Key Points
Detection of multiple HY-Abs at 3 months post-F→M HCT predicts cGVHD incidence, severity, and nonrelapse mortality.
Patients with a high HY score may be good candidates for cGVHD prevention trials, especially those targeting allogeneic B cells.
Abstract
Allogeneic antibodies against minor histocompatibility antigens encoded on the Y chromosome (HY-Abs) develop after hematopoietic cell transplant (HCT) of male recipients with female donors (F→M). However, the temporal association between HY-Ab development and chronic graft-versus-host disease (cGVHD) has yet to be elucidated. We studied 136 adult F→M HCT patients, with plasma prospectively collected through 3 years posttransplant, and measured immunoglobulin G against 6 H-Y antigens. Multiple HY-Abs were frequently detected beginning at 3 months posttransplant: 78 (57%) of F→M patients were seropositive for at least 1 of the 6 HY-Abs, and 3-month seropositivity for each HY-Ab was associated with a persistent seropositive response throughout the posttransplant follow-up period (P < .001 in each). There were no associations between pretransplant features and 3-month overall HY-Ab development. Detection of multiple HY-Abs at 3 months (represented by HY score) was significantly associated with an increased risk of cGVHD (P < .0001) and nonrelapse mortality (P < .01). Compared to clinical factors alone, the addition of HY score to clinical factors improved the predictive potential of cGVHD (P < .01). Monitoring HY-Ab development thus stratifies cGVHD risk in F→M HCT patients and may support preemptive prophylaxis therapy for cGVHD beginning at 3 months posttransplant.
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) can cure hematologic malignancies, but the allogeneic immune benefit often results in chronic graft-versus-host disease (cGVHD) development, with severe morbidity and mortality.1 The pathogenesis and optimal treatment of cGVHD remains to be elucidated. cGVHD affects a wide range of organs, and severe cGVHD remarkably impairs a recipient’s quality of life.2 Therefore, more efficient approaches to predict, prevent, and treat cGVHD should be explored.3,4
Established risk factors for development of cGVHD include acute GVHD (aGVHD), peripheral blood stem cell transplant, donor and recipient age, and sex mismatch.3-6 Sex-mismatched transplantation remains a model system for human cGVHD studies involving both biological and clinical analyses. Specifically, hematopoietic cell transplant (HCT) of male recipients with female donors (F→M) has been long-recognized as a significant risk factor for the development of cGVHD.5-7 The biological explanation is that naïve female-donor lymphocytes recognize several proteins encoded by the Y chromosome of a male recipient, which are called H-Y minor histocompatibility antigens. Our group has previously shown that alloantibodies against H-Y antigens (HY-Abs) were detected in 39 F→M recipients using enzyme-linked immunosorbent assay and that H-Y antigen–specific B cells developed in F→M HCT patients.8,9
Clinical manifestations of cGVHD are similar to those observed in connective tissue disease, including scleroderma and sicca syndrome, and autoreactive/alloreactive immune abnormalities have so far been proposed to play a pathogenic role in cGVHD.10,11 B-cell activating factor has been associated with active cGVHD.12 The fibrotic change in cGVHD, especially extensive cGVHD, was also associated with the production of autoantibodies against platelet-derived growth factor receptor.13 Rituximab, which depletes B cells in vivo, was reported to have a preventive and curative potential for GVHD.14-16 However, the optimal strategy for B-cell depletion has yet to be investigated. To optimize B-cell depletion, an effective immunologic guide to identifying patients most at risk for developing cGVHD is needed. HY-Abs may be a candidate for these necessary immunologic tools.
Our prior studies have demonstrated that HY-Abs were detected in F→M HCT patients,8,9 but the temporal association between HY-Ab development and cGVHD has yet to be established. In addition, prior studies were limited by antibody detection technologies and by measurement of HY-Abs in blood samples collected ≥9 months after HCT.8 Therefore, it remains unclear whether HY-Ab develops before cGVHD or as a consequence of cGVHD. In this study, HY-Abs in F→M HCT patients were prospectively monitored using our novel protein microarrays17 and then related to cGVHD development and other clinical outcomes.
Patients and methods
Patients and blood samples
We studied 136 adult F→M HCT patients who underwent allo-HCT between 2005 and 2012, who survived without relapse for at least 3 months post-HCT, and who had a minimum follow-up period of 1 year. Their plasma samples were prospectively collected at 2, 3, 6, and 9 months and at 1, 1.5, 2, 2.5, and 3 years post-HCT and cryopreserved until use. HY-Abs were measured in a total of 710 samples. In addition, the HY-Abs in F→M HCT patients were compared to those in male recipients with male donors (M→M) at 3 months (n = 60; see supplemental Table 1, available on the Blood Web site) and at 1 year post-HCT (including samples at 9 months post-HCT, n = 50). This study was approved by the Institutional Review Board of Stanford University. All patients gave written informed consent for the cryopreservation and analyses of blood samples in accordance with the Declaration of Helsinki.
Proteomic microarrays for the detection of HY-Abs
A panel of 6 H-Y antigens was tested in our proteomic micrroarray17: DBY (DEAD box 3 peptide, Y-linked, DDX3Y); UTY (ubiquitously transcribed tetratricopeptide repeat–containing, Y-linked); ZFY (zinc finger protein, Y-linked); RPS4Y (ribosomal protein S4, Y-linked); EIF1AY (eukaryotic translation initiation factor 1A, Y-linked); and SMCY (lysine [K]-specific demethylase 5D, KDM5D). Briefly, our H-Y microarray presented the 6 recombinant H-Y proteins and tested plasma samples diluted at 1:50. Because UTY and SMCY are too large to express efficiently, they were divided into 3 (UTY1-3) and 6 (SMCYa-f) overlapping fragments and assessed separately. Mean fluorescence intensity (MFI) of each spot was obtained. The threshold of each HY-Ab seropositivity was determined from 60 healthy men, and the positive cutoff was defined as the third quartile plus 2 times the interquartile range (Q3 + 2[IQR]). The relative HY-Ab quantification is a factor of each HY-Ab seropositivity threshold. Microarray data are available from the Dryad Digital Repository, doi:10.5061/dryad.bm714.
Definitions of categories and statistical analyses
The details of category definitions and statistical analyses are shown in supplemental Methods. Briefly, the 6 HY-Abs were evaluated as principal components, and the HY score was defined as the sum total of HY-Ab seropositivity. Regarding UTY and SMCY, those samples that were reactive with any one of the UTY1-3 or SMCYa-f fragments were scored as positive for UTY or SMCY, respectively. The HY score indicates recognition of 0 to 6 HY-Abs.
The cumulative incidence rates of events were estimated by the Gray method, and overall survival (OS) rates from HCT were derived by the Kaplan-Meier method with 95% confidence intervals (CI). In a multivariate analysis, the Cox proportional hazard model or the Fine and Gray method was used. A 2-tailed P value <.05 was considered significant. Receiver operating characteristic (ROC) curves were estimated, and areas under the curve (AUCs) were compared for cGVHD predictive potential based on HY score alone, clinical factors alone,5,6 and the combination of both. All analyses and data management were performed using Stata v.12.0 (StataCorp, College Station, TX); R v.3.0.1 (R Foundation, Austria); and EZR18 (Saitama Medical Center, Jichi Medical University, available at www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html).
Results
Patient characteristics
Patient characteristics are shown in Table 1. The median patient age was 53 years (range: 21-74 years). Of the 136 recipients, 128 (94%) received a peripheral blood stem cell transplant and 112 (83%) had donors that were HLA matched (related donor in 81 [60%], unrelated donor in 31 [23%]). More than half (53%) received reduced-intensity conditioning, with most receiving total lymphoid irradiation with ATG. Grade 2 aGVHD developed in 23 patients (17%), and grade 3 or 4 developed in 8 patients (6%).
Table 1.
Patient characteristics and their impact on the detection of H-Y antigen–specific antibodies at 3 months posttransplant
| N | Any HY-Ab seropositivity 3 mo post-HCT, n (%) | HY-Ab seronegativity 3 mo post-HCT, n (%) | P | |
|---|---|---|---|---|
| Total cohort | 136 | 78 (57%) | 58 (42%) | |
| Patient age, y | ||||
| <50 | 58 | 31 (40%) | 27 (47%) | .49 |
| ≥50 | 78 | 47 (60%) | 31 (53%) | |
| Donor age, y | ||||
| <50 | 92 | 50 (64%) | 42 (72%) | .46 |
| ≥50 | 43 | 27 (35%) | 16 (28%) | |
| Missing | 1 | |||
| Disease | ||||
| AML | 38 | 23 (29%) | 15 (26%) | .79 |
| ALL | 26 | 16 (21%) | 10 (17%) | |
| MDS, MPN | 19 | 12 (15%) | 7 (12%) | |
| Lymphoma | 48 | 25 (32%) | 23 (40%) | |
| Others | 5 | 2 (3%) | 3 (5%) | |
| Disease risk | ||||
| Standard | 105 | 57 (73%) | 48 (83%) | .22 |
| High | 31 | 21 (27%) | 10 (17%) | |
| CMV seropositivity | ||||
| D+R+ | 52 | 27 (35%) | 25 (43%) | .15 |
| D+R− | 24 | 18 (23%) | 8 (14%) | |
| D−R+ | 26 | 17 (22%) | 7 (12%) | |
| D−R− | 34 | 16 (21%) | 18 (31%) | |
| Donor type | ||||
| MRD | 81 | 48 (62%) | 33 (57%) | .88 |
| MUD | 33 | 19 (24%) | 14 (24%) | |
| MMRD | 4 | 2 (3%) | 2 (3%) | |
| MMUD | 18 | 9 (12%) | 9 (16%) | |
| Cell source | ||||
| Bone marrow | 8 | 4 (5%) | 4 (7%) | .72 |
| Peripheral blood | 128 | 74 (95%) | 54 (93%) | |
| GVHD prophylaxis | ||||
| CsA-based | 82 | 48 (62%) | 34 (59%) | .87 |
| Tac-based | 48 | 27 (35%) | 21 (36%) | |
| Others | 6 | 3 (4%) | 3 (5%) | |
| Conditioning | ||||
| MAC | 64 | 35 (45%) | 29 (50%) | .60 |
| RIC | 72 | 43 (55%) | 29 (50%) | |
| With ATG | 70 | 43 (55%) | 27 (47%) | .39 |
| Without ATG | 66 | 35 (45%) | 31 (53%) | |
| aGVHD | ||||
| Grade 0-1 | 105 | 66 (85%) | 39 (67%) | .044 |
| Grade 2 | 23 | 8 (10%) | 15 (26%) | |
| Grade 3-4 | 8 | 4 (5%) | 4 (7%) |
ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; ATG, anti-thymocyte globulin; CMV, cytomegalovirus; CsA, cyclosporine; D, donor CMV seropositivity; MAC, myeloablative conditioning; MDS, myelodysplastic syndrome; MMRD, HLA-mismatched related donor; MMUD, HLA-mismatched unrelated donor; MPN, myeloproliferative neoplasm; R, recipient CMV seropositivity; MRD, HLA-matched related donor; MUD, HLA-matched unrelated donor; RIC, reduced intensity conditioning; Tac, tacrolimus.
Persistent HY-Abs develop at 3 months post-F→M HCT
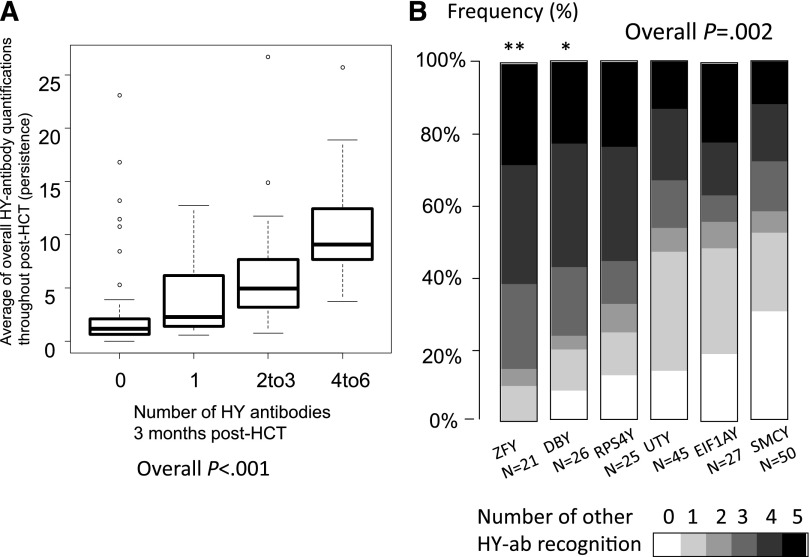
HY-Abs were measured using the H-Y protein microarray technology as previously described,17 and antibodies against each H-Y antigen were reported as a factor of HY-Ab seropositivity called the relative HY-Ab quantification. The temporal development and relative change of each HY-Ab quantification after F→M HCT is shown in Figure 1A. In general, although a few HY-Abs were detected at 2 months post-HCT, the majority of HY-Abs were first detected at 3 months post-HCT. Notably, only DBY-immunoglobulin (Ig)G and UTY-IgG prevalence increased beyond 1 year post-HCT (Figure 1B). The persistence of each HY-Ab was quantified as the average of the relative HY-Ab quantification measured at ≥3 months for all available samples. The average throughout 3 years post-HCT was greater at 3 months in patients testing HY-Ab seropositive than in those testing HY-Ab seronegative (P < .001 in each HY-Ab; supplemental Figure 1), thereby confirming the persistence of HY-Abs detected at 3 months. Furthermore, the detection of multiple HY-Abs at 3 months was significantly associated with higher overall relative HY-Ab quantifications (P < .001; Figure 2A). In summary, persistent and multiple HY-Abs were first detected at 3 months after F→M HCT.
Figure 1.
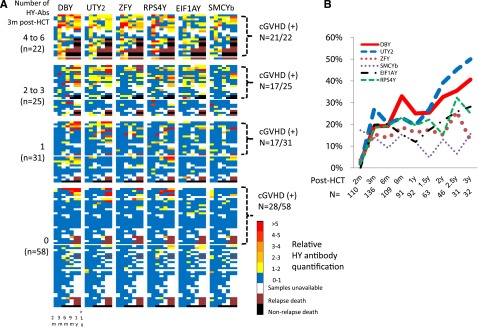
Recombinant H-Y protein microarray analysis. (A) Temporal changes of overall HY-Ab development throughout 3 years post-HCT. The various colors indicate relative antibody quantification as a factor of each HY-Ab–seropositive threshold. White squares indicate that samples were unavailable. Black and brown denote death and relapse, respectively. From left to right, the columns represent 2, 3, 6, and 9 months, 1 year, and >1 year posttransplant. UTY2 and SMCYb are shown as representatives of 3 UTY and 6 SMCY fragments, respectively. (B) Changes in prevalence of HY-Abs throughout 3 years post-HCT. UTY2 and SMCYb are shown as representatives of 3 UTY and 6 SMCY fragments, respectively. m, months.
Figure 2.
Detection of multiple HY-Abs. (A) Detection of multiple HY-Abs (HY score) at 3 months post-HCT was associated with their persistence (P < .001 by the Jonckheere-Terpstra test). A high average of overall relative HY-Ab quantifications throughout post-HCT indicates HY-Ab persistence as well as high intensity. (B) Association between individual HY-Ab and multiple recognitions. Overall P value was calculated by the Kruskal-Wallis test. **P < .01 and *P < .05 compared to SMCY by post-hoc test (the Steel-Dwass method).
Prevalence of HY-Abs
All HY-Abs except for RPS4Y-IgG were detected with a significantly higher frequency in F→M patients relative to M→M patients (P < .01 in each; Table 2). The HY-Abs most frequently detected in F→M patients at 3 months post-HCT were UTY (33%) and SMCY (37%) (Table 2). Although ZFY (15%) and DBY (19%) were less frequent, their detection was linked with detection of multiple HY-Abs (Figure 2B), suggesting a driver role for these 2 antigens in broad allogeneic antibody development. As expected, detection of multiple HY-Abs at 3 months was more frequently observed in F→M patients (35%) compared with M→M patients (10%; P < .001; Table2). Higher prevalence of HY-Abs in F→M HCT patients was also observed at 1 year post-HCT (Table2). In addition, relative HY-Ab quantifications in F→M HCT exceeded those in M→M HCT patients (supplemental Figure 2). We conclude that the HY-Ab seropositivity threshold based on nonparametric summaries (ie, Q3 + 2[IQR]) for each HY-Ab MFI provides increased sensitivity for persistent and prevalent alloimmune responses. In summary, 78 (57%) of the 136 F→M HCT patients tested seropositive for at least 1 of the 6 H-Y antigens at 3 months post-HCT, and 70% of HY-Ab–seropositive patients were detected at 3 months. In effort to maximize lead time for cGVHD prediction, the subsequent analysis explores the significance of clinically important 3-month allogeneic antibody detection.
Table 2.
Comparison of HY-Ab development between male patients with female (F→M) and male donors (M→M) at 3 months and at 1 year post-HCT
| Hy-Ab development | F→M at 3 mo (n = 136) | M→M at 3 mo (n = 60) | P | F→M at 1 y (n=92) | M→M at 1 y (n=50) | P |
|---|---|---|---|---|---|---|
| DBY | 26 (19%) | 3 (5%) | .009 | 23 (25%) | 3 (6%) | .006 |
| UTY* | 45 (33%) | 7 (12%) | .002 | 22 (24%) | 1 (2%) | .005 |
| RPS4Y | 25 (18%) | 8 (13%) | .4 | 18 (20%) | 5 (10%) | .16 |
| ZFY | 21 (15%) | 0 (0%) | <.001 | 15 (16%) | 0 (0%) | .001 |
| EIF1AY | 27 (20%) | 0 (0%) | <.0001 | 11 (12%) | 0 (0%) | .008 |
| SMCY* | 50 (37%) | 8 (13%) | .001 | 28 (30%) | 7 (14%) | .04 |
| Multiple HY-Abs (≥2) | 47 (35%) | 6 (10%) | <.001 | 25 (27%) | 3 (6%) | .002 |
Values are n (%) unless otherwise noted.
Any seropositivity against 3 UTY and 6 SMCY fragments were scored as positive of UTY and SMCY, respectively.
Pretransplant characteristics did not associate with 3-month HY-Ab detection
There were no positive associations between pretransplant clinical factors and overall 3-month HY-Ab development (Table 1). However, F→M HCT patients who developed grade 2 to 4 aGVHD were less likely to have HY-Abs detected at 3 months post-HCT (Table 1). Of the 31 patients with severe aGVHD, only 12 (39%) had 3-month HY-Abs detected, but 12 more subsequently seroconverted, providing an overall HY-Ab prevalence of 78%. Considering each H-Y antigen individually, only recipient CMV seropositivity was associated with UTY- or ZFY-IgG detection (P = .03 and P < .01, respectively; supplemental Table 2). In summary, HY-Ab development was not associated with pre-HCT clinical features.
The detection of individual HY-Abs at 3 months predicts cGVHD development and nonrelapse mortality (NRM)
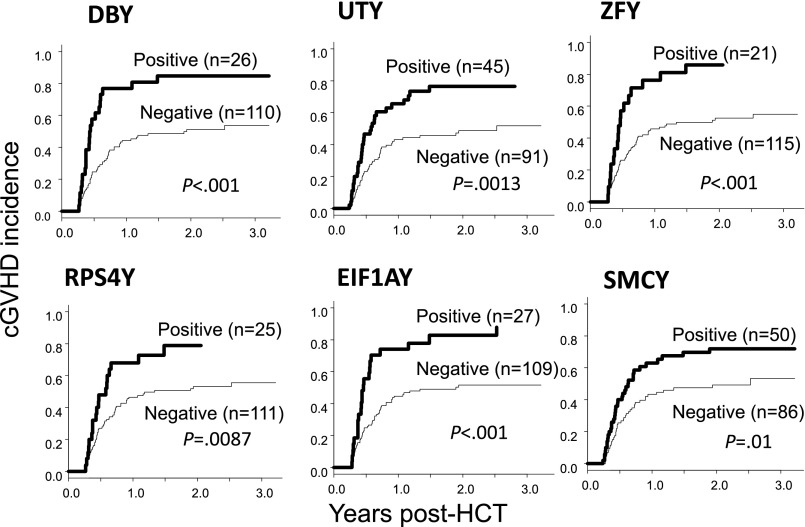
The detection of HY-Abs at 3 months was significantly related to clinical outcomes. Of the 136 patients, 83 (61%) experienced cGVHD, and their 1-year cGVHD cumulative incidence was 51% (95% CI: 42-59). Considering each HY-Ab separately, relative HY-Ab quantification was higher in cGVHD patients. F→M patients who tested seropositive were more likely to develop cGVHD, and their cumulative 1-year cGVHD incidence ranged from 70% to 80% (Figure 3; supplemental Table 3). Multivariate analysis demonstrated that all individual HY-Abs (except RPS4Y-IgG) detected at 3 months showed significant association with cGVHD (Table 3). Of the 6 HY-Abs, the greatest HR was observed with ZFY-IgG (HR: 3.62, P < .001) and DBY-IgG detection (HR: 3.03, P < .001).
Figure 3.
Cumulative cGVHD incidence. Graphs compare cumulative cGVHD incidence of individual HY-Abs in seropositive and seronegative patients at 3 months post-HCT.
Table 3.
Multivariate analyses for the impact of individual H-Y antigen IgGs on clinical outcomes
| Serostatus | n | HR* (95% CI) | P | |
|---|---|---|---|---|
| cGVHD | ||||
| DBY | Negative | 110 | 1 | |
| Positive | 26 | 3.03 (1.58-5.8) | .0009 | |
| EIF1AY | Negative | 109 | 1 | |
| Positive | 27 | 2.39 (1.48-3.88) | .0004 | |
| RPS4Y | Negative | 111 | 1 | |
| Positive | 25 | 1.55 (0.84-2.85) | .16 | |
| SMCY | Negative | 86 | 1 | |
| Positive | 50 | 1.99 (1.24-3.17) | .004 | |
| UTY | Negative | 91 | 1 | |
| Positive | 45 | 2.43 (1.39-4.25) | .0018 | |
| ZFY | Negative | 115 | 1 | |
| Positive | 21 | 3.62 (1.81-7.22) | .0003 | |
| NRM | ||||
| DBY | Negative | 110 | 1 | |
| Positive | 26 | 7.28 (1.93-27.5) | .0034 | |
| EIF1AY | Negative | 109 | 1 | |
| Positive | 27 | 4.90 (1.61-15.0) | .0053 | |
| RPS4Y | Negative | 111 | 1 | |
| Positive | 25 | 3.42 (1.15-10.1) | .027 | |
| SMCY | Negative | 86 | 1 | |
| Positive | 50 | 5.78 (1.30-25.7) | .021 | |
| UTY | Negative | 91 | 1 | |
| Positive | 45 | 6.93 (1.81-26.5) | .0047 | |
| ZFY | Negative | 115 | 1 | |
| Positive | 21 | 6.00 (1.34-27.0) | .019 | |
| Relapse | ||||
| DBY | Negative | 110 | 1 | |
| Positive | 26 | 0.50 (0.16-1.54) | .23 | |
| EIF1AY | Negative | 109 | 1 | |
| Positive | 27 | 0.65 (0.26-1.62) | .35 | |
| RPS4Y | Negative | 111 | 1 | |
| Positive | 25 | 0.52 (0.17-1.60) | .25 | |
| SMCY | Negative | 86 | 1 | |
| Positive | 50 | 0.79 (0.40-1.56) | .5 | |
| UTY | Negative | 91 | 1 | |
| Positive | 45 | 0.47 (0.21-1.02) | .06 | |
| ZFY | Negative | 115 | 1 | |
| Positive | 21 | 0.53 (0.16-1.71) | .29 | |
| OS | ||||
| DBY | Negative | 110 | 1 | |
| Positive | 26 | 1.97 (0.96-4.03) | .064 | |
| EIF1AY | Negative | 109 | 1 | |
| Positive | 27 | 1.10 (0.52-2.31) | .8 | |
| RPS4Y | Negative | 111 | 1 | |
| Positive | 25 | 1.40 (0.67-2.93) | .37 | |
| SMCY | Negative | 86 | 1 | |
| Positive | 50 | 1.63 (0.87-3.05) | .13 | |
| UTY | Negative | 91 | 1 | |
| Positive | 45 | 0.92 (0.49-1.75) | .8 | |
| ZFY | Negative | 115 | 1 | |
| Positive | 21 | 1.60 (0.76-3.35) | .22 |
HR, hazard ratio.
HRs of seropositivity of individual H-Y antigen IgGs are shown after adjusting for age of patient and donor, disease, disease risk, CMV serostatus, donor types, cell sources, usage of total body irradiation, usage of anti-thymocyte globulin, and grade of aGVHD.
The overall NRM was 11% (95% CI: 6-18) at 3 years post-HCT. Similar to cGVHD, all individual HY-Abs detected at 3 months were associated with an increased risk for NRM (Table 3). The greatest NRM HRs were observed with DBY-IgG (HR: 7.28, P < .01) and UTY-IgG (HR: 6.93, P < .01). Regarding relapse and OS, patients with UTY-IgG tended to have lower relapse risk (HR: 0.47, P = .06), and those with DBY-IgG tended to have an inferior (although not significant) survival risk (HR: 1.97, P = .06; Table 3).
Three-month HY-Abs and target organs of cGVHD at diagnosis
cGVHD organ involvement at diagnosis was associated with antigen-specific 3-month HY-Abs by least absolute shrinkage and selection operator (LASSO) analysis (supplemental Figure 3) within the current F→M HCT patients. For example, DBY-IgG demonstrated reproducible associations with skin, liver, and gastrointestinal involvement in replicated bootstrap analysis. Likewise, ZFY-IgG and UTY-IgG individually were associated with oral pharyngeal and liver involvement; EIF1AY-IgG was associated with skin, oral pharyngeal, ocular, and liver involvements. A different analysis to identify organ-specific HY-Ab alloimmunity compared the prevalence of organ involvements in 3-month HY-Ab–positive F→M patients to that in seronegative M→M patients (supplemental Table 4) and demonstrated the similar cGVHD organ-specific HY-Ab associations described above. These concordant results suggest that H-Y antigens may have organ-specific immunogenicity in certain cGVHD involvements. However, these results require independent validation.
HY score is an additive indicator of the development of multiple HY-Abs
We hypothesized that antibody detection of multiple H-Y antigens indicates stronger allogeneic immunity and would predict greater clinical impact. The HY score is the sum of 0 to 6 HY-Abs detected at 3 months post-HCT. As with individual HY-Abs, the HY score was also not associated with pretransplant clinical factors except CMV serostatus. CMV-seropositive patients tended to have a higher HY score at 3 months post-HCT (HY score of 4-6 in 18 [23%] of 78 CMV-positive patients and in 4 [7%] of 58 CMV-negative patients; P = .018). In addition, patients who experienced CMV reactivation also had a higher HY score of 4 to 6 (P = .033).
Three-month HY score predicts cGVHD development and NRM
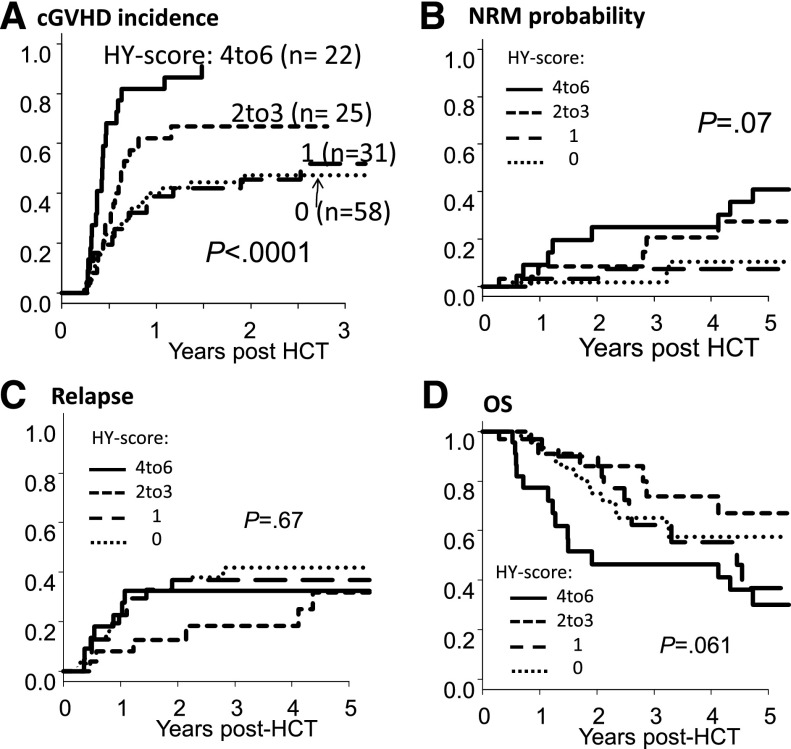
Patients with a higher 3-month HY score developed cGVHD more frequently. The 1-year cGVHD cumulative incidence was 82% (95% CI: 56-93) for an HY score of 4 to 6; 62% (95% CI: 39-79) for an HY score of 2 to 3; 39% (95% CI: 22-56) for an HY score of 1; and 40% (95% CI: 27-53) for an HY score of 0 (P < .001; Figure 4A). The cGVHD incidence of HY-Ab–seronegative patients (HY score of 0) was not significantly different from that of M→M patients (35% at 1 year; P = .87). In multivariate analysis, a higher 3-month HY score was significantly associated with an increased risk of cGVHD development (HR: 1.37, P < .0001; Table 4).
Figure 4.
HY score strongly associates with cGVHD and NRM. Cumulative incidences of cGVHD (A), NRM (B), relapse incidence (C), and OS (D) according to HY score at 3 months post-HCT. Univariate P value is shown.
Table 4.
Impact of HY-score at 3 months post-HCT on clinical outcomes in multivariate analyses
| HY score* | cGVHD | NRM | Relapse | OS | ||||
|---|---|---|---|---|---|---|---|---|
| HR† (95% CI) | P | HR† (95% CI) | P | HR† (95% CI) | P | HR† (95% CI) | P | |
| As a continuous variable | 1.37 (1.19-1.59) | <.0001 | 1.66 (1.17-2.35) | .0043 | 0.83 (0.66-1.04) | .11 | 1.11 (0.94-1.31) | .2 |
| As a group variable | ||||||||
| 0 | 1 | Ref. | 1 | Ref. | 1 | Ref. | 1 | Ref. |
| 1 | 1.11 (0.55-2.24) | .77 | 0.73 (0.09-5.96) | .77 | 0.97 (0.45-2.09) | .94 | 0.92 (0.40-2.11) | .85 |
| 2-3 | 2.19 (1.17-4.11) | .014 | 13.4 (1.99-89.7) | .0076 | 0.42 (0.17-1.02) | .06 | 0.62 (0.24-1.62) | .33 |
| 4-6 | 5.00 (2.30-10.9) | <.0001 | 20.0 (2.70-148) | .0034 | 0.56 (0.18-1.73) | .32 | 1.78 (0.79-4.01) | .16 |
Ref., reference.
HY score is the cumulative number of HY-Abs detected 3 mo post-HCT.
HRs are shown after adjusting for age of patient and donor, disease, disease risk, CMV serostatus, donor types, cell sources, usage of total body irradiation, usage of anti-thymocyte globulin, and grade of aGVHD.
NRM rates were also increased in patients with a higher 3-month HY score. Although the NRM difference was borderline significant in the univariate analysis (Figure 4B), the multivariate analysis showed that a high HY score was associated with an increased risk of NRM (HR: 1.66, P = .0043; Table 4). These associations between 3-month HY score and cGVHD/NRM did not change after additionally adjusting for other possible factors such as conditioning intensity and ABO mismatch.19
When we considered the HY score stratified into 4 groups, there was no significant difference in relapse (Figure 4C; Table 4) or OS (Figure 4D; Table 4). However, when we compared OS between 2 groups of HY scores (≥4 vs ≤3), an HY score of 4 to 6 was significantly associated with inferior survival (HR: 2.09, P = .047). Furthermore, when we analyzed those who survived beyond day 180 (excluding early relapse), 3-month HY score was significantly associated with reduced risk of subsequent relapse after 6 months (HR: 0.76, P = .043), consistent with our prior publication.8
In summary, HY score quantifies development of allogeneic antibodies against multiple H-Y antigens after F→M HCT and strongly associates with cGVHD and NRM.
Three-month HY score was associated with cGVHD severity at diagnosis
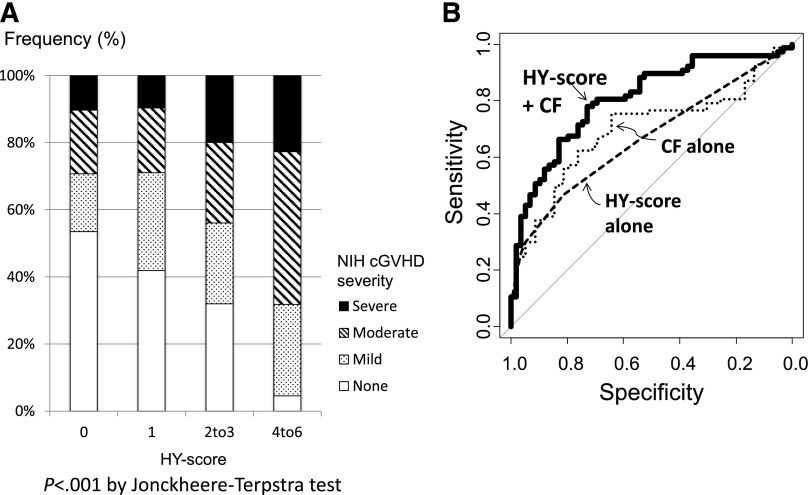
Considering cGVHD severity at diagnosis, F→M patients with a high 3-month HY score developed a higher grade of cGVHD (P < .001; Figure 5A). Of the 22 patients with an HY score of ≤4, 23% developed severe cGVHD, 46% developed moderate, 27% developed mild, and only 1 patient (5%) never developed cGVHD as scored by National Institutes of Health consensus criteria.20 In contrast, of the 58 patients with an HY score of 0, only 6 patients (10%) developed severe cGVHD, and more than half never developed cGVHD.
Figure 5.
Severity of cGVHD and ROC curve analysis. (A) Association between the severity of cGVHD at diagnosis and HY score at 3 months post-HCT. (B) Predictive potential for cGVHD by ROC curve analysis, comparing HY score alone, clinical factors (CF) alone, and their combination. The AUCs of HY score and clinical factors alone were 0.67 and 0.69, respectively; but when combined, the AUC increased to 0.81 (P < .01 in each comparison with the combination). Clinical factors included age of patient and donor, diagnosis of chronic myeloid leukemia, disease risk, CMV serostatus, donor types, cell sources, usage of total body irradiation, usage of anti-thymocyte globulin, and grade of aGVHD.5,6 NIH, National Institutes of Health.
Adding 3-month HY score to clinical factors improves prediction for cGVHD development
We compared the cGVHD predictive potential for 3-month HY score alone, clinical factors alone, and the combination of both by ROC curve analysis. The AUCs of 3-month HY score and clinical factors alone were 0.67 and 0.69, respectively. But ROC curve analysis combining HY score with clinical factors increased the AUC to 0.81 (P < .01 in each comparison with the combination; Figure 5B). When we focused on HY-Abs and only pretransplant factors, excluding aGVHD from clinical factors, the predictive value was preserved in our cohort (AUC: 0.81). This lack of aGVHD impact may be due to our overall low aGVHD incidence.
HY score at 6 and 9 months post-HCT was associated with subsequent cGVHD risk
In 61 of 83 cGVHD patients (74%), HY-Abs were detected before cGVHD diagnosis. In those 58 F→M HCT patients who tested HY-Ab negative at 3 months, the subsequent detection of HY-Abs and high relative HY-Ab quantification at ≥6 months was also associated with cGVHD development. We assessed the impact of HY score at 6 and 9 months post-HCT in relation to concurrent or subsequent cGVHD development among 6-month and 9-month cGVHD-free survivors, respectively. In multivariate analyses, the HY score at either 6 or 9 months was associated with an increased risk for subsequent cGVHD development (6 months [n = 79] HR: 1.62 (95% CI: 1.20-2.19), P = .0016; 9 months [n = 49] HR: 2.96 (95% CI: 1.04-8.42), P = .042). These increasing HRs of cGVHD development when measured closer to cGVHD onset also support our hypothesis that alloreactive antibodies play a pathogenic role in cGVHD development.
Discussion
We have established HY-Ab development in F→M HCT patients as a robust allogeneic immune assay with important clinical consequences. This study shows that 3-month HY-Ab detection stratifies risk of F→M HCT patients for overall cGVHD incidence, severity, and closely related NRM. Considering the frequent adverse effects of long-term corticosteroid therapy, a risk-adapted strategy is desired for the prevention of cGVHD development. We believe that the detection of HY-Abs 3 months before cGVHD development may provide a chance for intervention in future prospective clinical trials.
Our prior work at the Dana-Farber Cancer Institute in 2005 demonstrated that HY-Abs developed in F→M HCT patients,8 but the temporal association of HY-Ab and cGVHD development remained to be elucidated because the studies were limited to the measurement of blood samples collected ≥9 months after HCT. To investigate the biology of human allogeneic HCT, we have established a clinically well-characterized research sample repository since 2005 at Stanford University, and ∼1000 HCT patients have been prospectively collected thus far. Using this Stanford repository, we validated the association of HY-Ab and cGVHD development in an independent cohort by studying 136 F→M HCT patients with plasma prospectively collected, with a 1-year minimum clinical follow-up. In this study, using our H-Y microarray measurement platform in a blinded manner,17 we show that multiple and frequent HY-Abs were first detected at 3 months post-HCT, with very few detected at 2 months post-HCT. In general, IgG may not return to normal for 3 to 12 months,21,22 and hypogammaglobulinemia is reported to occur at a median of 56 days post-HCT.23 Humoral reconstitution post-HCT might require ≥3 months, although the true reason is unclear. It would be worthy to investigate and verify HY-Ab–specific B-cell development in the context of total B-cell reconstitution after various conditioning methods of allo-HCT to validate the results from this Stanford study. Of the 136 F→M patients, 57% had at least 1 of 6 HY-Abs at 3 months post-HCT, and the frequency of HY-Ab detection, especially multiple detections, was significantly higher in F→M patients than in M→M patients, which is consistent with our previous report.8 These are allogeneic antibody responses because they are elicited by sex-mismatch HCT and preferentially recognize H-Y protein and not their H-X homologs as shown in our previous study.8 In general, HY-Abs tended to persist after they developed. Nonetheless, our investigation identified a group who had HY-Abs disappear after detection at 3 months but often recurred later, and not surprisingly, many of these patients received rituximab as a treatment of cGVHD. Therapeutic rituximab decreases both allo-reactive B cells and HY-Abs, but they may recur 12 to 15 months after administration.24 We believe that this phenomenon should be addressed in prospective rituximab trials. Somewhat paradoxically, patients who experienced severe aGVHD were less likely to develop HY-Abs at 3 months, although they were shown to subsequently develop HY-Abs ≥6 months post-HCT. Of note, 28 of 31 patients with grade 3/4 aGVHD were still on steroid therapy at 3 months, and there was a significant inverse relation between the detection of HY-Abs at 3 months and steroid therapy (P = .011). It is possible that the low 3-month HY-Ab detection rate in aGVHD patients is due to the humoral immune suppressive effect of corticosteroids, which is then removed with subsequent steroid withdrawal. CMV seropositivity or reactivation, however, was associated with a high HY score. One possible explanation is that immune reconstitution against CMV might stimulate or enhance alloreactive immunity. Viral infections can induce HLA class II on nonhematopoietic tissues, making them targets for alloreactive CD4+ T cells that may elicit aGVHD.25 Similarly, CMV inflammation via alloreactive CD4+ T cells may augment H-Y humoral immunity indirectly, although the mediators remain to be determined.
This study of 136 F→M HCT shows 3-month HY-Ab detection has 3 clinical implications. First, each HY-Ab is additive for cGVHD prediction, and multiple H-Y alloimmunity quantified by HY score stratifies risk of cGVHD development as a predictive biomarker. Indeed, our 3-month HY score demonstrated excellent predictive potential for cGVHD development in combination with clinical factors. We could further identify patients at risk for cGVHD by considering clinical factors among patients with a low HY score, whereas the 3-month HY score alone could stratify those at high-risk for cGVHD. Furthermore, the HY score might also play a role as a diagnostic biomarker for cGVHD severity, because a higher HY score was also associated with cGVHD severity, indicating stronger alloimmunity. Our binomial analysis for each HY-Ab was determined based on a nonparametric description (Q3 + 2[IQR]) from 60 healthy men because there is no gold standard for HY-Ab–seropositive threshold. This analysis provided sensitive HY-Ab detection and relation to clinical outcomes. Because our MFI data will be uploaded on the online repository (Dryad), a further suitable decision rule for seropositive cutoff may be developed from other readers.
Second, individual allogeneic responses to H-Y antigens may play a role as a diagnostic biomarker for specific organ involvement of cGVHD. A possible explanation for the specificity would be the relative difference of H-Y protein expression among these cGVHD-involved tissues. Another ongoing study has collected skin biopsy samples from cGVHD patients, and will investigate the difference in gene expression of H-Y proteins between patients with cGVHD and normal subjects. Alternatively, H-Y antigen immunogenicity may vary by tissues depending on T-cell homing and antigen presentation cell frequencies. T cells responding to DBY peptides were CD4+-restricted T cells, and these CD4+ helper T cells are thought to promote IgG production by activated B cells in germinal centers. 26 Ubiquitous expression and efficient HLA presentation of H-Y antigens in involved organs may drive B-cell response, with high resultant HY-Abs. It would be worthwhile to assess the relationship between HLA-restricted T-cell response and HY-Ab development.
Third, testing 3-month HY score also stratifies F→M HCT patients for NRM. Patients who had an HY score of 4 to 6 were at high risk for NRM (HR: 20.0) in comparison with those having an HY score of 0 in our cohort. This high incidence of NRM in patients with an HY score of 4 to 6 would result in inferior OS compared with patients with an HY score of ≤3. This prognostic potential of 3-month HY-Abs should be validated in a multicenter observational study. After qualification, the HY score may be applied to a risk-adapted strategy as a predictive and prognostic biomarker for cGVHD. High-risk patients would be the best candidates for a trial of preemptive cGVHD prevention using novel B-cell interventions beginning at 3 months post-HCT. On the other hand, 3-month HY score was significantly associated with a subsequent relapse incidence among 6-month disease-free survivors but not relapse occurring before 6 months. Therefore, survival ≥6 months may be required for effective graft-versus-tumor benefit to develop through H-Y immunity.
In total, the increasing HY-Ab prevalence detected in advance of cGVHD development supports a pathogenic role of alloreactive B cells in human cGVHD, as has been reported in animal models.27,28 Murine alloantibody deposition is observed in cGVHD-involved organs, and allo-HCT in mice that cannot produce IgG demonstrates reduced cGVHD incidence.27 Our human study is unable to provide cGVHD pathogenicity of HY-Abs. However, HY-Ab detection preceded cGVHD diagnosis in 74% of cGVHD patients. Considering an opsonic effect of immunoglobulins, we hypothesize that HY-Abs may enhance inflammation and deteriorate tissue damages, eventually leading to cGVHD development. The correlative biological analysis of HY-Abs supports the allogeneic B-cell pathogenic role in independent prospective trials for cGVHD prophylaxis and treatment.9,24,29,30 HY-Abs would serve as a prognostic biomarker for cGVHD and an immunologic indicator.
Our study has several limitations. First, in this cohort, half of our patients received ATG, which contributes to lower incidence of severe aGVHD and NMR.31,32 A pathogenic B-cell role in cGVHD might have become apparent under the unique T-cell suppressive circumstance by ATG. However, it is relevant that ATG usage did not associate with HY-Ab development. Second, the clinical utility of HY-Ab testing is limited to male patients with female donors (F→M HCT). F→M HCT usually accounts for only 25% of patients undergoing allo-HCT, but in general, F→M HCT is an established independent risk factor for cGVHD.5-7 Furthermore, HY-Abs would play a role not only as a biomarker but also as a direct assessment of alloimmunity. Investigating HY-Abs in F→M HCT is an important model of humoral alloimmunity.33 This study is the first to comprehensively address the tempo of HY-Ab development. Now, B-cell–targeted drugs including rituximab, ofatumumab, and ibrutinib are currently being tested for cGVHD efficacy. HY-Abs may be evaluated as a predictive biomarker in these ongoing clinical trials, and anti–B-cell therapeutic depletion would be related to cGVHD clinical response, providing additional support for allogeneic B cells in cGVHD development.
In summary, HY-Abs were detected in F→M HCT patients prior to cGVHD development, and detection of multiple HY-Abs at 3 months after F→M HCT significantly predicted cGVHD and NRM. Monitoring HY-Ab development stratifies cGVHD risk in F→M patients and, thus, HY-Abs represent a promising cGVHD biomarker. Furthermore, this study would suggest a pathogenic role of alloreactive B-cell response and may support B-cell depletion therapy beginning at 3 months post-HCT to prevent cGVHD development.
Acknowledgments
The authors thank the patients who participated in this study for giving their precious samples, and the BMT nurses, patient coordinators, and staff at Stanford University Medical Center who made this work possible.
This work was supported by National Institutes of Health National Heart, Lung, and Blood Institute grant R21 HL084318, National Institutes of Health National Cancer Institute grant P01 CA049605, and Stanford University Cancer Institute support grant 1P030CA124435-01 (D.B.M.). H.N. was a recipient of a Japan Herpesvirus Infection Forum Scholarship Award, and D.B.M. was an American Society of Hematology Scholar Award recipient.
Footnotes
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.N. designed the study, collected the clinical data, analyzed the data, and wrote the manuscript; L.T. analyzed the data and revised the manuscript; B.S., K.S., S.P., J.P., C.E.R., and R.P. collected the samples and analyzed the data; F.W. and J.C. printed and processed the H-Y microarray method; K.S. and J.M.O. collected the clinical data; T.K. and E.H.W. provided the SMCY protein profiles and revised the manuscript; and D.B.M. designed the project, analyzed the data, wrote the manuscript, and was responsible for the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David B. Miklos, Division of Blood and Marrow Transplantation, Stanford University School of Medicine, 269 West Campus Dr, CCSR #2205, Stanford, CA 94305; e-mail: dmiklos@stanford.edu.
References
- 1.Powles R. 50 years of allogeneic bone-marrow transplantation. Lancet Oncol. 2010;11(4):305–306. doi: 10.1016/S1470-2045(10)70001-2. [DOI] [PubMed] [Google Scholar]
- 2.Pidala J, Anasetti C, Jim H. Quality of life after allogeneic hematopoietic cell transplantation. Blood. 2009;114(1):7–19. doi: 10.1182/blood-2008-10-182592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9(4):215–233. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 4.Inamoto Y, Flowers ME. Treatment of chronic graft-versus-host disease in 2011. Curr Opin Hematol. 2011;18(6):414–420. doi: 10.1097/MOH.0b013e32834ba87d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flowers ME, Inamoto Y, Carpenter PA, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117(11):3214–3219. doi: 10.1182/blood-2010-08-302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanda J, Nakasone H, Atsuta Y, et al. Risk factors and organ involvement of chronic GVHD in Japan. Bone Marrow Transplant. 2014;49(2):228–235. doi: 10.1038/bmt.2013.151. [DOI] [PubMed] [Google Scholar]
- 7.Randolph SS, Gooley TA, Warren EH, Appelbaum FR, Riddell SR. Female donors contribute to a selective graft-versus-leukemia effect in male recipients of HLA-matched, related hematopoietic stem cell transplants. Blood. 2004;103(1):347–352. doi: 10.1182/blood-2003-07-2603. [DOI] [PubMed] [Google Scholar]
- 8.Miklos DB, Kim HT, Miller KH, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105(7):2973–2978. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahaf B, Yang Y, Arai S, Herzenberg LA, Herzenberg LA, Miklos DB. H-Y antigen-binding B cells develop in male recipients of female hematopoietic cells and associate with chronic graft vs. host disease. Proc Natl Acad Sci USA. 2013;110(8):3005–3010. doi: 10.1073/pnas.1222900110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimabukuro-Vornhagen A, Hallek MJ, Storb RF, von Bergwelt-Baildon MS. The role of B cells in the pathogenesis of graft-versus-host disease. Blood. 2009;114(24):4919–4927. doi: 10.1182/blood-2008-10-161638. [DOI] [PubMed] [Google Scholar]
- 11.Ratanatharathorn V, Pavletic S, Uberti JP. Clinical applications of rituximab in allogeneic stem cell transplantation: anti-tumor and immunomodulatory effects. Cancer Treat Rev. 2009;35(8):653–661. doi: 10.1016/j.ctrv.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Sarantopoulos S, Stevenson KE, Kim HT, et al. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clin Cancer Res. 2007;13(20):6107–6114. doi: 10.1158/1078-0432.CCR-07-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Svegliati S, Olivieri A, Campelli N, et al. Stimulatory autoantibodies to PDGF receptor in patients with extensive chronic graft-versus-host disease. Blood. 2007;110(1):237–241. doi: 10.1182/blood-2007-01-071043. [DOI] [PubMed] [Google Scholar]
- 14.Cutler C, Miklos D, Kim HT, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108(2):756–762. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ratanatharathorn V, Logan B, Wang D, et al. Center for International Blood and Marrow Transplant Research, Milwaukee, WI, USA. Prior rituximab correlates with less acute graft-versus-host disease and better survival in B-cell lymphoma patients who received allogeneic peripheral blood stem cell transplantation. Br J Haematol. 2009;145(6):816–824. doi: 10.1111/j.1365-2141.2009.07674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Dorp S, Pietersma F, Wölfl M, et al. Rituximab treatment before reduced-intensity conditioning transplantation associates with a decreased incidence of extensive chronic GVHD. Biol Blood Marrow Transplant. 2009;15(6):671–678. doi: 10.1016/j.bbmt.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Wadia PP, Sahaf B, Miklos DB. Recombinant antigen microarrays for serum/plasma antibody detection. Methods Mol Biol. 2011;723:81–104. doi: 10.1007/978-1-61779-043-0_7. [DOI] [PubMed] [Google Scholar]
- 18.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Logan AC, Wang Z, Alimoghaddam K, et al. ABO mismatch is associated with increased nonrelapse mortality after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21(4):746–754. doi: 10.1016/j.bbmt.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Noel DR, Witherspoon RP, Storb R, et al. Does graft-versus-host disease influence the tempo of immunologic recovery after allogeneic human marrow transplantation? An observation on 56 long-term survivors. Blood. 1978;51(6):1087–1105. [PubMed] [Google Scholar]
- 22.Elfenbein GJ, Anderson PN, Humphrey RL, et al. Immune system reconstitution following allogeneic bone marrow transplantation in man: a multiparameter analysis. Transplant Proc. 1976;8(4):641–646. [PubMed] [Google Scholar]
- 23.Frangoul H, Min E, Wang W, et al. Incidence and risk factors for hypogammaglobulinemia in pediatric patients following allo-SCT. Bone Marrow Transplant. 2013;48(11):1456–1459. doi: 10.1038/bmt.2013.76. [DOI] [PubMed] [Google Scholar]
- 24.Sahaf B, Arai S, Otani J, Schoenrock K, Logan A, Miklos DB. Rituximab provides steroid-sparing therapy in new-onset chronic graft-versus-host disease [abstract]. Biol Blood Marrow Transplant. 2013;19(2):S140. Abstract 55.
- 25.Stevanovic S, van Bergen CA, van Luxemburg-Heijs SA, et al. HLA class II upregulation during viral infection leads to HLA-DP-directed graft-versus-host disease after CD4+ donor lymphocyte infusion. Blood. 2013;122(11):1963–1973. doi: 10.1182/blood-2012-12-470872. [DOI] [PubMed] [Google Scholar]
- 26.Zorn E, Miklos DB, Floyd BH, et al. Minor histocompatibility antigen DBY elicits a coordinated B and T cell response after allogeneic stem cell transplantation. J Exp Med. 2004;199(8):1133–1142. doi: 10.1084/jem.20031560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srinivasan M, Flynn R, Price A, et al. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood. 2012;119(6):1570–1580. doi: 10.1182/blood-2011-07-364414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young JS, Wu T, Chen Y, et al. Donor B cells in transplants augment clonal expansion and survival of pathogenic CD4+ T cells that mediate autoimmune-like chronic graft-versus-host disease. J Immunol. 2012;189(1):222–233. doi: 10.4049/jimmunol.1200677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arai S, Sahaf B, Narasimhan B, et al. Prophylactic rituximab after allogeneic transplantation decreases B-cell alloimmunity with low chronic GVHD incidence. Blood. 2012;119(25):6145–6154. doi: 10.1182/blood-2011-12-395970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cutler C, Kim HT, Bindra B, et al. Rituximab prophylaxis prevents corticosteroid-requiring chronic GVHD after allogeneic peripheral blood stem cell transplantation: results of a phase 2 trial. Blood. 2013;122(8):1510–1517. doi: 10.1182/blood-2013-04-495895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohrt HE, Turnbull BB, Heydari K, et al. TLI and ATG conditioning with low risk of graft-versus-host disease retains antitumor reactions after allogeneic hematopoietic cell transplantation from related and unrelated donors. Blood. 2009;114(5):1099–1109. doi: 10.1182/blood-2009-03-211441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Messina G, Giaccone L, Festuccia M, et al. Gruppo Italiano Trapianti di Midollo. Multicenter experience using total lymphoid irradiation and antithymocyte globulin as conditioning for allografting in hematological malignancies. Biol Blood Marrow Transplant. 2012;18(10):1600–1607. doi: 10.1016/j.bbmt.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Popli R, Sahaf B, Nakasone H, Lee JY, Miklos DB. Clinical impact of H-Y alloimmunity. Immunol Res. 2014;58(2-3):249–258. doi: 10.1007/s12026-014-8514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]