Abstract
Taxane-based chemotherapy for the treatment of breast cancer is associated with fluid retention in the extremities; however, its association with development of breast cancer-related lymphedema is unclear. We sought to determine if adjuvant taxane-based chemotherapy increased risk of lymphedema or mild swelling of the upper extremity. 1121 patients with unilateral breast cancer were prospectively screened for lymphedema with perometer measurements. Lymphedema was defined as a relative volume change (RVC) of ≥10 % from preoperative baseline. Mild swelling was defined as RVC 5- <10 %. Clinicopathologic characteristics were obtained via medical record review. Kaplan–Meier and Cox proportional hazard analyses were performed to determine lymphedema rates and risk factors. 29 % (324/1121) of patients were treated with adjuvant taxane-based chemotherapy. The 2-year cumulative incidence of lymphedema in the overall cohort was 5.27 %. By multivariate analysis, axillary lymph node dissection (ALND) (p < 0.0001), higher body mass index (p = 0.0007), and older age at surgery (p = 0.04) were significantly associated with increased risk of lymphedema; however, taxane chemotherapy was not significant when compared to no chemotherapy and non-taxane chemotherapy (HR 1.14, p = 0.62; HR 1.56, p = 0.40, respectively). Chemotherapy with docetaxel was significantly associated with mild swelling on multivariate analysis in comparison to both no chemotherapy and non-taxane chemotherapy groups (HR 1.63, p = 0.0098; HR 2.15, p = 0.02, respectively). Patients who receive taxane-based chemotherapy are not at an increased risk of lymphedema compared to patients receiving no chemotherapy or non-taxane adjuvant chemotherapy. Those treated with docetaxel may experience mild swelling, but this does not translate into subsequent lymphedema.
Keywords: Lymphedema, Breast cancer, Taxane chemotherapy, Arm swelling, Quality of life
Introduction
As the survival from early breast cancer continues to improve, the effects of post-treatment-related complications on long-term quality of life (QOL) have become increasingly important. Women treated for breast cancer face a lifetime risk of developing lymphedema, which is a chronic swelling of the arms, breast, or trunk due to an accumulation of lymphatic fluid in the interstitial tissues along with tissue remodeling and increased fibrosis. This condition is one of the most feared side effects of breast cancer treatment and is known to have a profoundly negative impact on QOL [1–6]. According to a recent meta-analysis, approximately one in five survivors will develop lymphedema [7].
Axillary lymph node dissection (ALND), regional lymph node radiation (RLNR), and higher body mass index (BMI) at time of diagnosis are well-established risk factors for development of lymphedema [1, 3, 8–20]. Some studies have reported increased incidence of lymphedema after chemotherapy [1, 7, 8, 13, 21–27]; however, other studies have not supported these findings [12, 28–30].These results warrant further investigation regarding the relationship between adjuvant chemotherapy and lymphedema.
Taxane-based chemotherapy is routinely used in the treatment of high-risk breast cancer and has been shown to improve both disease-free survival and overall survival. [31–35]. A common side effect of taxane-based chemotherapy, specifically docetaxel, is increased extracellular fluid (ECF) which often presents as fluid retention in the extremities [36–39]. Patients typically receive premedication with corticosteroids to prevent or delay onset of taxane-induced fluid retention while receiving treatment [36, 40]. However, it is unclear if taxane chemotherapy causes long-term arm swelling after completion of treatment.
Little data exists regarding the association between taxane-based chemotherapy and lymphedema development in breast cancer survivors. To date, only three studies have examined this relationship and all report that taxane-based chemotherapy increases the risk of lymphedema [41–43]. However, these studies are limited by lack of pre-operative arm volume measurement, varying definitions of lymphedema, small sample size, and limited long-term follow-up.
Since generalized fluid retention is common following taxane chemotherapy, we postulated that adjuvant taxane-based chemotherapy may overwhelm the compromised lymphatic vessels from breast and/or axillary surgery and therefore increase risk of lymphedema. We sought to determine whether taxane-based chemotherapy for the treatment of breast cancer is associated with increased risk of lymphedema in a large cohort of patients prospectively screened for arm volume changes. Additionally, we sought to investigate the relationship between taxane-based chemotherapy with mild arm swelling versus chronic arm swelling, and determine if type of taxane (paclitaxel vs. docetaxel) affected lymphedema risk.
Materials and methods
Study design
Per standard of care at our institution, all newly diagnosed breast cancer patients undergo routine screening for lymphedema with serial perometer arm volume measurements. The perometer is an optoelectronic device that uses infrared beams to measure and calculate overall limb volume [44–46]. Bilateral arm volume measurements are obtained pre-operatively, post-operatively, after completion of chemotherapy and/or radiation, and at regular follow-up oncology visits. This screening protocol was approved by the Partners Healthcare Institutional Review Board and has been previously published [47] [Clinicaltrials.gov Identification number NCT01521741].
Patient population
We identified 1121 women diagnosed with unilateral breast cancer between 2005 and 2012 who underwent surgery and prospective screening for lymphedema at our institution. All patients had a baseline arm volume measurement and ≥18 months of post-operative follow-up. Clinicopathologic characteristics, patient demographics, and treatment data were collected via medical record review. Arm measurements obtained after bilateral breast surgery or diagnosis of metastasis were excluded.
Lymphedema definition and measurement
Arm volume was quantified using the previously validated relative volume change (RVC) equation, which calculates change in volume compared to a pre-operative measurement [47]. Briefly, RVC = [(A(2)U(1)/U(2)A(1)) − 1], where A(1), A(2) are the preoperative (1) and postoperative (2) arm volumes on the surgical side and U(1), U(2) are arm volumes on the contralateral side at corresponding time points. The RVC equation accounts for asymmetry between the arms prior to surgery and utilizes the contralateral arm as a control to account for factors unrelated to lymphedema that may cause change in arm size such as weight gain or loss. Lymphedema was defined as a ≥ 10 % RVC occurring >3 months post-operatively. This definition was based on the scientific consensus in the literature which commonly utilizes a ≥ 10 % increase in the affected limb as criteria for diagnosing lymphedema [7, 12, 48, 49].
For the present study, we also investigated the risk of mild swelling as defined by RVC ≥ 5 to <10 %.
Chemotherapy
Taxane-based chemotherapy was classified as regimens containing docetaxel (Taxotere), paclitaxel (Taxol), or albumin-bound paclitaxel (Abraxane). Dexamethasone premedication was administered per institutional standard for each regimen. Patients who received neoadjuvant chemotherapy were excluded from this analysis.
Statistical analysis
Patient characteristics were summarized and compared between patients who did and did not receive taxane-based chemotherapy via Chi square and Wilcoxon tests. Two-year cumulative incidence of lymphedema, defined as RVC ≥ 10 % measured at least 3 months after surgery, was calculated within each taxane group using the Kaplan–Meier method. Median time from surgery to onset of lymphedema was calculated among patients who developed lymphedema. Univariate and multivariate Cox proportional hazard models were used to evaluate the association between lymphedema risk and use of taxane-based chemotherapy, as well as other risk factors. Time-dependent covariates were included for use of systemic therapies and radiation fields such that cases were included in the unexposed group prior to initiation of a given treatment and then were included in the exposed group after treatment began. The effects of treatment with paclitaxel and docetaxel were evaluated both combined (i.e. receiving either agent versus neither) and separately (paclitaxel versus docetaxel versus no taxane treatment). Multivariate models were derived using backwards selection, starting with a model that included all variables that were significant (p < 0.1) in the univariate analysis, and removing non-significant variables one at a time until only significant variables (p < 0.05) remained. Two-way interactions were evaluated for all covariates included in the resulting model. An additional analysis was conducted to assess the relationship between taxane use and risk of low level swelling, defined as 5 % ≤ RVC < 10 % measured at least 3 months after surgery. Patients with RVC ≥ 10 % were excluded from this analysis.
Results
Patient population
Arm volume measurements from 1121 patients were included with a median post-operative follow-up of 39.7 months (range 7.7–103.3). All patients underwent unilateral breast surgery with 76 % (854/1121) lumpectomy and 24 % (267/1121) mastectomy. 66 % (738/1121) underwent sentinel lymph node biopsy (SLNB) and 20 % (219/1121) had axillary lymph node dissection (ALND). 14 % (164/1121) did not have any nodal surgery, largely due to diagnosis of ductal carcinoma in situ. Of the 219 patients treated with ALND, 73 % (159/219) subsequently received taxane chemotherapy compared to 7 % (16/219) treated with non-taxane chemotherapy and 20 % (44/219) did not receive adjuvant chemotherapy. Out of 738 patients who had SLNB, 22 % (165/738) received taxane chemotherapy, 6 % (46/738) received non-taxane chemotherapy, and 71 % (527/738) received no chemotherapy. Clinicopathologic factors of patients with and without taxane-based chemotherapy are listed in Table 1.
Table 1.
Clinicopathologic characteristics of study population (n = 1121), adjuvant taxane patients (n = 324) compared with patients who received either adjuvant chemotherapy without taxane or no chemotherapy (n = 797)
| Entire cohort n = 1121 |
Adjuvant taxane n = 324 |
No adjuvant taxane n = 797 |
p valuea | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Median age at surgery (months) | 57 | 53 (24–78) | 59 (30–89) | <0.0001 |
| Median pre-operative body mass index (BMI)b (kg/m2) | 26.3 | 26.6 (16.8–50.4) | 26.2 (16.5–55.7) | 0.75 |
| Median post-operative follow up (months) | 39.7 | 42.0 (18–100.4) | 38.5 (7.7–103.3) | 0.05 |
| Breast surgery | <0.0001 | |||
| Lumpectomy | 854 (76 %) | 207 (64 %) | 647 (81 %) | |
| Mastectomy | 267 (24 %) | 117 (36 %) | 150 (19 %) | |
| Axillary surgery | <0.0001 | |||
| None | 164 (14 %) | 0 (0 %) | 164 (21 %) | |
| Sentinel lymph node biopsy (SLNB) | 738 (66 %) | 165 (51 %) | 573 (72 %) | |
| Axillary lymph node dissection (ALND) | 219 (20 %) | 159 (49 %) | 60 (8 %) | |
| Tumor type | <0.0001 | |||
| Invasive Carcinoma | 925 (83 %) | 320 (99 %) | 605 (76 %) | |
| Ductal Carcinoma in Situ (DCIS) | 196 (17 %) | 4 (1 %) | 192 (24 %) | |
| Pathologic characteristics | ||||
| Median invasive tumor size, cm‡ | 1.4 (0.05–12.5) | 1.9 (0.2–12.5) | 1.1 (0.05–10.5) | <0.0001 |
| Median number lymph nodes dissected | 2 (0–43) | 6 (1–43) | 1 (0–26) | <0.0001 |
| Median number positive lymph nodes | 0 (0–39) | 1 (0–39) | 0 (0–26) | <0.0001 |
| Radiation therapy | <0.0001 | |||
| None | 216 (19 %) | 40 (12 %) | 176 (22 %) | |
| Partial Breast Irradiation (PBI) | 96 (9 %) | 1 (0.3 %) | 95 (12 %) | |
| Breast + Chest Wall only | 640 (57 %) | 148 (46 %) | 492 (62 %) | |
| Breast + Chest Wall + Nodal Radiation (RLNR) | 167 (15 %) | 133 (41 %) | 34 (4 %) | |
| Adjuvant chemotherapy | <0.0001 | |||
| Yes | 386 (34 %) | 324 (100 %) | 62 (8 %) | |
| No | 735 (66 %) | 0 (0 %) | 735 (92 %) | |
| Adjuvant hormonal therapy | 0.20 | |||
| Yes | 874 (78 %) | 79 (24 %) | 167 (21 %) | |
| No | 246 (22 %) | 244 (76 %) | 630 (79 %) | |
| Herceptin-based chemotherapy | <0.0001 | |||
| Yes | 87 (8 %) | 75 (23 %) | 12 (2 %) | |
| No | 1031 (92 %) | 248 (77 %) | 783 (98 %) | |
a P value for test of association between characteristic and receipt of taxane
b17 values missing for BMI
29 % (324/1121) of the cohort received adjuvant taxane chemotherapy, 6 % (62/1121) received non-taxane chemotherapy, and the remaining 66 % (735/1121) received no chemotherapy. Out of the 324 patients who received taxane-based chemotherapy, 56 % (181/324) were treated with paclitaxel, 40 % (131/324) with docetaxel, and 3 % (9/324) with albumin-bound paclitaxel. 3 patients received a combination of the above types of taxane-containing regimens due to intolerance of initial taxane administered.
Cumulative incidence of lymphedema
The two-year cumulative incidence of lymphedema was 5.27 % (95 % CI 4.10–6.76 %) for the overall cohort. By chemotherapy group, the cumulative incidence of lymphedema was 10.29 % (95 % CI 7.43–14.18 %) for those receiving taxane chemotherapy compared to 4.87 % (95 % CI 1.60–14.33) for those receiving non-taxane chemotherapy and 3.07 % (95 % CI 2.03–4.63 %) for those who did not receiving chemotherapy (Table 2).
Table 2.
Two-year cumulative incidence of lymphedema (RVC ≥ 10 %) overall and by chemotherapy group
| N | 2-Year cumulative incidence (%) | 95 % Confidence interval | |
|---|---|---|---|
| Entire cohort | 1121 | 5.27 | 4.10–6.76 |
| No chemotherapy | 735 | 3.07 | 2.03–4.63 |
| Non-taxane chemotherapy | 62 | 4.87 | 1.60–14.33 |
| Taxane-based chemotherapy | 324 | 10.29 | 7.43–14.18 |
Cumulative incidence of mild swelling
The two-year cumulative incidence of mild swelling was 16.37 % for the overall cohort. For patients receiving taxane chemotherapy, the cumulative incidence of mild swelling was 22.76 % (95 % CI 18.19–28.28 %) compared to 7.05 % (95 % CI 2.71–17.71 %) for the non-taxane chemotherapy group and 14.64 % (95 % CI 12.20–17.53 %) for those who did not receive any chemotherapy (Table 3).
Table 3.
Two-year cumulative incidence of mild swelling (RVC 5- < 10 % RVC) overall and by chemotherapy group
| N | 2-Year cumulative incidence (%) | 95 % Confidence interval | |
|---|---|---|---|
| Entire cohort | 1121 | 16.37 | 14.22–18.80 |
| No chemotherapy | 735 | 14.64 | 12.20–17.53 |
| Non-taxane chemotherapy | 62 | 7.05 | 2.71–17.71 |
| Taxane-based chemotherapy | 324 | 22.76 | 18.19–28.28 |
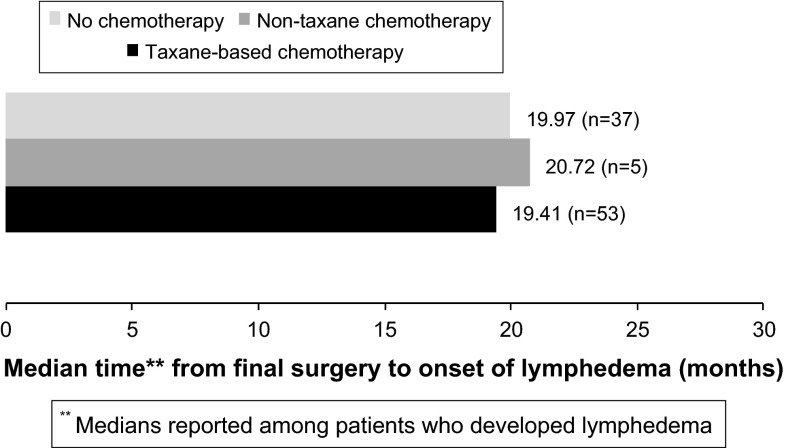
Timing of swelling
Among patients who developed lymphedema, median time from final surgery to onset of lymphedema was 19.97 months in the no chemotherapy group, 20.72 months in the non-taxane chemotherapy group, and 19.41 months in the taxane chemotherapy group. Among those who developed mild swelling, median time from final surgery to onset of mild swelling was 19.28 months in the no chemotherapy group, 44.21 months in the non-taxane chemotherapy group and 14.54 months in the taxane chemotherapy group (Fig. 1).
Fig. 1.
Median time to onset of lymphedema (RVC ≥ 10 %) by chemotherapy group
Univariate analysis
By univariate analysis, adjuvant taxane-based chemotherapy was associated with a significantly increased risk of lymphedema, as defined by RVC ≥ 10 %, compared to no chemotherapy (HR 2.61, p < 0.0001) as well as non-taxane chemotherapy (HR 2.90, p = 0.0412). In addition, an analysis of paclitaxel and docetaxel as individual agents showed that both were significantly associated with lymphedma risk compared to those who did not receive chemotherapy (HR 2.00, p = 0.0053; HR 2.54, p = 0.0004, respectively). However, when compared to the non-taxane chemotherapy group, only docetaxel was significant for increased lymphedema risk (HR 3.12, p = 0.0392). Other significant risk factors included: higher pre-operative BMI, ALND, greater number of lymph nodes (LNs) removed, invasive versus ductal carcinoma in situ pathology, greater number of positive LNs, and RLNR (Table 4).
Table 4.
Univariate and multivariate analysis of characteristics associated with risk of lymphedema (RVC ≥ 10 %)
| Univariate results | Multivariate resultsc | |||
|---|---|---|---|---|
| Hazard ratio (95 % CI) | p value | Hazard ratio (95 % CI) | p value | |
| Patient characteristics | ||||
| Age at surgery (years)a | 1.02 (1.00–1.03) | 0.1080 | 1.02 (1.00–1.04) | 0.0433 |
| Pre-operative BMIa (kg/m2) | 1.07 (1.04–1.10) | <0.0001 | 1.05 (1.02–1.09) | 0.0007 |
| Surgical characteristics | ||||
| Axillary surgery | ||||
| SLNB versus no axillary surgery | 0.83 (0.36–1.89) | 0.6507 | b | – |
| ALND versus no axillary surgery | 6.31 (2.88–13.80) | <0.0001 | 7.32 (3.16–17.01) | <0.0001 |
| ALND versus SLNB/no axillary surgery | 7.37 (4.86–11.19) | <0.0001 | 8.19 (5.12–13.10) | <0.0001 |
| Pathologic characteristics | ||||
| Invasive vs. DCIS | 2.20 (1.06–4.53) | 0.0335 | 1.05 (0.49–2.25) | 0.9041 |
| Number positive lymph nodesa | 1.10 (1.07–1.13) | <0.0001 | 1.02 (0.98–1.06) | 0.4337 |
| Systemic therapy | ||||
| Adjuvant taxane-based chemotherapy | ||||
| Yes versus no chemo | 2.61 (1.73–3.95) | <0.0001 | 1.14 (0.69–1.87) | 0.6188 |
| Yes versus non-taxane chemo | 2.90 (1.04–8.08) | 0.0412 | 1.56 (0.56–4.37) | 0.3988 |
| Paclitaxel | ||||
| Yes versus no chemo | 2.00 (1.23–3.24) | 0.0053 | 0.81 (0.46–1.41) | 0.4473 |
| Yes versus non-taxane chemo | 2.45 (0.85–7.07) | 0.0984 | 1.26 (0.43–3.65) | 0.6725 |
| Docetaxel | ||||
| Yes versus no chemo | 2.54 (1.52–4.26) | 0.0004 | 1.25 (0.71–2.18) | 0.4374 |
| Yes versus non-taxane chemo | 3.12 (1.06–9.20) | 0.0392 | 1.95 (0.65–5.85) | 0.2334 |
| Non-taxane chemotherapy | ||||
| Yes versus no chemo | 0.90 (0.32–2.52) | 0.8417 | 0.64 (0.22–1.83) | 0.4056 |
| Hormonal therapy | ||||
| Yes versus no | 1.59 (0.90–2.80) | 0.1098 | – | |
| Herceptin-based chemotherapy | ||||
| Yes versus no | 1.10 (0.55–2.20) | 0.7778 | – | – |
| Radiation therapy | ||||
| Yes versus no | 1.06 (0.65–1.75) | 0.8056 | – | – |
| RLNR versus breast+chest wall/none | 4.32 (2.82–6.63) | <0.0001 | 1.29 (0.77– 2.16) | 0.3354 |
CI confidence interval, BMI body mass index, SLNB sentinel lymph node biopsy, ALND axillary lymph node dissection, DCIS ductal carcinoma in situ, RLNR regional lymph node radiation
aAge at surgery, pre-operative BMI, and number of positive lymph nodes were analyzed as continuous variables such that the hazard ratios reflect the change in lymphedema risk associated with a 1-unit increase in the variable
b"–" indicates the specified variable/comparison was not analyzed
c2 Separate models, each including age at surgery, BMI and ALND were used to estimate the hazard ratios for (1) adjuvant taxane-based chemo and (2) individual effects of paclitaxel and docetaxel
Univariate analysis of mild swelling, as defined by RVC ≥ 5 % to <10 %. indicated that taxane chemotherapy was associated with a borderline significant increase in risk of mild swelling compared to no chemotherapy (HR 1.31, p = 0.0512), and a significant increase in risk of mild swelling compared to non-taxane chemotherapy (HR 1.86, p = 0.0398). Additionally, comparison of paclitaxel and docetaxel as individual agents showed docetaxel, but not paclitaxel to be significantly associated with mild swelling when compared to non-taxane chemotherapy (HR 2.31, p = 0.0107; HR 1.65, p = 0.1163, respectively). Docetaxel was also associated with a significant increase in risk for mild swelling compared to no chemotherapy (HR 1.62, p = 0.0084), however, paclitaxel was not (HR 1.16, p = 0.3882). Other significant risk factors for mild swelling included older age at surgery, ALND, greater number of LNs removed, and greater number of positive LNs (Table 5).
Table 5.
Univariate and multivariate analysis of characteristics associated with risk of mild swelling (5 ≤ 10 % RVC)
| Univariate results | Multivariate resultsc | |||
|---|---|---|---|---|
| Hazard Ratio (95 % CI) | p value | Hazard Ratio (95 % CI) | p value | |
| Patient characteristics | ||||
| Age at surgery (years)a | 1.02 (1.01–1.03) | 0.0011 | 1.02 (1.01–1.03) | 0.0003 |
| Pre-operative BMIa (kg/m2) | 1.02 (1.00–1.04) | 0.1366 | 1.01 (0.99–1.03) | 0.4157 |
| Surgical characteristics | ||||
| Axillary surgery | ||||
| SLNB versus no axillary surgery | 0.90 (0.63–1.29) | 0.5631 | –b | – |
| ALND versus no axillary surgery | 1.36 (0.89–2.07) | 0.1527 | 1.35 (0.84–2.18) | 0.2188 |
| ALND versus SLNB/no axillary surgery | 1.48 (1.10–2.00) | 0.0105 | 1.47 (1.05–2.07) | 0.0266 |
| Pathologic characteristics | ||||
| Invasive vs. DCIS | 1.42 (0.99–2.04) | 0.0572 | 1.27 (0.87–1.84) | 0.2152 |
| Number positive lymph nodesa | 1.07 (1.03–1.11) | 0.0002 | 1.04 (0.99–1.00) | 0.0603 |
| Systemic therapy | ||||
| Adjuvant taxane-based chemo | ||||
| Yes versus no chemo | 1.31 (1.00–1.71) | 0.0512 | 1.33 (0.97–1.83) | 0.0778 |
| Yes versus non-taxane chemo | 1.86 (1.03–3.35) | 0.0398 | 1.74 (0.95–3.13) | 0.0732 |
| Paclitaxel | ||||
| Yes versus no-chemo | 1.16 (0.83–1.62) | 0.3882 | 1.13 (0.77–1.66) | 0.5428 |
| Yes versus non-taxane chemo | 1.65 (0.88–3.07) | 0.1163 | 1.49 (0.79–2.80) | 0.2174 |
| Docetaxel | ||||
| Yes versus no-chemo | 1.62 (1.13–2.32) | 0.0084 | 1.63 (1.13–2.36) | 0.0098 |
| Yes versus non-taxane chemo | 2.31 (1.21–4.38) | 0.0107 | 2.15 (1.13–4.09) | 0.0195 |
| Non-taxane chemotherapy | ||||
| Yes versus no chemo | 0.70 (0.40, 1.24) | 0.2265 | 0.75 (0.42–1.35) | 0.3473 |
| Hormonal therapy | ||||
| Yes versus no | 1.05 (0.78–1.41) | 0.7639 | – | – |
| Herceptin-based chemotherapy | ||||
| Yes versus no | 0.80 (0.50–1.30) | 0.3644 | – | – |
| Radiation therapy | ||||
| Yes versus no | 1.04 (0.77–1.40) | 0.8106 | – | – |
| RLNR versus breast + chest wall/none | 1.18 (0.80–1.74) | 0.4043 | 0.82 (0.51–1.31) | 0.4064 |
CI confidence interval, BMI body mass index, SLNB sentinel lymph node biopsy, ALND axillary lymph node dissection, DCIS ductal carcinoma in situ, RLNR regional lymph node radiation
aAge at surgery, pre-operative BMI, and number of positive lymph nodes were analyzed as continuous variables such that the hazard ratios reflect the change in lymphedema risk associated with a 1-unit increase in the variable
b"–" indicates the specified variable/comparison was not analyzed
c2 Separate models, each including age at surgery, BMI and ALND were used to estimate the hazard ratios for (1) adjuvant taxane-based chemo and (2) individual effects of paclitaxel and docetaxel
Multivariate analysis
Receipt of taxane-based chemotherapy did not remain significantly associated with increased risk of lymphedema compared to no chemotherapy (HR 1.14, p = 0.6188) and non-taxane chemotherapy (HR 1.56, p = 0.3988) on multivariate analysis. Neither paclitaxel nor docetaxel was significantly associated with increased lymphedema risk when analyzed as individual agents (Table 4). Risk factors that were associated with lymphedema included ALND (HR 8.19, p < 0.0001), higher pre-operative BMI (HR 1.05, p = 0.0007), and older age at surgery (HR 1.02, p = 0.0433) (Table 4).
Adjuvant taxane-based chemotherapy associated with a borderline increase in risk of mild swelling compared to no chemotherapy (HR 1.33, p = 0.0778) and non-taxane chemotherapy (HR 1.74, p = 0.0732). Docetaxel was significantly associated with increased risk of mild swelling when compared to no chemotherapy (HR 1.63, p = 0.0098) as well as to non-taxane chemotherapy (HR 2.15, p = 0.0195). Paclitaxel, however, was not associated with risk of mild swelling in comparison to either the no chemotherapy group or the non-taxane chemotherapy group (HR 1.13, p = 0.5428; HR 1.49, p = 0.2174, respectively) (Table 5). Older age at surgery (HR 1.02, p = 0.0003) and ALND (HR 1.47, p = 0.0266) were also significantly associated with increased risk of mild swelling on multivariate analysis.
Discussion
In this cohort of 1121 patients prospectively screened for lymphedema with perometer measurements, adjuvant taxane-based chemotherapy with either paclitaxel or docetaxel was not significantly associated with an increased risk of lymphedema (RVC ≥ 10 %) compared to no adjuvant chemotherapy as well as non-taxane chemotherapy. However, adjuvant chemotherapy with docetaxel was a significant risk factor for mild arm swelling (5 to <10 % RVC) compared to no chemotherapy as well as non-taxane chemotherapy (p = 0.0098, p = 0.0195, respectively). Additional risk factors for mild arm swelling were older age at surgery and ALND. Consistent with the literature, ALND, pre-operative BMI ≥ 30, and older age at surgery were independent risk factors for lymphedema (RVC ≥ 10 %).
Currently, taxane-based chemotherapy is administered for node-positive and high-risk node-negative breast cancer, as it has been shown to significantly reduce mortality [31–35, 38, 50–53]. The use of anthracycline-alone regimens in breast cancer treatment has declined [54], which has resulted in a growing increase of taxane-based chemotherapy for early stage breast cancer. Therefore, a full understanding of the QOL and long-term implications of taxanes is necessary.
Docetaxel has relatively greater hematologic toxicity and is more commonly associated with edema than paclitaxel [53]. To reduce incidence and severity of edema, coticosteroids are routinely administered [40]. Due to similarities between the mechanism of fluid retention and development of breast cancer-related lymphedema [55, 56] the association between taxane-based chemotherapy (specifically docetaxel) and lymphedema warrants further investigation. A recent review comparing adjuvant chemotherapy with and without docetaxel in breast cancer patients showed that patients receiving docetaxel consistently had increased rates of edema compared to patients receiving docetaxel-free chemotherapy [57]. This review included studies comparing generalized edema in docetaxel and docetaxel-free groups, but did not specifically report on lymphedema of the arm. The relationship between docetaxel and upper extremity lymphedema is unclear, as the only studies in the literature examining this are case reports [58, 59].
The association of breast cancer-related lymphedema with taxane-based chemotherapy has been reported, but not widely studied. In 2013, Kilbreath et al. analyzed 160 women for lymphedema with bioimpedence spectroscopy (BIS) as part of a larger randomized study which evaluated effect of exercise after breast surgery. Measurements were taken at 1, 3, 9, and 15 months post-operatively and lymphedema was defined according to previously established cutoffs. On multivariate analysis, patients who received taxane chemotherapy had a 7-fold greater risk of swelling in the arm at 9-months after surgery compared to women who did not receive taxane chemotherapy (HR 7.4, p < 0.001) [42]. However, taxane chemotherapy did not remain significant at 15 months post-operative, leading to the conclusion that taxanes cause transient swelling in the at-risk arm.
A similar study by Jung et al. evaluating patients who underwent ALND showed that taxane-based chemotherapy was an independent risk factor for lymphedema on multivariate analysis [41]. In 848 patients evaluated post-operatively for a one-time lymphedema event as well as for persistent lymphedema, taxane-based chemotherapy was associated with higher incidence of lymphedema (HR = 1.69, p = 0.03 for lymphedema event; HR 2.07, p = 0.04 for persistent lymphedema) [41]. However, patients did not undergo a pre-operative arm measurement and lymphedema was defined using a wide range of both objective and subjective criteria. Interestingly, in our analysis, taxane-based chemotherapy was not associated with increased risk of lymphedema. Possible explanations for this include low cumulative incidence of lymphedema of the entire cohort and the impact of multivariable analysis adjusted for factors known to increase risk of lymphedema such as ALND (Table 3).
Most recently, Lee et al. reported on lymphedema following taxane chemotherapy in women with early stage breast cancer. 63 patients were assessed with BIS after axillary surgery, before taxane-chemotherapy, and 6 months after completion of chemotherapy. Results showed that taxane chemotherapy increased incidence of lymphedema of the ipsilateral arm and persisted for at least 6 months after completion of chemotherapy, whereas generalized edema in the legs resolved during this timeframe [43]. This series was limited by small sample size and lack of long-term follow-up. In our series the median time to lymphedema in all cohorts was between 19 and 21 months post-surgery, therefore the increased swelling that this study reports could be related to transient edema.
The results of our study show adjuvant docetaxel increases risk of mild swelling; however, multivariate analysis indicates that this does not subsequently lead to increased risk of lymphedema. These findings are consistent with the reported side effects of docetaxel treatment, and further the understanding of the relationship between docetaxel and breast cancer-related lymphedema. More importantly, because docetaxel is not an independent risk factor for lymphedema and the median time to onset of lymphedema was similar across chemotherapy groups (Fig. 1), results of this study suggest that it is largely ALND that elevates risk of lymphedema, not receipt of taxane chemotherapy.
As many patients who receive ALND are also subsequently treated with taxanes due to more advanced disease, these individuals should still be closely monitored for development of lymphedema. The strong association between adjuvant docetaxel and mild swelling highlights the need to distinguish between minor increases in arm volume versus chronic lymphedema. Because of our prospective screening and quantified method of measuring arm volume, the results of this study fill a gap in the literature regarding risk of lymphedema after receiving taxane-based chemotherapy. Furthermore, our data better informs clinicians of docetaxel-related arm edema, and can help educate patients on treatment-related risk factors for lymphedema.
Due to the non-randomized selection of patients for taxane-based chemotherapy versus non-taxane-based chemotherapy, there are limitations in the present study. At our institution, few patients receive chemotherapy without a taxane (i.e. anthracycline alone); out of 1121 patients eligible for this analysis, only 62 received adjuvant non-taxane chemotherapy (5.5 %). Although a larger percentage of patients receiving non-taxane-based chemotherapy would have allowed for a more accurate analysis on the effects of taxane, the minimal usage of anthracyclines alone in our cohort reflects standard practice and guidelines for systemic treatment. The nature of our screening protocol calls for arm volume assessments to be taken before and after completion of chemotherapy, but not during; therefore, the data that are reported in this study does not include any arm volume changes that occurred while receiving active taxane chemotherapy. Taking these factors into account, there are many areas for future research.
The current study also has several strengths. We utilized a large cohort of patients prospectively screened for changes in arm volume with a perometer, a device with demonstrated validity for lymphedema assessment [44, 45, 60, 61]. This cohort of 1121 patients represents one of the largest in the lymphedema literature, and to our knowledge, the largest in which risk of lymphedema was evaluated for association with adjuvant taxane-based chemotherapy. Of note, patients receiving neoadjuvant chemotherapy were excluded in order to solely assess the hypothesis that fluid retention from chemotherapy may overwhelm compromised lymphatic vessels after surgery, and could therefore lead to chronic lymphedema. All patients in our study underwent a pre-operative arm volume measurement and regular post-operative screening, with a median follow-up of over 3 years. The importance of obtaining pre-operative assessments to account for asymmetry between arms and adjustment for factors unrelated to lymphedema has been previously demonstrated [47, 62, 63]. In addition, we utilized a validated formula to measure arm volume differences, accounting for baseline pre-operative differences as well as weight changes [64]. Lymphedema was defined as ≥10 % RVC, which has been widely used in the literature [7, 12, 48].
Our study also separates paclitaxel and docetaxel for risk of lymphedema due to the well known differences in adverse events related to these therapeutic agents. Additionally, distinction between mild and chronic edema was evaluated in both univariate and multivariate models. Mild swelling was defined as RVC ≥ 5 to <10 % based on our previously published analysis of 1173 patients in which we found that a measurement of ≥5 to <10 % RVC at >3 months post-operative was significantly associated with an increased risk of progression to ≥10 % [65]. As the importance of detecting sublinical edema has been cited in the literature [63, 66–68], we sought to determine if patients receiving taxane-based chemotherapy were more likely to exhibit these low level arm volume increases compared to patients who did not receive taxane-based chemotherapy. Further, we sought to determine if mild swelling after taxane chemotherapy led to progressive lymphedema or if it was transient (never progressing to RVC ≥ 10 %). Results of our analyses suggest that docetaxel, but not paclitaxel is associated with risk of mild swelling, but that neither taxane is a risk factor for development of lymphedema.
Conclusions
In conclusion, multivariate analysis of 1121 patients prospectively screened for lymphedema via perometry demonstrated that taxane-based chemotherapy did not increase risk of lymphedema. Athough docetaxel was found to be a significant risk factor for mild swelling, it did not correlate with progressive lymphedema. These findings can be utilized for patient counseling and education regarding common side effects while undergoing taxane-based chemotherapy. Although arm volume changes should be regularly monitored for early signs of progression, it is important for clinicians to differentiate between treatment-related risk factors for developing chronic edema versus mild or transient edema that may resolve without intervention. This may help patients avoid costly treatment expenses and potentially reduce fear of lymphedema.
Acknowledgments
The study described was supported by Award Number R01CA139118 (AGT), Award Number P50CA089393 (AGT) from the National Cancer Institute and the Adele McKinnon Research Fund for Breast Cancer-Related Lymphedema. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Conflict of interest
The authors have no conflicts of interest to disclose.
References
- 1.Paskett ED, Naughton MJ, McCoy TP, et al. The epidemiology of arm and hand swelling in premenopausal breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2007;16:775–782. doi: 10.1158/1055-9965.EPI-06-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakorafas GH, Peros G, Cataliotti L, et al. Lymphedema following axillary lymph node dissection for breast cancer. Surg Oncol. 2006;15:153–165. doi: 10.1016/j.suronc.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Hayes SC, Janda M, Cornish B, et al. Lymphedema after breast cancer: incidence, risk factors, and effect on upper body function. J Clin Oncol. 2008;26:3536–3542. doi: 10.1200/JCO.2007.14.4899. [DOI] [PubMed] [Google Scholar]
- 4.Jager G, Doller W, Roth R. Quality-of-life and body image impairments in patients with lymphedema. Lymphology. 2006;39:193–200. [PubMed] [Google Scholar]
- 5.Ridner SH. The psycho-social impact of lymphedema. Lymphat Res Biol. 2009;7:109–112. doi: 10.1089/lrb.2009.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jammallo LS, Miller CL, Horick NK, et al. Factors associated with fear of lymphedema after treatment for breast cancer. Oncol Nurs Forum. 2014;41:473–483. doi: 10.1188/14.ONF.473-483. [DOI] [PubMed] [Google Scholar]
- 7.DiSipio T, Rye S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14:500–515. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 8.Norman SA, Localio AR, Kallan MJ, et al. Risk factors for lymphedema after breast cancer treatment. Cancer Epidemiol Biomarkers Prev. 2010;19:2734–2746. doi: 10.1158/1055-9965.EPI-09-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang EJ, Park WB, Seo KS, et al. Longitudinal change of treatment-related upper limb dysfunction and its impact on late dysfunction in breast cancer survivors: a prospective cohort study. J Surg Oncol. 2010;101:84–91. doi: 10.1002/jso.21435. [DOI] [PubMed] [Google Scholar]
- 10.Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25:3657–3663. doi: 10.1200/JCO.2006.07.4062. [DOI] [PubMed] [Google Scholar]
- 11.Park JH, Lee WH, Chung HS. Incidence and risk factors of breast cancer lymphoedema. J Clin Nurs. 2008;17:1450–1459. doi: 10.1111/j.1365-2702.2007.02187.x. [DOI] [PubMed] [Google Scholar]
- 12.Tsai RJ, Dennis LK, Lynch CF, et al. The risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factors. Ann Surg Oncol. 2009;16:1959–1972. doi: 10.1245/s10434-009-0452-2. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed RL, Schmitz KH, Prizment AE, et al. Risk factors for lymphedema in breast cancer survivors, the Iowa Women’s Health Study. Breast Cancer Res Treat. 2011;130:981–991. doi: 10.1007/s10549-011-1667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crosby MA, Card A, Liu J, et al. Immediate breast reconstruction and lymphedema incidence. Plast Reconstr Surg. 2012;129:789e–795e. doi: 10.1097/PRS.0b013e31824a2ab1. [DOI] [PubMed] [Google Scholar]
- 15.Swenson KK, Nissen MJ, Leach JW, et al. Case-control study to evaluate predictors of lymphedema after breast cancer surgery. Oncol Nurs Forum. 2009;36:185–193. doi: 10.1188/09.ONF.185-193. [DOI] [PubMed] [Google Scholar]
- 16.Soran A, D’Angelo G, Begovic M, et al. Breast cancer-related lymphedema—what are the significant predictors and how they affect the severity of lymphedema? Breast J. 2006;12:536–543. doi: 10.1111/j.1524-4741.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 17.Nesvold IL, Dahl AA, Lokkevik E, et al. Arm and shoulder morbidity in breast cancer patients after breast-conserving therapy versus mastectomy. Acta Oncol. 2008;47:835–842. doi: 10.1080/02841860801961257. [DOI] [PubMed] [Google Scholar]
- 18.Ozaslan C, Kuru B. Lymphedema after treatment of breast cancer. Am J Surg. 2004;187:69–72. doi: 10.1016/j.amjsurg.2002.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Warren LE, Miller CL, Horick N, et al. The impact of radiation therapy on the risk of lymphedema after treatment for breast cancer: a prospective cohort study. Int J Radiat Oncol Biol Phys. 2014;88:565–571. doi: 10.1016/j.ijrobp.2013.11.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jammallo LS, Miller CL, Singer M, et al. Impact of body mass index and weight fluctuation on lymphedema risk in patients treated for breast cancer. Breast Cancer Res Treat. 2013;142:59–67. doi: 10.1007/s10549-013-2715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Togawa K, Ma H, Sullivan-Halley J, et al. Risk factors for self-reported arm lymphedema among female breast cancer survivors: a prospective cohort study. Breast Cancer Res. 2014;16:414-014-0414-x. doi: 10.1186/s13058-014-0414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu YQ, Xie YH, Liu FH, et al. Systemic analysis on risk factors for breast cancer related lymphedema. Asian Pac J Cancer Prev. 2014;15:6535–6541. doi: 10.7314/APJCP.2014.15.16.6535. [DOI] [PubMed] [Google Scholar]
- 23.Lee SH, Min YS, Park HY, et al. Health-related quality of life in breast cancer patients with lymphedema who survived more than one year after surgery. J Breast Cancer. 2012;15:449–453. doi: 10.4048/jbc.2012.15.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haddad P, Farzin M, Amouzegar-Hashemi F, et al. A multicentre cross-sectional study of arm lymphedema four or more years after breast cancer treatment in Iranian patients. Breast Cancer. 2010;17:281–285. doi: 10.1007/s12282-009-0165-1. [DOI] [PubMed] [Google Scholar]
- 25.Shah C, Wilkinson JB, Baschnagel A, et al. Factors associated with the development of breast cancer-related lymphedema after whole-breast irradiation. Int J Radiat Oncol Biol Phys. 2012;83:1095–1100. doi: 10.1016/j.ijrobp.2011.09.058. [DOI] [PubMed] [Google Scholar]
- 26.Shih YC, Xu Y, Cormier JN, et al. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2-year follow-up study. J Clin Oncol. 2009;27:2007–2014. doi: 10.1200/JCO.2008.18.3517. [DOI] [PubMed] [Google Scholar]
- 27.Kim M, Kim SW, Lee SU, et al. A model to estimate the risk of breast cancer-related lymphedema: combinations of treatment-related factors of the number of dissected axillary nodes, adjuvant chemotherapy, and radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86:498–503. doi: 10.1016/j.ijrobp.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Gartner R, Jensen MB, Kronborg L, et al. Self-reported arm-lymphedema and functional impairment after breast cancer treatment—a nationwide study of prevalence and associated factors. Breast. 2010;19:506–515. doi: 10.1016/j.breast.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Monleon S, Murta-Nascimento C, Bascuas I et al (2014) Lymphedema predictor factors after breast cancer surgery: a survival analysis. Lymphat Res Biol. doi:10.1089/lrb.2013.0042 [DOI] [PubMed]
- 30.Meeske KA, Sullivan-Halley J, Smith AW, et al. Risk factors for arm lymphedema following breast cancer diagnosis in Black women and White women. Breast Cancer Res Treat. 2009;113:383–391. doi: 10.1007/s10549-008-9940-5. [DOI] [PubMed] [Google Scholar]
- 31.Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 32.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Peto R, Davies C, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–2313. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 34.Martin M, Segui MA, Anton A, et al. Adjuvant docetaxel for high-risk, node-negative breast cancer. N Engl J Med. 2010;363:2200–2210. doi: 10.1056/NEJMoa0910320. [DOI] [PubMed] [Google Scholar]
- 35.Mamounas EP, Bryant J, Lembersky B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol. 2005;23:3686–3696. doi: 10.1200/JCO.2005.10.517. [DOI] [PubMed] [Google Scholar]
- 36.Goble S, Bear HD. Emerging role of taxanes in adjuvant and neoadjuvant therapy for breast cancer: the potential and the questions. Surg Clin N Am. 2003;83:943–971. doi: 10.1016/S0039-6109(03)00071-9. [DOI] [PubMed] [Google Scholar]
- 37.Ohsumi S, Shimozuma K, Ohashi Y, et al. Subjective and objective assessment of edema during adjuvant chemotherapy for breast cancer using taxane-containing regimens in a randomized controlled trial: the national surgical adjuvant study of breast cancer 02. Oncology. 2012;82:131–138. doi: 10.1159/000336480. [DOI] [PubMed] [Google Scholar]
- 38.Qin YY, Li H, Guo XJ, et al. Adjuvant chemotherapy, with or without taxanes, in early or operable breast cancer: a meta-analysis of 19 randomized trials with 30698 patients. PLoS ONE. 2011;6:e26946. doi: 10.1371/journal.pone.0026946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bronstad A, Berg A, Reed RK. Effects of the taxanes paclitaxel and docetaxel on edema formation and interstitial fluid pressure. Am J Physiol Heart Circ Physiol. 2004;287:H963–H968. doi: 10.1152/ajpheart.01052.2003. [DOI] [PubMed] [Google Scholar]
- 40.Piccart MJ, Klijn J, Paridaens R, et al. Corticosteroids significantly delay the onset of docetaxel-induced fluid retention: final results of a randomized study of the European Organization for Research and Treatment of Cancer Investigational Drug Branch for Breast Cancer. J Clin Oncol. 1997;15:3149–3155. doi: 10.1200/JCO.1997.15.9.3149. [DOI] [PubMed] [Google Scholar]
- 41.Jung SY, Shin KH, Kim M, et al. Treatment factors affecting breast cancer-related lymphedema after systemic chemotherapy and radiotherapy in stage II/III breast cancer patients. Breast Cancer Res Treat. 2014;148:91–98. doi: 10.1007/s10549-014-3137-x. [DOI] [PubMed] [Google Scholar]
- 42.Kilbreath SL, Lee MJ, Refshauge KM, et al. Transient swelling versus lymphoedema in the first year following surgery for breast cancer. Support Care Cancer. 2013;21:2207–2215. doi: 10.1007/s00520-013-1770-2. [DOI] [PubMed] [Google Scholar]
- 43.Lee MJ, Beith J, Ward L, et al. Lymphedema following taxane-based chemotherapy in women with early breast cancer. Lymphat Res Biol. 2014;12:282–288. doi: 10.1089/lrb.2014.0030. [DOI] [PubMed] [Google Scholar]
- 44.Stanton AW, Northfield JW, Holroyd B, et al. Validation of an optoelectronic limb volumeter (Perometer) Lymphology. 1997;30:77–97. [PubMed] [Google Scholar]
- 45.Tierney S, Aslam M, Rennie K, et al. Infrared optoelectronic volumetry, the ideal way to measure limb volume. Eur J Vasc Endovasc Surg. 1996;12:412–417. doi: 10.1016/S1078-5884(96)80005-0. [DOI] [PubMed] [Google Scholar]
- 46.O’Toole J, Jammallo LS, Skolny MN, et al. Lymphedema following treatment for breast cancer: a new approach to an old problem. Crit Rev Oncol Hematol. 2013;88:437–446. doi: 10.1016/j.critrevonc.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ancukiewicz M, Russell TA, Otoole J, et al. Standardized method for quantification of developing lymphedema in patients treated for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:1436–1443. doi: 10.1016/j.ijrobp.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Armer JM, Stewart BR. A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphat Res Biol. 2005;3:208–217. doi: 10.1089/lrb.2005.3.208. [DOI] [PubMed] [Google Scholar]
- 49.Specht MC, Miller CL, Russell TA, et al. Defining a threshold for intervention in breast cancer-related lymphedema: what level of arm volume increase predicts progression? Breast Cancer Res Treat. 2013;140:485–494. doi: 10.1007/s10549-013-2655-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mackey JR, Martin M, Pienkowski T, et al. Adjuvant docetaxel, doxorubicin, and cyclophosphamide in node-positive breast cancer: 10-year follow-up of the phase 3 randomised BCIRG 001 trial. Lancet Oncol. 2013;14:72–80. doi: 10.1016/S1470-2045(12)70525-9. [DOI] [PubMed] [Google Scholar]
- 51.Ferguson T, Wilcken N, Vagg R, et al. Taxanes for adjuvant treatment of early breast cancer. Cochrane Database Syst Rev. 2007;4:CD004421. doi: 10.1002/14651858.CD004421.pub2. [DOI] [PubMed] [Google Scholar]
- 52.De Laurentiis M, Cancello G, D’Agostino D, et al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol. 2008;26:44–53. doi: 10.1200/JCO.2007.11.3787. [DOI] [PubMed] [Google Scholar]
- 53.Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giordano SH, Lin YL, Kuo YF, et al. Decline in the use of anthracyclines for breast cancer. J Clin Oncol. 2012;30:2232–2239. doi: 10.1200/JCO.2011.40.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Semb KA, Aamdal S, Oian P. Capillary protein leak syndrome appears to explain fluid retention in cancer patients who receive docetaxel treatment. J Clin Oncol. 1998;16:3426–3432. doi: 10.1200/JCO.1998.16.10.3426. [DOI] [PubMed] [Google Scholar]
- 56.Behar A, Pujade-Lauraine E, Maurel A, et al. The pathophysiological mechanism of fluid retention in advanced cancer patients treated with docetaxel, but not receiving corticosteroid comedication. Br J Clin Pharmacol. 1997;43:653–658. doi: 10.1046/j.1365-2125.1997.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hugenholtz-Wamsteker W, Robbeson C, Nijs J et al (2014) The effect of docetaxel on developing oedema in patients with breast cancer: a systematic review. Eur J Cancer Care (Engl). doi:10.1111/ecc.12261 [DOI] [PubMed]
- 58.Park SI, Jeon WH, Jeung HJ, et al. Clinical features of docetaxel chemotherapy-related lymphedema. Lymphat Res Biol. 2014;12:197–202. doi: 10.1089/lrb.2013.0037. [DOI] [PubMed] [Google Scholar]
- 59.Vignes S, Lebrun-Vignes B. Sclerodermiform aspect of arm lymphoedema after treatment with docetaxel for breast cancer. J Eur Acad Dermatol Venereol. 2007;21:1131–1133. doi: 10.1111/j.1468-3083.2006.02119.x. [DOI] [PubMed] [Google Scholar]
- 60.Taylor R, Jayasinghe UW, Koelmeyer L, et al. Reliability and validity of arm volume measurements for assessment of lymphedema. Phys Ther. 2006;86:205–214. [PubMed] [Google Scholar]
- 61.Deltombe T, Jamart J, Recloux S, et al. Reliability and limits of agreement of circumferential, water displacement, and optoelectronic volumetry in the measurement of upper limb lymphedema. Lymphology. 2007;40:26–34. [PubMed] [Google Scholar]
- 62.Ancukiewicz M, Miller CL, Skolny MN, et al. Comparison of relative versus absolute arm size change as criteria for quantifying breast cancer-related lymphedema: the flaws in current studies and need for universal methodology. Breast Cancer Res Treat. 2012;135:145–152. doi: 10.1007/s10549-012-2111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stout Gergich NL, Pfalzer LA, McGarvey C, et al. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer. 2008;112:2809–2819. doi: 10.1002/cncr.23494. [DOI] [PubMed] [Google Scholar]
- 64.Ancukiewicz M, Russell TA, Otoole J, et al. Standardized method for quantification of developing lymphedema in patients treated for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:1436–1443. doi: 10.1016/j.ijrobp.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Specht MC, Miller CL, Skolny MN, et al. Residual lymph node disease after neoadjuvant chemotherapy predicts an increased risk of lymphedema in node-positive breast cancer patients. Ann Surg Oncol. 2013;20:2835–2841. doi: 10.1245/s10434-012-2828-y. [DOI] [PubMed] [Google Scholar]
- 66.Soran A, Ozmen T, McGuire KP, et al. The importance of detection of subclinical lymphedema for the prevention of breast cancer-related clinical lymphedema after axillary lymph node dissection; a prospective observational study. Lymphat Res Biol. 2014;12:289–294. doi: 10.1089/lrb.2014.0035. [DOI] [PubMed] [Google Scholar]
- 67.Ostby PL, Armer JM, Dale PS, et al. Surveillance recommendations in reducing risk of and optimally managing breast cancer-related lymphedema. J Pers Med. 2014;4:424–447. doi: 10.3390/jpm4030424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cormier JN, Xing Y, Zaniletti I, et al. Minimal limb volume change has a significant impact on breast cancer survivors. Lymphology. 2009;42:161–175. [PMC free article] [PubMed] [Google Scholar]