation analyses did, however, underscore the importance of considering the impact of depression on various aspects of social functioning in schizotypy. Depression mediated the relationship between schizotypy and QOLI total life satisfaction for the entire sample, indicating that schizotypal traits exert their influence on life satisfaction via depression. Depression also mediated the relationship between schizotypy and SAS-SR peer social functioning. Academic social functioning was the only subscale of the SAS-SR for which schizotypal traits were a better predictor than depressive symptoms, possibly reflecting the impact of cognitive disorganization and disinhibition aspects of schizotypy on academic functioning (e.g., difficulty keeping up with schoolwork demands, conflicts with fellow students).
The positive association between irony sensitivity and schizotypy in this sample was unexpected and intriguing. It may be a chance finding resulting from multiple comparisons; alternatively, the relationship may be indicative of vigilance toward social threat in schizotypy, as the ironic statements were often sarcastic slights against the story characters. The results of a follow-up study in our laboratory support the latter interpretation; schizotypal traits were associated with “excessive” ToM (i.e., reading “too much” into the intentions of others), as assessed using the Movie for the Assessment of Social Cognition (Divilbiss et al., 2009; Dziobek et al., 2006). The O-LIFE subscale most strongly associated with enhanced irony sensitivity was IN, a facet of schizotypy that is not assessed in questionnaires derived from the traditional three-factor model of schizotypy (i.e., positive, negative, and disorganized schizotypy). In contrast with the current dimensional findings, Langdon and Coltheart (2004) found that extreme high scorers on a schizotypy scale were significantly less sensitive to ironic statements than extreme low scorers. Despite the presence of significant group differences, correlations between individual facets of schizotypy and irony sensitivity were not significant; thus, it is possible that the relationship between schizotypal traits and irony sensitivity is curvilinear (i.e., ToM impairment evident only for individuals with extreme elevations in schizotypy), generally consistent with the scatterplot in Figure 1. These findings, taken together, suggest the possibility of a “threshold effect,” whereby schizotypal traits confer no detrimental effects or even a slight advantage for some aspects of ToM up to a point and ToM is negatively impacted only after surpassing a symptomatic threshold. This is a question for future research. It must be acknowledged, however, that differences in performance on the Irony Perception Task between the current sample and that of Langdon and Coltheart’s (2004) sample (i.e., higher mean hit and false alarm rates) may be attributable to differences between the briefer, 72-item version of this task used in the current study versus the 96-item version used by Langdon and Coltheart (2004).
Previous studies of the relationship between neurocognition and schizotypy have yielded mixed findings (e.g., Gooding et al., 1999; Jahshan and Sergi, 2007; Lenzenweger and Korfine, 1994; Spitznagel and Suhr, 2002). In the current sample, no relationship between schizotypy and neurocognitive functioning was found. Neurocognitive dysfunction may be associated with negative aspects of schizotypy (Gooding et al., 1999); the null findings of the current study in this regard may be attributable to the absence of extreme scorers on the IA subscale of the O-LIFE. Likewise, it is possible that ToM impairment is more strongly associated with negative schizotypy. Future studies that selectively recruit “negative schizotypes” could explore these hypotheses further. General intellectual ability was associated with all ToM tasks, underscoring the importance of considering IQ and verbal ability when investigating ToM (Brüne, 2003; Happé, 1995).
There were several limitations to the current study. First, the range of scores on the IA subscale of the O-LIFE was restricted; hence, the impact of “negative” schizotypy on neurocognition, ToM, and social functioning could not be adequately evaluated in this sample. Likewise, the range of the total scores for the O-LIFE was slightly restricted; thus, it is unclear whether the relationships found in the current sample would remain consistent for individuals exhibiting higher levels of schizotypal traits. In future research, the recruitment of extreme scorers on the schizotypy measure might elucidate the reasons why some investigators have found relationships between schizotypy and ToM impairments, whereas others have not (e.g., possible curvilinear relationship between ToM and schizotypy). Another potential limitation of the study is related to the tasks used to assess ToM abilities. Although the tasks used in the current study were selected because they had been used with undergraduate samples in the literature (Langdon and Coltheart, 2004; Pickup, 2006), they may not have been sensitive enough to detect subtle ToM differences potentially associated with gradations of schizotypy in an essentially normal college sample. In addition, the ToM tasks showed minimal association with each other. It is possible that these tasks tap distinct ToM domains (e.g., cognitive versus affective empathy; Shamay-Tsoory et al., 2007); further study of the validity of these ToM tasks is warranted.
In sum, within a dimensional framework, no associations were found between schizotypy and impaired ToM or neurocognition. Rather, schizotypal traits were associated with enhanced performance on one social cognitive task that involved detection of ironic statements. However, schizotypy was strongly associated with both depression and social functioning impairment. Exploratory analyses revealed that depression mediated the relationship between schizotypy and quality of life and between schizotypy and facets of social functioning.
ACKNOWLEDGMENTS
The authors thank the following undergraduate research assistants who provided tremendous help in data collection: Tara Baluck, Audrey Norris, Kym Redden, and Patrick Sullivan.
This project was supported by Kent State University and National Institute of Mental Health (NIMH) Grant R01-MH58783.
The NIMH had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
To determine whether discrepancy between performance on ToM tasks and their respective control tasks varied as a function of schizotypy, additional correlational analyses were conducted using residual scores for a) the irony sensitivity task and b) ToM stories task, where performance on their respective control tasks (i.e., sensitivity for literal and metaphor conditions for irony task and physical stories for ToM stories) was regressed out (Chapman and Chapman, 1973; Cronbach and Furby, 1970; Pike, 1992). The results of these analyses did not appreciably differ from the reported analyses which used raw scores.
The results of subsequent correlational analyses controlling for the impact of cognitive variables on the relationships between schizotypy and ToM task performance did not differ substantially from those reported above.
DISCLOSURE
The authors have nothing to disclose.
REFERENCES
- Addington J, Girard TA, Christensen BK, Addington D. Social cognition mediates illness-related and cognitive influences on social function in patients with schizophrenia-spectrum disorders. J Psychiatry Neurosci. 2010;35:49–54. doi: 10.1503/jpn.080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J, Saeedi H, Addington D. Facial affect recognition: A mediator between cognitive and social functioning in psychosis? Schizophr Res. 2006;85:142–150. doi: 10.1016/j.schres.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The ‘reading the mind in the eyes’ test revised version: A study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001;42:241–251. [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. The Beck Depression Inventory-II. San Antonio, TX: Harcourt Assessment; 1996. [Google Scholar]
- Bowie CR, Leung WW, Reichenberg A, McClure MM, Patterson TL, Heaton RK, Harvey PD. Predicting schizophrenia patients’ real-world behavior with specific neuropsychological and functional capacity measures. Biol Psychiatry. 2008;63:505–511. doi: 10.1016/j.biopsych.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüne M. Theory of mind and the role of IQ in chronic disorganized schizophrenia. Schizophr Res. 2003;60:57–64. doi: 10.1016/s0920-9964(02)00162-7. [DOI] [PubMed] [Google Scholar]
- Brüne M. ‘Theory of mind’ in schizophrenia: A review of the literature. Schizophr Bull. 2005;31:21–42. doi: 10.1093/schbul/sbi002. [DOI] [PubMed] [Google Scholar]
- Brüne M, Bodenstein L. Proverb comprehension reconsidered;‘theory of mind’ and the pragmatic use of language in schizophrenia. Schizophr Res. 2005;75:233–239. doi: 10.1016/j.schres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. Problems in the measurement of cognitive deficit. Psychol Bull. 1973;79:380–385. doi: 10.1037/h0034541. [DOI] [PubMed] [Google Scholar]
- Claridge G. Theoretical background and issues. In: Claridge G, editor. Schizotypy: Implications for illness and health. Oxford, UK: Oxford University Press; 1997. pp. 3–18. [Google Scholar]
- Corcoran R, Mercer G, Frith CD. Schizophrenia, symptomatology and social inference: Investigating ‘theory of mind’ in people with schizophrenia. Schizophr Res. 1995;17:5–13. doi: 10.1016/0920-9964(95)00024-g. [DOI] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: A review. Schizophr Bull. 2006;32:44–63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig JS, Hatton C, Craig FB, Bentall RP. Persecutory beliefs, attributions and theory of mind: comparison of patients with paranoid delusions, Asperger’s syndrome and healthy controls. Schizophr Res. 2004;69:29–33. doi: 10.1016/S0920-9964(03)00154-3. [DOI] [PubMed] [Google Scholar]
- Cronbach LJ, Furby L. How we should measure ‘change’Vor should we? Psychol Bull. 1970;74:68–80. [Google Scholar]
- Divilbiss M, Aakre J, Seghers J, McCleery A, Schumann EB, Docherty N. Poster presented at: the 23rd Annual Meeting of the Society for Research in Psychopathology. Minneapolis, MN: 2009. Schizotypy and social functioning: Implications for emotional distress. [Google Scholar]
- Dziobek I, Fleck S, Kalbe E, Rogers K, Hassenstab J, Brand M, Kessler J, Woike JK, Wolf OT, Convit A. Introducing MASC: A movie for the assessment of social cognition. J Autism Dev Disord. 2006;36:623–636. doi: 10.1007/s10803-006-0107-0. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happé F, Frith U, Baker SC, Dolan RJ, Frackowiak RS, Frith CD. Other minds in the brain: A functional imaging study of ‘theory of mind’ in story comprehension. Cognition. 1995;57:109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Frisch M. The quality of life inventory. Minneapolis, MN: National Computer Systems; 1994. [Google Scholar]
- Gooding DC, Kwapil TR, Tallent KA. Wisconsin card sorting deficits in schizotypic individuals. Schizophr Res. 1999;40:201–209. doi: 10.1016/s0920-9964(99)00124-3. [DOI] [PubMed] [Google Scholar]
- Green MF, Olivier B, Crawley JN, Penn DL, Silverstein S. Social cognition in schizophrenia: Recommendations from the measurement and treatment research to improve cognition in schizophrenia new approaches conference. Schizophr Bull. 2005;31:882–887. doi: 10.1093/schbul/sbi049. [DOI] [PubMed] [Google Scholar]
- Happé FGE. The role of age and verbal ability in the theory of mind task performance of subjects with autism. Child Dev. 1995;66:843–855. [PubMed] [Google Scholar]
- Henry JD, Bailey PE, Rendell PG. Empathy, social functioning and schizotypy. Psychiatry Res. 2008;160:15–22. doi: 10.1016/j.psychres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Herold R, Tenyi T, Lenard K, Trixler M. Theory of mind deficit in people with schizophrenia during remission. Psychol Med. 2002;32:1125–1129. doi: 10.1017/s0033291702005433. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RM, Montgomery SA, Keller MB, Kasper S, Schatzberg AF, Möller HJ, Baldwin D, Humble M, Versiani M, Montenegro R, Bourgeois M. Social functioning in depression: A review. J Clin Psychiatry. 2000;61:268–275. doi: 10.4088/jcp.v61n0405. [DOI] [PubMed] [Google Scholar]
- Jahshan CS, Sergi M. Theory of mind, neurocognition, and functional status in schizotypy. Schizophr Res. 2007;89:278–286. doi: 10.1016/j.schres.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Kongs S, Thompson L, Iverson G, Heaton R. Wisconsin Card Sorting Test–64 card computerized version [computer program] Odessa, FL: Psychological Assessment Resources; 2000a. [Google Scholar]
- Kongs S, Thompson L, Iverson G, Heaton R. Professional manual. Lutz, FL: Psychological Assessment Resources; 2000b. Wisconsin Card Sorting Test—64 card version. [Google Scholar]
- Langdon R, Coltheart M. Mentalising, schizotypy, and schizophrenia. Cognition. 1999;71:43–71. doi: 10.1016/s0010-0277(99)00018-9. [DOI] [PubMed] [Google Scholar]
- Langdon R, Coltheart M. Recognition of metaphor and irony in young adults: The impact of schizotypal personality traits. Psychiatry Res. 2004;125:9–20. doi: 10.1016/j.psychres.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Langdon R, Coltheart M, Ward PB, Catts SV. Disturbed communication in schizophrenia: The role of poor pragmatics and poor mind-reading. Psychol Med. 2002;32:1273–1284. doi: 10.1017/s0033291702006396. [DOI] [PubMed] [Google Scholar]
- Lenzenweger MF, Korfine L. Perceptual aberrations, schizotypy, and the Wisconsin card sorting test. Schizophr Bull. 1994;20:345–357. doi: 10.1093/schbul/20.2.345. [DOI] [PubMed] [Google Scholar]
- Lewandowski KE, Barrantes-Vidal N, Nelson-Gray RO, Clancy C, Kepley HO, Kwapil TR. Anxiety and depression symptoms in psychometricallyidentified schizotypy. Schizophr Res. 2006;83:225–235. doi: 10.1016/j.schres.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Mason O, Claridge G, Jackson M. New scales for the assessment of schizotypy. Pers Individ Differ. 1995;18:7–13. [Google Scholar]
- Mason O, Claridge G. The Oxford-Liverpool inventory of feelings and experiences (O-LIFE): Further description and extended norms. Schizophr Res. 2006;82:203–211. doi: 10.1016/j.schres.2005.12.845. 2006. [DOI] [PubMed] [Google Scholar]
- Meyer J, Shean G. Social-cognitive functioning and schizotypal characteristics. J Psychol. 2006;140:199–207. doi: 10.3200/JRLP.140.3.199-207. [DOI] [PubMed] [Google Scholar]
- Penn DL, Corrigan PW, Bentall R, Racenstein JM, Newman L. Social cognition in schizophrenia. Psychol Bull. 1997;121:114–132. doi: 10.1037/0033-2909.121.1.114. [DOI] [PubMed] [Google Scholar]
- Penn DL, Spaulding W, Reed D, Sullivan M. The relationship of social cognition toward behavior in chronic schizophrenia. Schizophr Res. 1996;20:327–335. doi: 10.1016/0920-9964(96)00010-2. [DOI] [PubMed] [Google Scholar]
- Pickup GJ. Theory of mind and its relation to schizotypy. Cogn Neuropsychiatry. 2006;11:177–192. doi: 10.1080/13546800444000236. [DOI] [PubMed] [Google Scholar]
- Pickup GJ, Frith CD. Theory of mind impairments in schizophrenia: Symptomatology, severity and specificity. Psychol Med. 2001;31:207–220. doi: 10.1017/s0033291701003385. [DOI] [PubMed] [Google Scholar]
- Pike GR. Lies, damn lies, and statistics revisited: A comparison of three methods of representing change. Res High Educ. 1992;33:71–84. [Google Scholar]
- Sarfati Y, Hardy-Bayle MC, Brunet E, Widlocher D. Investigating theory of mind in schizophrenia: Influence of verbalization in disorganized and non-disorganized patients. Schizophr Res. 1999;37:183–190. doi: 10.1016/s0920-9964(98)00154-6. [DOI] [PubMed] [Google Scholar]
- Sergi MJ, Rassovsky Y, Nuechterlein KH, Green MF. Social perception as a mediator of the influence of early visual processing on functional status in schizophrenia. Am J Psychiatry. 2006;163:448–454. doi: 10.1176/appi.ajp.163.3.448. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, Levkovitz Y. The neuroanatomical basis of affective mentalizing in schizophrenia: Comparison of patients with schizophrenia and patients with localized prefrontal lesions. Schizophr Res. 2007;90:274–283. doi: 10.1016/j.schres.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Shipley WC. A self-administering scale for measuring intellectual impairment and deterioration. J Psychol. 1940;9:371–377. [Google Scholar]
- Spitznagel MB, Suhr JA. Executive function deficits associated with symptoms of schizotypy and obsessive-compulsive disorder. Psychiatry Res. 2002;110:151–163. doi: 10.1016/s0165-1781(02)00099-9. [DOI] [PubMed] [Google Scholar]
- Vauth R, Rüsch N, Wirtz M, Corrigan PW. Does social cognition influence the relation between neurocognitive deficits and vocational functioning in schizophrenia? Psychiatry Res. 2004;128:155–165. doi: 10.1016/j.psychres.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Verdoux H, van Os J, Maurice-Tilson S, Gay B, Salamon R, Bourgeois ML. Increased occurrence of depression in psychosis-prone subjects: A follow-up study in primary care settings. Compr Psychiatry. 1999;40:462–468. doi: 10.1016/s0010-440x(99)90091-3. [DOI] [PubMed] [Google Scholar]
- Versmissen D, Janssen I, Myin-Germeys I, Mengelers R, Campo JA, van Os J, Krabbendam L. Evidence for a relationship between mentalising deficits and paranoia over the psychosis continuum. Schizophr Res. 2008;99:103–110. doi: 10.1016/j.schres.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Weismann MM, Bothwell S. Assessment of social adjustment by patient self-report. Arch Gen Psychiatry. 1976;33:1111–1115. doi: 10.1001/archpsyc.1976.01770090101010. [DOI] [PubMed] [Google Scholar]
- Weschler D. Weschler Adult Intelligence Scale–Third Edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Zachary RA. Shipley Institute of Living Scale: Revised manual. Los Angeles, CA: Western Psychological Services; 1986. [Google Scholar]