Abstract
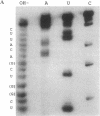
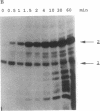
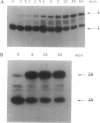
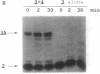
In an attempt to synthesize an oligoribonucleotide by run-off transcription by bacteriophage T7 RNA polymerase, a major transcript was produced that was much longer than expected. Analysis of the reaction indicated that the product resulted from initial DNA-directed run-off transcription followed by RNA template-directed RNA synthesis. This reaction occurred because the RNA made from the DNA template displayed self-complementarity at its 3' end and therefore could form an intra- or intermolecular primed template. In reactions containing only an RNA template, the rate of incorporation of NTPs was quite comparable to DNA-dependent transcription. RNA template-directed RNA synthesis has been found to occur with a great number of oligoribonucleotides, even with primed templates that are only marginally stable. In one instance, we observed a multistep extension reaction converting the oligonucleotide into a final product longer than twice its original length. Presumably, such a process could have generated some of the RNAs found to be efficiently replicated by T7 RNA polymerase.
Full text
PDF
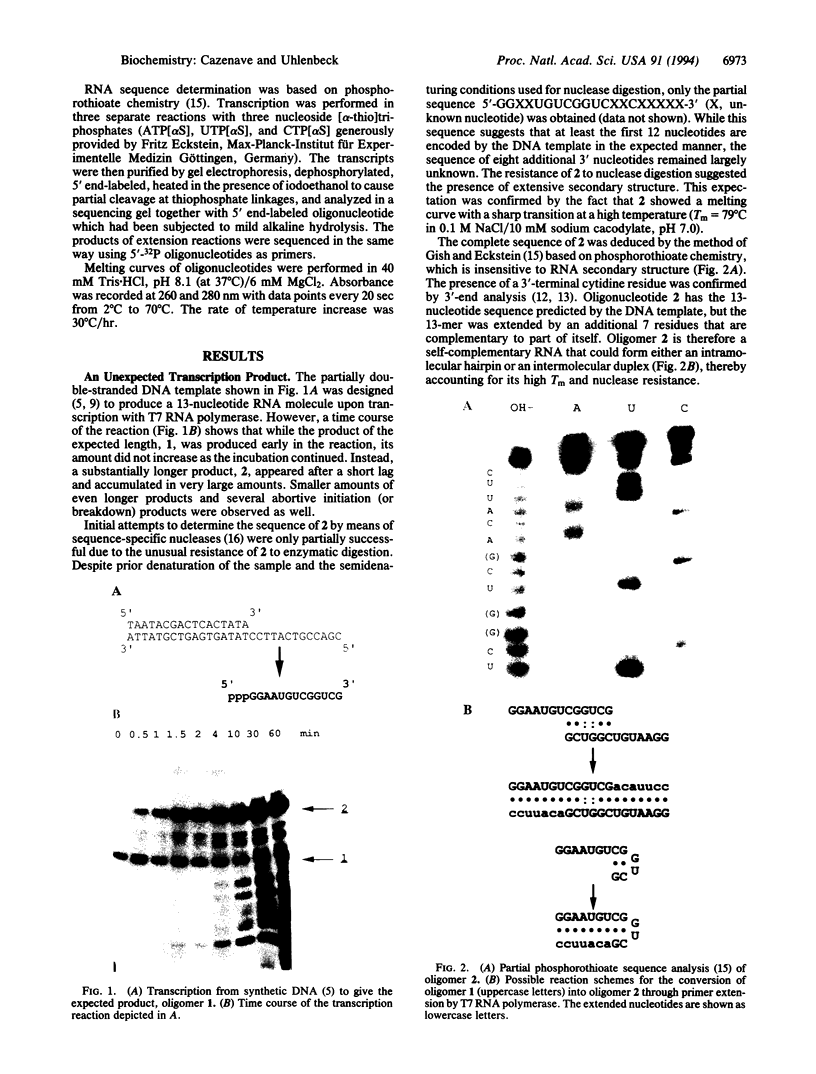

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod V. D., Kramer F. R. Transcription from bacteriophage T7 and SP6 RNA polymerase promoters in the presence of 3'-deoxyribonucleoside 5'-triphosphate chain terminators. Biochemistry. 1985 Oct 8;24(21):5716–5723. doi: 10.1021/bi00342a005. [DOI] [PubMed] [Google Scholar]
- Daube S. S., von Hippel P. H. Functional transcription elongation complexes from synthetic RNA-DNA bubble duplexes. Science. 1992 Nov 20;258(5086):1320–1324. doi: 10.1126/science.1280856. [DOI] [PubMed] [Google Scholar]
- Daube S. S., von Hippel P. H. RNA displacement pathways during transcription from synthetic RNA-DNA bubble duplexes. Biochemistry. 1994 Jan 11;33(1):340–347. doi: 10.1021/bi00167a044. [DOI] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. Enzymatic oligoribonucleotide synthesis with T4 RNA ligase. Biochemistry. 1978 May 30;17(11):2069–2076. doi: 10.1021/bi00604a008. [DOI] [PubMed] [Google Scholar]
- Gish G., Eckstein F. DNA and RNA sequence determination based on phosphorothioate chemistry. Science. 1988 Jun 10;240(4858):1520–1522. doi: 10.1126/science.2453926. [DOI] [PubMed] [Google Scholar]
- Gott J. M., Willis M. C., Koch T. H., Uhlenbeck O. C. A specific, UV-induced RNA-protein cross-link using 5-bromouridine-substituted RNA. Biochemistry. 1991 Jun 25;30(25):6290–6295. doi: 10.1021/bi00239a030. [DOI] [PubMed] [Google Scholar]
- Johnston R. F., Pickett S. C., Barker D. L. Autoradiography using storage phosphor technology. Electrophoresis. 1990 May;11(5):355–360. doi: 10.1002/elps.1150110503. [DOI] [PubMed] [Google Scholar]
- Konarska M. M., Sharp P. A. Replication of RNA by the DNA-dependent RNA polymerase of phage T7. Cell. 1989 May 5;57(3):423–431. doi: 10.1016/0092-8674(89)90917-3. [DOI] [PubMed] [Google Scholar]
- Konarska M. M., Sharp P. A. Structure of RNAs replicated by the DNA-dependent T7 RNA polymerase. Cell. 1990 Nov 2;63(3):609–618. doi: 10.1016/0092-8674(90)90456-o. [DOI] [PubMed] [Google Scholar]
- Krupp G. RNA synthesis: strategies for the use of bacteriophage RNA polymerases. Gene. 1988 Dec 10;72(1-2):75–89. doi: 10.1016/0378-1119(88)90129-1. [DOI] [PubMed] [Google Scholar]
- Krupp G. Unusual promoter-independent transcription reactions with bacteriophage RNA polymerases. Nucleic Acids Res. 1989 Apr 25;17(8):3023–3036. doi: 10.1093/nar/17.8.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchino Y., Nishimura S. Enzymatic RNA sequencing. Methods Enzymol. 1989;180:154–163. doi: 10.1016/0076-6879(89)80099-0. [DOI] [PubMed] [Google Scholar]
- Martin C. T., Coleman J. E. Kinetic analysis of T7 RNA polymerase-promoter interactions with small synthetic promoters. Biochemistry. 1987 May 19;26(10):2690–2696. doi: 10.1021/bi00384a006. [DOI] [PubMed] [Google Scholar]
- Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987 Nov 11;15(21):8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan J. F., Uhlenbeck O. C. Synthesis of small RNAs using T7 RNA polymerase. Methods Enzymol. 1989;180:51–62. doi: 10.1016/0076-6879(89)80091-6. [DOI] [PubMed] [Google Scholar]
- Moore M. J., Sharp P. A. Site-specific modification of pre-mRNA: the 2'-hydroxyl groups at the splice sites. Science. 1992 May 15;256(5059):992–997. doi: 10.1126/science.1589782. [DOI] [PubMed] [Google Scholar]
- Moroney S. E., Piccirilli J. A. Abortive products as initiating nucleotides during transcription by T7 RNA polymerase. Biochemistry. 1991 Oct 22;30(42):10343–10349. doi: 10.1021/bi00106a036. [DOI] [PubMed] [Google Scholar]
- Puglisi J. D., Tinoco I., Jr Absorbance melting curves of RNA. Methods Enzymol. 1989;180:304–325. doi: 10.1016/0076-6879(89)80108-9. [DOI] [PubMed] [Google Scholar]
- Ruffner D. E., Uhlenbeck O. C. Thiophosphate interference experiments locate phosphates important for the hammerhead RNA self-cleavage reaction. Nucleic Acids Res. 1990 Oct 25;18(20):6025–6029. doi: 10.1093/nar/18.20.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharmeen L., Taylor J. Enzymatic synthesis of RNA oligonucleotides. Nucleic Acids Res. 1987 Aug 25;15(16):6705–6711. doi: 10.1093/nar/15.16.6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg W. L., Uhlenbeck O. C. Specific replacement of functional groups of uridine-33 in yeast phenylalanine transfer ribonucleic acid. Biochemistry. 1985 May 21;24(11):2705–2712. doi: 10.1021/bi00332a017. [DOI] [PubMed] [Google Scholar]