Abstract
Background
African Americans are reported to be more sensitive to pain than European Americans. Pain sensitivity has been shown to be genetically linked in animal models and is likely to be in humans.
Methods
11,239 self-identified African American post menopausal women enrolled in the Women’s Health Initiative had percentage African ancestry determined by ancestry informative markers, “Pain Construct” measurements and covariate information. They answered 5 questions about specific types and location of pain, such as joint, neck, low back, headache, and urinary. They also answered 2 questions which were used to derive a “Pain Construct”, a measure of general pain scored on a scale of 1 to 100. Associations were tested in linear regression models adjusting for age, self-reported medical conditions, neighborhood socio-economic status, education, and depression.
Results
In the unadjusted model of the 5 specific types of pain measures, greater pain perception was associated with a higher proportion of African ancestry. However, some of the specific types of pain measures were no longer associated with African ancestry after adjustment for other study covariates. The Pain Construct was statistically significantly associated with African ancestry in both the unadjusted [Beta = −0.132, 95% confidence interval (C I) = −099 – −0.164; r = −0.075, 95% CI −0.056 – −0.093] and the adjusted models (Beta = −0.069 95% CI = −0.04 – 0.10).
Conclusions
Greater African ancestry was associated with higher levels of self-reported pain although this accounted for only a minor fraction of the overall variation in the Pain Construct.
Keywords: Pain, African ancestry
INTRODUCTION
Literature suggests self-identified African Americans report more pain than European Americans. This is reported for multiple types of pain. Reports address different types of artificially induced pain; one of the earliest publications looked at sensitivity to applied heat to the skin.(Chapman 1944) This was repeated more recently with similar results.(Sheffield, Biles et al. 2000) In a clinical population with pain, induced ischemia was better tolerated by European Americans than African Americans. (Edwards, Doleys et al. 2001) An experimental study to test differences in central pain-inhibitory mechanisms found that non-Hispanic whites could be conditioned to experience less pain through ischemic conditioning than African Americans.(Campbell, France et al. 2008) Evaluation of pain in the elderly using the McGill Pain Questionnaire showed increased pain perception among African Americans,(Johnson-Umezulike 1999) reinforcing the idea that questionnaire data parallels experimental data.
There is limited research relating human genetic differences to pain perception; ethnicity has always been self-defined. In mouse models numerous genes have been identified which relate to pain.(Lacroix-Fralish, Ledoux et al. 2007) For humans there are isolated reports that differences in allele frequencies of SNPs in the opiate receptor gene OPRM1 are associated with required pain medications dosages, meta-analyses failed to show any significant results.(Walter and Lotsch 2009) A genome-wide association study examining coding sequence variations suggested specific genetic polymorphisms in the angiotensen pathway which correlate with pain sensation in humans.(Geisser, Gracely et al. 2007) To our knowledge, to date, no specific genetic link between African ancestry and pain has been established. Prior research has focused on self-identified ancestry and not genetically identified ancestry between different ethnicities. A review published in 2014 which looked at 180 published manuscripts addressing pain and ethnicity did not mention genetically identified ethnicity.(Lavin and Park 2014)
The Women’s Health Initiative offers an opportunity to investigate the association of African American ancestry with self-reported pain. Women answered questions about the pain they experienced. This information can be cross referenced with percentage Sub-Saharan African Ancestry and adjusted for multiple covariates. We hypothesized that higher percentage African ancestry is associated with higher levels of self-reported pain.
Methods
Participants
WHI recruited postmenopausal women aged 50 to 75 from 40 clinical centers across the United States to participate in 3 overlapping clinical trials (CT) and an observational study (OS). The total number of women recruited was 161,856. Of these women 14,618 self-reported as being African American. Recruitment started in 1993 and lasted 5 years. For an overview of recruitment and design see the referenced papers.(Group 1998) Only data from women who self-reported as African American and for whom pain measurements were available were considered in these analyses. Not all women who self-reported as African American had available DNA or DNA which could be genotyped and not all women had pain measurements. Thus, only 11,239 women were included in these analyses. All of the data is taken from the first clinical visit; the analysis is cross-sectional. Table 1 shows the distribution of the available variables used in the primary analysis. We note that the characteristics of the self-identified African American women that were not included in the analyses were similar to those included in our studies (Supplementary Table 1)
Table 1.
Descriptive Statistics of Variables
| Variable | Mean | Standard deviation |
|---|---|---|
| Pain Construct (range, 0–100) | 70.4 | 26.5 |
| Age at screening (N = 9921) | 61.5 | 7.1 |
| Shortened CES-D (Depression) | 0.1 | 0.2 |
| Percent African Ancestry (N= 9921) | 75.4 | 15.1 |
| SES* | 0.6 | 0.1 |
| Number | Percent | |
| Diabetes ever | 1346 | 12.0 |
| Education | ||
| High School or below | 2762 | 24.9 |
| Vocational | 1389 | 12.5 |
| Some College, including associate | 2938 | 26.5 |
| College, BA/BS and above | 4014 | 36.2 |
SES normalized to have values between 0 and 1
Pain Measures
1. Pain Construct
Uses a 100 point score which combines the 2 questions below, a and b taken directly from the SF36.(Ware and Sherbourne 1992) More pain was coded as a lower score for the Pain Construct. They were graded from 100, for no pain, to 0 for the most pain with 75, 50 and 25 in between and the responses to the two questions added together and divided by 2.
-
a)
How much body pain, During the past four weeks, how much bodily pain have you had? (0–5, None to severe.)
-
b)
How much did pain interfere: During the past four weeks, how much did pain interfere with your normal work (both outside your home and at home)? (0–5, None to severe.)
This format, a lower score being indicative of more pain, was used to remain consistent with prior usage in WHI.(Rubin, Landfair et al. 2010) (Wyshak In press)
Other Pain Measurements
The following questions helped evaluate specific types and location of pain.
-
a)
Headaches or migraines: …mark the one oval that best describes how bothersome the symptom was during the past 4 weeks for you. (0–3, None to severe.)
-
b)
Low back pain: … mark the one oval that best describes how bothersome the symptom was during the past 4 weeks for you. (0–3, None to severe.)
-
c)
Neck pain: …mark the one oval that best describes how bothersome the symptom was during the past 4 weeks for you. (0–3, None to severe.)
-
d)
Joint pain or stiffness: …mark the one oval that best describes how bothersome the symptom was during the past 4 weeks for you (0–3, None to severe.)
-
d)
Pain/burning while urinating: …mark the one oval that best describes how bothersome the symptom was during the past 4 weeks for you. (0–3, None to severe.)
The specific measures of pain were used to validate the Pain Construct and not individually tested against ancestry.
Ancestry Estimation
We estimated the proportion of African and European ancestry using a validated set of ancestry informative markers (AIMs) (Kosoy, Nassir et al. 2009, Nassir, Kosoy et al. 2009) that included 92 SNPs enabling accurate estimation of ancestry proportions in African Americans. Genotyping was performed by our group as previously described (Nassir, Qi et al. 2012) using the TaqMan® OpenArrays® system (https://products.appliedbiosystems.com/ab/en/US/adirect/ab?cmd=catNavigate2&catID=605783) (ABI, Foster City, CA, USA). These SNP AIMs were chosen to distinguish continental ancestries (here, sub-Saharan African vs. European) and under the analytic conditions used do not distinguish intra-group differences in either sub-Saharan African origins (e.g. different tribal groups) or European origins (e.g. nationalities). The mean call rate was 97.1%. All AIM SNPs were in Hardy Weinberg equilibrium (P>.005) in parental populations, and the mean width of the 90% Bayesian confidence intervals (CIs) was 0.2 for ancestry estimates in our studies of groups of African and Hispanic Americans. (Qi, Nassir et al. 2012) While the mean width of CIs are larger for smaller AIM sets than larger sets of AIMs (the mean width of 90% CI was 0.1 for sets containing >1500 AIMs), the ancestry proportions estimated in individuals assessed with these highly selected AIMs are strongly correlated with larger AIMs sets. Correlations with larger sets of SNP AIMs (>500 SNPs) are >0.9 in testing with Mexican American and African American admixed populations (mean difference in ancestry fraction <0.05) (MFS, unpublished data). For the current study we did not include assessment of Amerindian ancestry since the African American participants showed a very low frequency of ancestry from this population (Amerindian ancestry, mean = 0.019, standard deviation = 0.025). In addition, assessment of other continental ancestries using STRUCTURE (v2.3.3) (Falush, Stephens et al. 2003) analyses also showed a negligible assignment of East Asian ancestry (mean = 0.007, standard deviation = 0014. Thus, the African American cohort studied here showed over 97% of the ancestry assignment determined by AIMs was contributed by African and European ancestry.
The African and European ancestry contribution (a proportion ranging from 0% to 100%) to each self-identified African American woman was assessed using STRUCTURE (v2.3.3)(Falush, Stephens et al. 2003) analyses of genotyping results with AIMs as previously described.(Nassir, Qi et al. 2012) The analyses were performed under the assumption of two populations (K = 2) with 100,000 replicates and 100,000 burn-in cycles and representatives of parental population groups as previously described.(Falush, Stephens et al. 2003) The results were consistent with < 0.02 difference between each of three independent runs. We considered only African ancestry in data analyses because the results for European ancestry accounted for the difference between African ancestry and 100% and would have given complimentary but inverse results.
Other Variables
Covariates were derived from WHI questionnaires. Age was ascertained at the same time as the pain questions. Depression was measured by the CES-D short form.(Andresen, Malmgren et al. 1994, Tuunainen, Langer et al. 2001) See the Online Appendix for the actual questions and the algorithm used to calculate this score. The mean score = 0.047 with a standard deviation of 0.145. A score ≥0.06 is considered depressed. Education was recorded as: 1 = high school or below; 2 = vocational; 3 = some college (includes associate degree); and 4 = college degree or beyond. Neighborhood Socio-economic status (nSES) was obtained using a standardized geocoding protocol (Whitsel, Quibrera et al. 2006),(Whitsel, Rose et al. 2004) which linked individual WHI OS and CT participant addresses to year 2000 U.S. Census Federal Information Processing Standards (FIPS) codes and tract-level socioeconomic data. nSES has been shown in multiple studies to correlate strongly with various medical outcomes. In older individuals with osteoarthritis (Knight, Callahan et al, 2011) nSES has been shown to be strongly associated with pain and disability. Similarly in older individual nSES has been linked to the progression of renal failure.(Merkina,_Diez Roux, 2007). In a sub-study of WHI, using the same algorithm nSES as used in this manuscript, nSES was associated with lower cognitive function.(Shih, Ghosh-Dastidar et al.) Family income is available at baseline in WHI but was felt be a poor reflection of the life time economic or social status of the participants, more than half of whom were no longer living with their former partner. A summary measure of each participant’s neighborhood socioeconomic environment was estimated from the tract-level data using six variables representing several dimensions of wealth and income: 1) log of median household income; 2) log of median value of housing units; 3) percentage of households receiving interest, dividend, or net rental income; 4) percentage of adults > 25 years of age who had completed high school; 5) percentage of adults > 25 years of age who had completed college; and 6) percentage of employed persons > 16 years of age in executive, managerial, or professional specialty occupations. The six variables were converted into standardized (Z) scores by subtracting the population-specific mean from the value associated with each participant’s census tract and then dividing the difference by the population-specific standard deviation (SD). The transformation was performed separately within the OS and CT and generated six Z scores, each of which indicated the deviation of the tract level value from the corresponding, population-specific mean and summed to zero across the population. A neighborhood summary Z score was then constructed by summing the six Z scores. Since we aimed at controlling for nSES among combined populations of both the OS and CT participants we included an indicator for study cohort in the regression analyses. Medical Conditions were self-reported at baseline and those medical conditions with a prevalence of greater than 5%, were included in the analysis. These variables were: glaucoma, cataracts, migraine headaches, asthma, stomach or duodenal ulcer, diverticulitis, cardiovascular disease, gallbladder disease or gallstones, arthritis, cancer, broken bone and diabetes. Those taking hypoglycemic medications at baseline were also classified as diabetic.
Statistical Methods
We conducted statistical analyses using SAS version 9.3 (SAS Institute, Cary, NC). All statistical tests were two sided and P<.05 was considered statistically significant. Descriptive statistics were obtained for continuous (mean and standard error) and categorical variables (frequency and percentage), respectively. Since nSES was calculated separately for OS and CT participants, the values of nSES were normalized to be between 0 and 1 for summary statistics. Polychoric correlation between different pain variables was also obtained. A categorical variable for the Pain Construct was used to permit reporting and comparison with dichotomous and multileveled variables for individual types of pain in the same figure or table. nSES was divided by its standard deviation consistent with our previous studies.(Qi, Nassir et al. 2012) We also investigated the associations between pain construct and nSES, and education, respectively, with and without controlling for ancestry and other covariates using linear regression models.
To investigate the association between the Pain Construct and African ancestry proportion, we obtained coefficient estimates and 95% confidence intervals (CIs) using linear regression models. The coefficient estimates described the effect of 10% increments in African ancestry. We considered the following analysis models: 1) African ancestry and age at entry; 2) African ancestry, age at entry, and nSES; 3) African ancestry, age at entry, nSES and education; 4) African ancestry, age at entry and medical conditions; 5) African ancestry, age at entry, medical conditions, and shortened CES-D; 6) African ancestry, age at entry, medical conditions, shortened CES-D and nSES; 7) African ancestry, age at entry, nSES, medical conditions, education and shortened CES-D; and 8) African ancestry, age at entry, nSES, medical conditions, shortened CES-D, and education.
We studied the association between pain variables and ancestry using multinomial logistic models, with and without adjusting for other covariates. Multinomial logistic models generalize logistic regression by allowing response variables with more than two levels, and comparing different levels of the response variables to the reference level. For joint pain/stiffness and lower back pain, the response variable in the models had four categories, “no pain”, “mild”, “moderate” and “severe” where “no pain” was the reference level. For headache/migraines, neck pain, pain/burning while urinating and pain construct, we combined the category(ies) which had less than 5% frequency with the adjacent category, and used the following categories in the regression models: for headache/migraines and neck pain, “no pain”, “mild”, and “moderate or severe”; for pain/burning while urinating, “no pain” and “pain” were used with, “no pain” was the reference level; and for Pain Construct, the values 0, 12.5 and 25 were combined as one category to represent the most pain, and 100 (least pain) was used as the reference level. The above eight models for Pain Construct and African ancestry were fitted for each of the pain variables, and ORs and 95% CIs were obtained for the African ancestry proportion.
Results
Association of Pain Construct and Ancestry
For our main analyses we examined the association of the Pain Construct with ancestry. The distribution of African ancestry in the study cohort is shown in Figure 1A. We examined the relationship without covariates by comparing the Pain Construct indices with different deciles of African ancestry (Figure 1B). Increasing levels of pain (lower pain construct value) correlated with higher deciles of African ancestry (r = −0.075, 95% confidence limit = −0.056 – −0.093) This correlation of the Pain Consruct with ancestry was stronger than age (r = −0.057, 95%CI =−0.039 – −0.076) but weaker than that with nSES measurement of socioeconomic status (r =−0.132, 95%CI = 0.114 – 0.150) and much weaker than that with the CES-D measurement of depression (r =− 0.255, 95%CI = −0.238 – −0.273) in this dataset.
Figure 1. Relationship of African ancestry with Pain Construct.
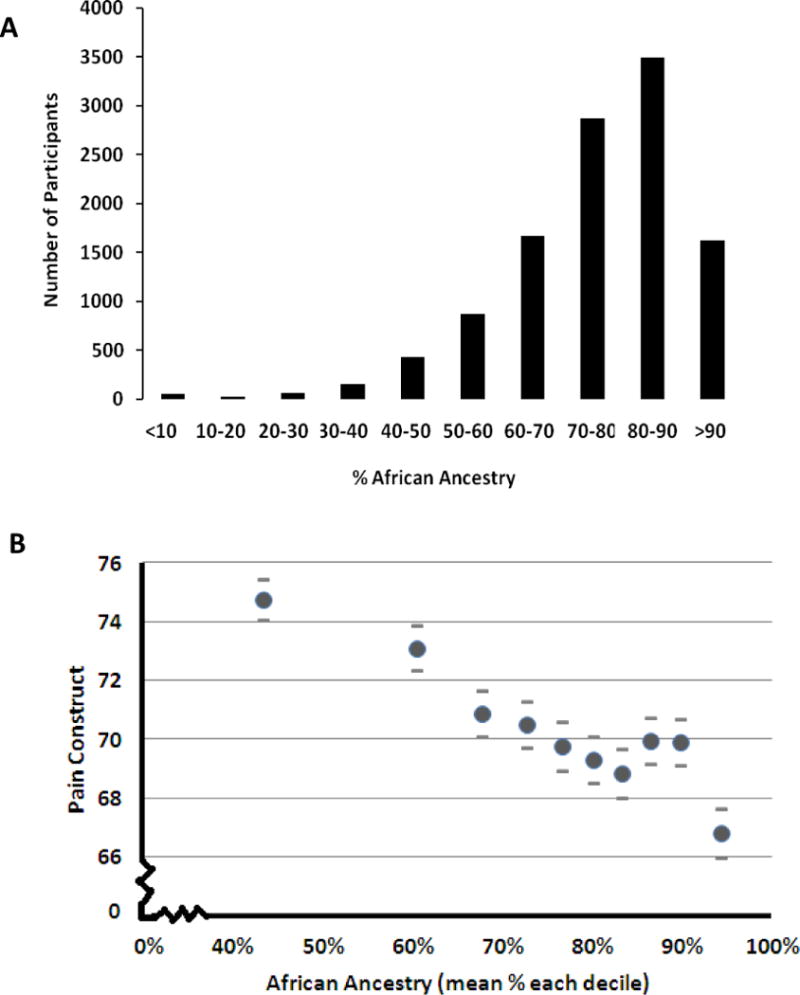
A) A histogram showing the distribution of African ancestry in the study subjects. B) The mean unadjusted levels of pain and standard errors of the mean (ordinate) are shown for each decile of ancestry (Less pain has a greater value). The Pain Construct combines scores from questionaires measuring how much body pain and how much pain interferes with normal work (for details see Methods).
In order to discern if the association of ancestry and self-reported pain was mediated through other associated variables multiple models were constructed (Table 2). Ancestry was included in each model as was age at entry and they were sequentially adjusted for different covariates. Each model remained significant after adjusting for the potential covariates. Both measures of nSES and Education decreased the size of the effect, however, the association remained highly significant (p <0.0001). In the most inclusive model with age, nSES, education, depression and medical conditions the association between percent African ancestry and the Pain Construct remained statistically significant at a level of p<0.0001. Another way to view this is that in the fully adjusted model a one unit increase in African ancestry (here defined as a 10% increment in African ancestry), the pain construct score was reduced by 0.67 while holding all other variables in the model constant.
Table 2.
Associations with Pain Construct
| Model | Beta | 95% CI | p value | |
|---|---|---|---|---|
| a. African Admixture | ||||
| AFRa | −1.32 | −1.64 | −0.99 | <0.0001 |
| AFR, Age | −1.41 | −1.74 | −1.08 | <0.0001 |
| AFR, Age, Depressionb | −1.27 | −1.59 | −0.95 | <0.0001 |
| AFR, Age, nSESc | −1.01 | −1.34 | −0.68 | <0.0001 |
| AFR, Age, nSES, Educationd | −0.75 | −1.08 | −0.42 | <0.0001 |
| AFR, Age, nSES, Depression | −0.91 | −1.24 | −0.59 | <0.0001 |
| AFR, Age, nSES, Education, Depression | −0.7 | −1.03 | −0.37 | <0.0001 |
| AFR, Age, Medical Conditionse | −1.07 | −1.38 | −0.76 | <0.0001 |
| AFR, Age, Medical Conditions, nSES | −0.81 | −1.12 | −0.05 | <0.0001 |
| AFR, Age, Medical Conditions, Education | −0.74 | −1.05 | −0.43 | <0.0001 |
| AFR, Age, Medical Conditions, Depression | −0.98 | −1.28 | −0.67 | <0.0001 |
| AFR, Age, Medical Conditions, Depression, nSES | −0.73 | −1.04 | −0.42 | <0.0001 |
| AFR, Age, Medical Conditions, Depression, Education | −0.70 | −1.00 | −0.39 | <0.0001 |
| AFR, Age, Medical Conditions, Depression, nSES, Education | −0.69 | −1.00 | −0.37 | <0.0001 |
| b. Neighborhood Socio Economic Status | ||||
|---|---|---|---|---|
| nSES | 3.49 | 3 | 3.97 | <0.0001 |
| nSES, Age | 3.41 | 2.93 | 3.9 | <0.0001 |
| nSES, Age, AFR | 3.12 | 2.63 | 3.62 | <0.0001 |
| nSES, Age, AFR, EDU | 2 | 1.49 | 2.51 | <0.0001 |
| nSES, Age, AFR, EDU, Depression | 1.88 | 1.38 | 2.38 | <0.0001 |
| nSES, Age, AFR, EDU, Medical Conditions, Depression | 1.97 | 1.51 | 2.44 | <0.0001 |
| c. Education | ||||
|---|---|---|---|---|
| Education | <0.0001 | |||
| vocational school | 3.41 | 1.73 | 5.09 | <0.0001 |
| some college including associate degree | 6.88 | 5.53 | 8.24 | <0.0001 |
| college degree BA/BS and above | 12.52 | 11.26 | 13.78 | <0.0001 |
| Education, Age | <0.0001 | |||
| vocational school | 3.18 | 1.5 | 4.86 | 0.0002 |
| some college including associate degree | 6.49 | 5.13 | 7.85 | <0.0001 |
| college degree BA/BS and above | 12.23 | 10.97 | 13.5 | <0.0001 |
| Education, Age, AFR | <0.0001 | |||
| vocational school | 3.17 | 1.5 | 4.85 | 0.0002 |
| some college including associate degree | 6.14 | 4.78 | 7.5 | <0.0001 |
| college degree BA/BS and above | 11.75 | 10.48 | 13.02 | <0.0001 |
| Education, Age, AFR, nSES | <0.0001 | |||
| vocational school | 2.78 | 1.11 | 4.46 | 0.001 |
| some college including associate degree | 5.38 | 4.01 | 6.76 | <0.0001 |
| college degree BA/BS and above | 10.39 | 9.08 | 11.71 | <0.0001 |
| Education, Age, AFR, nSES, Depression | <0.0001 | |||
| vocational school | 1.53 | −0.14 | 3.19 | 0.07 |
| some college including associate degree | 4.09 | 2.72 | 5.45 | <0.0001 |
| college degree BA/BS and above | 8.52 | 7.2 | 9.83 | <0.0001 |
| Education, Age, AFR, nSES, Medical Conditions, Depression | <0.0001 | |||
| vocational school | 1.03 | −0.57 | 2.63 | 0.21 |
| some college including associate degree | 3.46 | 2.15 | 4.77 | <0.0001 |
| college degree BA/BS and above | 7.1 | 5.84 | 8.36 | <0.0001 |
African Ancestry,
Based on the CES-D short form.
Neighborhood Socio Economic Status normalized to have values between 0 and 1,
Education: high school or below, vocational, some college including associate degree, college degree or beyond.
Self reported conditions present in >5% of subjects; Glaucoma, Asthma, Emphysema, Cancer, Broken bone, Diabetes, Stomach or duodenal ulcer, Diverticulitis, Cardiovascular disease, Gallbladder disease, Arthritis.
nSES and Education are Associated with Pain Construct
We also examined whether nSES and education were associated with the Pain Construct, see Tables 2b and 2c. Both these measures were significantly associated with or without adjusting for the covariates. Those in the lower nSES categories reported more pain (p<0.0001). Similarly, those with less education reported more pain. Both nSES and education were largely unaffected when considering ancestry as a covariate. As indicated above nSES did have a stronger correlation with the Pain Construct than ancestry (r = 0.132 vs r = 0.075).
Other Measures of Pain Correlate with the Pain Construct
We also explored whether specific types of self-reported pain were associated with ancestry. All of the available measures of self-reported pain were evaluated. Each showed the same direction of association as the Pain Construct (i.e. increasing levels of pain were associated with higher deciles of African ancestry)(Figure 2). Figure 2 shows the odds ratio and statistical significance for the highest level of each specific pain variable compared to no pain as a function of ancestry. The combined highest three deciles of the Pain Construct were compared to no pain. Supplementary Tables 2–7 provide complete results with all levels of pain and 95% confidence intervals. We note that the scales for these measures are more constrained than that of the Pain Construct and may thus not yield as significant results. These more specific measures of pain were correlated with the Pain Construct, the components of the Pain Construct and each other; the p values for these associations were all <0.0001 (Table 3).
Figure 2. Association of different measures of pain with African ancestry.
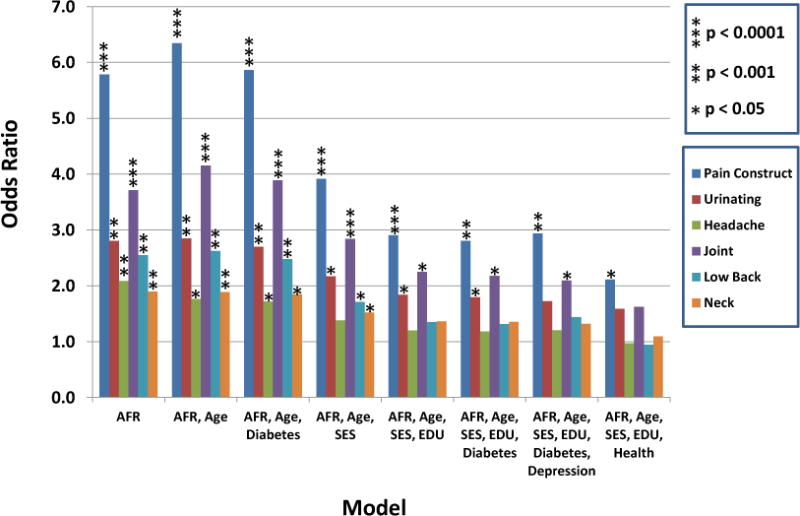
The ordinate provides the Odds Ratios for the color coded pain measurement with the corresponding significance of association shown in the figure key. For each measurement except the Pain Construct the highest level of each specific pain variable was compared to no pain as a function of ancestry. For the Pain Construct the combined highest three deciles of pain were compared to no pain to enable comparison of these results.
Discussion
We found that The Pain Construct, and each of the individual measures of types and location of pain showed increasing pain perception with increasing African ancestry in post-menopausal African American women. These are findings may help to unravel some of the differences in pain perception. Our results are consistent with previous studies that have reported increased pain sensitivity in African Americans compared with European Americans. (Chapman 1944, Johnson-Umezulike 1999, Sheffield, Biles et al. 2000, Edwards, Doleys et al. 2001, Campbell, France et al. 2008) These associations were partially attenuated by considering a variety of potential confounders that yielded lower beta coefficients but nonetheless remained statistically significant, p<0.0001. This is similar to the findings by Golightly et al when looking at findings related to osteoarthritis. They were unable to diminish the pain and disability association by adjusting for multiple socio-demographic variables including, age, nSES and education.(Golightly and Dominick 2005)
By examining genetically defined ancestry rather than self-identified race we found associations where others did not. In a younger population, adjustment for nSES made statistically insignificant an association the authors had found between race and pain perception.(Green, Hart-Johnson, 2012) Using structural analysis these authors came to the conclusion that “Black race was only indirectly associated with sensory pain through neighborhood SES.” We also note that using family income instead of nSES did not appreciably change the results reported here (Supplementary Table 8). Although it is possible that unmeasured societal factors or differences in self-reported levels of pain may underlie these differences, this association with ancestry suggests that there may be genetic factors that either directly or indirectly influence pain sensitivity.
Previous studies have demonstrated that differences in pain perception can be mediated by discrimination (Burgess, Grill et al. 2009) and our findings should not be taken to negate this important social problem. However, the findings of an association between pain perception and ethnicity can open the door for a search for specific pathways to pain which are mediated by genetic differences associated with ethnicity. If genetically controlled pathways to pain are different these pathways may be susceptible to different interventions. We know that there are multiple different clinical entities which are associated with ethnicity; these run the gambit from sickle cell anemia to differences in fracture risk or risk of atrial fibrillation. It seems reasonable to suggest that future interventions to mediate pain may take advantage of these different pathways.
The study suffers from a number of limitations. There was no actual experimentally induced pain, thus the main analyses are all limited to self-reported perceived pain measurements derived from questionnaires. Previous studies have suggested that the general pain questions correlate with induced pain responses.(Geisser, Gracely et al. 2007) However, these studies were not conducted in a population that directly corresponds to the WHI cohort. It is important to remember that self-perceived pain is what individuals suffer from, thus it has a meaning in and of itself. In addition, although the constructed models were adjusted for available covariates, hypothetical confounders may be unmeasured covariates that play an important role in the association. In fact, the current study also found an association of the Pain Construct with nSES and education that could not be explained by ancestry. Not surprisingly this suggests that multiple factors may underlie perceptions of pain.
Equally we must emphasize that only a small amount of the large variation of the Pain Construct measurement can be explained by ancestry information; nor for that matter the other factors included in the current study (e.g. nSES, age and depression). The individual correlation coefficient between ancestry and the Pain Construct was small (r=0.075) indicating that a direct linear correlation is marginal although it was significant (p<0.0001). Whether this simply indicate noise in the crude Pain Construct measurement and unreliability of individual determinations, a non-linear relationship of these two parameters, or the importance of other unmeasured factors is not clear. The strong ecological correlation [correlation of deciles of ancestry with the Pain Construct (r=0.93) and quartiles (r= 0.95)] is supportive of our general conclusion that ancestry is associated with pain perception, as were the findings of association of ancestry with specific types of self-reported pain. Thus, it is clear that future studies using more sensitive and quantitative measures of pain will be necessary to more definitively examine the factors that determine individual differences in pain perception. This is critically important, if future efforts are directed towards identifying specific genetic factors that may underlie pain sensitivity.
Another limitation is the impossibility of knowing which health factors might predispose individuals to pain. For some specific conditions, for example diabetes, it is probably reasonable to assume that the health factor is potentially in the causal pathway. In contrast, for depression it is not clear whether it is in the causal pathway or whether models including measures of depression are over-adjusting for factors which are either co-linear with ancestry and pain or in fact secondary to perceptions of pain. We note that in the fully adjusted model while the association remains significant, p<0.0001 the beta coefficient is decreased. This decreased beta coefficient may be a function of over adjustment. For example, when an individual is in pain she may show evidence of depression, thus including depression in this model decreases the association between pain and ancestry.
Strengths of the study included the large number of participants from several study sites across the United States. Close to ten thousand women were included in the study. We believe our ability to genetically identify the percentage of African ancestry for each participant provides a clearer assessment of the genetics of pain than self-reported ethnicity or race and partially ameliorates cultural differences.
It is important to note that this is an epidemiologic study and relates to populations, not individuals. No clinical implications are appropriate to make based on these results. The perception of pain is individual and influenced by multiple factors. Our findings that pain perception is associated with ancestry may have future research implications but should not influence the treatment of any specific patient.
This study does not negate the value of further emphasis on factors other than ancestry that are clearly of major importance for pain perception in different population groups. In fact, our study is consistent with other factors including socioeconomic status having a substantially larger impact on pain perception as evidence by the stronger correlation of the Pain Construct with nSES than with ancestry. Even stronger correlations were seen with collinear morbidities (e.g. depression), however as discussed above it is unclear whether these are the cause or effect of pain perception. Thus, our study while supporting a role for ancestry (and presumably genetic factors) also indicates that factors other than ancestry account for a substantially larger fraction of the individual (and population) differences in pain perception.
As with much research one is left with more questions than answers. African ancestry and self-perceived pain were associated but the pathway was not clear. Does it relate to a “pain” gene(s) or does it relate through complex social factors that were not measured in our study? Multiple genes related to pain have been found in animal models and human studies have suggested possible genetic links to heat sensitivity.(Lacroix-Fralish, Ledoux et al. 2007) The current study suggests that further genetic studies examining measures of pain in African American women may be particularly valuable in ascertaining specific gene variations that underlie pain sensitivity. However, as discussed above the effect size was relatively small and the development of better and more quantitative Pain Constructs or measurements will be critical in further advancing the current studies.
Supplementary Material
Acknowledgments
We thank the participants of the WHI and acknowledge the contributions of WHI investigators for the development of study. This work was supported by the National Institutes of Health NHLBI BAA contract no. HHSN268200764319C. The study design was approved by the NHLBI as part of a BAA for the Women’s Health Initiative. The Women’s Health Initiative provided access to clinical data and DNA samples under appropriate institutional review board approval. The Women’s Health Initiative Publication and Presentation Committee reviewed and approved the manuscript for submission. The NHLBI was not otherwise involved in the design and conduct of the study, or in the analysis of data or preparation of the manuscript.
References
- Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- Burgess DJ, Grill J, Noorbaloochi S, Griffin JM, Ricards J, van Ryn M, Partin MR. The effect of perceived racial discrimination on bodily pain among older African American men. Pain Med. 2009;10(8):1341–1352. doi: 10.1111/j.1526-4637.2009.00742.x. [DOI] [PubMed] [Google Scholar]
- Campbell CM, France CR, Robinson ME, Logan HL, Geffken GR, Fillingim RB. Ethnic differences in diffuse noxious inhibitory controls. J Pain. 2008;9(8):759–766. doi: 10.1016/j.jpain.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman WP, Jones CM. Variations in cutaneous and visceral pain sensitivity in normal subjects. Journal of Clinical Investigation. 1944;23(81) doi: 10.1172/JCI101475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RR, Doleys DM, Fillingim RB, Lowery D. Ethnic differences in paintolerance: clinical implications in a chronic pain population. Psychosom Med. 2001;63(2):316–323. doi: 10.1097/00006842-200103000-00018. [DOI] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164(4):1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisser ME, Gracely RH, Giesecke T, Petzke FW, Williams DA, Clauw DJ. The association between experimental and clinical pain measures among persons with fibromyalgia and chronic fatigue syndrome. Eur J Pain. 2007;11(2):202–207. doi: 10.1016/j.ejpain.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Golightly YM, Dominick KL. Racial variations in self-reported osteoarthritis symptom severity among veterans. Aging Clin Exp Res. 2005;17(4):264–269. doi: 10.1007/BF03324608. [DOI] [PubMed] [Google Scholar]
- Green CR, Hart-Johnson T. The association between race and neighborhoodsocioeconomic status in younger Black and White adults with chronic pain. J Pain. 2012 Feb;13(2):176–86. doi: 10.1016/j.jpain.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Johnson-Umezulike JM. A comparison of pain perception of elderly African Americans and Caucasians. Nursingconnections. 1999;12(2):5–12. [PubMed] [Google Scholar]
- Knight JB, Callahan LF, Luong ML, Shreffler J, Schoster B, Renner JB, Jordan JM. The association of disability and pain with individual and community socioeconomic status in people with hip osteoarthritis. Open Rheumatol J. 2011;5:51–8. doi: 10.2174/1874312901105010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosoy R, Nassir R, Tian C, White PA, Butler LM, Silva G, Kittles R, Alarcon-Riquelme ME, Gregersen PK, Belmont JW, De La Vega FM, Seldin MF. Ancestry informative marker sets for determining continental origin and admixture proportions in common populations in America. Hum Mutat. 2009;30(1):69–78. doi: 10.1002/humu.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix-Fralish ML, Ledoux JB, Mogil JS. The Pain Genes Database: An interactive web browser of pain-related transgenic knockout studies. Pain. 2007;131(1–2):3e 1–4. doi: 10.1016/j.pain.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Lavin R, Park J. A characterization of pain in racially and ethnically diverse older adults: a review of the literature. J Appl Gerontol. 2014;33(3):258–290. doi: 10.1177/0733464812459372. [DOI] [PubMed] [Google Scholar]
- Merkin SS, Diez Roux AV, Coresh J, Fried LF, Jackson SA, Powe NR. Individual and neighborhood socioeconomic status and progressive chronic kidney disease in an elderly population: The Cardiovascular Health Study. Soc Sci Med. 2007 Aug;65(4):809–21. doi: 10.1016/j.socscimed.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Nassir R, Kosoy R, Tian C, White PA, Butler LM, Silva G, Kittles R, Alarcon-Riquelme ME, Gregersen PK, Belmont JW, De La Vega FM, Seldin MF. An ancestry informative marker set for determining continental origin: validation and extension using human genome diversity panels. BMC Genet. 2009;10:39. doi: 10.1186/1471-2156-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassir R, Qi L, Kosoy R, Garcia L, Allison M, Ochs-Balcom HM, Tylavsky F, Manson JE, Shigeta R, Robbins J, Seldin MF. Relationship between adiposity and admixture in African-American and Hispanic-American women. Int J Obes (Lond) 2012;36(2):304–313. doi: 10.1038/ijo.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Nassir R, Kosoy R, Garcia L, Curb JD, Tinker L, Howard BV, Robbins J, Seldin MF. Relationship between diabetes risk and admixture in postmenopausal AfricanAmerican and Hispanic-American women. Diabetologia. 2012;55(5):1329–1337. doi: 10.1007/s00125-012-2486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin JP, Landfair AS, Shestak K, Lane D, Valoski A, Chang Y, Tindle HA, Kuller LH. Health characteristics of postmenopausal women with breast implants. Plast Reconstr Surg. 2010;125(3):799–810. doi: 10.1097/PRS.0b013e3181cb5e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield D, Biles PL, Orom H, Maixner W, Sheps DS. Race and sex differences in cutaneous pain perception. Psychosom Med. 2000;62(4):517–523. doi: 10.1097/00006842-200007000-00010. [DOI] [PubMed] [Google Scholar]
- Shih RA, Ghosh-Dastidar B, Margolis KL, Slaughter ME, Jewell A, Bird CE, Eibner C, Denburg NL, Ockene J, Messina CR, Espeland MA. Neighborhood socioeconomic status and cognitive function in women. Am J Public Health. 2011 Sep;101(9):1721–8. doi: 10.2105/AJPH.2011.300169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuunainen A, Langer RD, Klauber MR, Kripke DF. Short version of the CES-D (Burnam screen) for depression in reference to the structured psychiatric interview. Psychiatry Res. 2001;103(2–3):261–270. doi: 10.1016/s0165-1781(01)00278-5. [DOI] [PubMed] [Google Scholar]
- Walter C, Lotsch J. Meta-analysis of the relevance of the OPRM1 118A>G genetic variant for pain treatment. Pain. 2009;146(3):270–275. doi: 10.1016/j.pain.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- Whitsel EA, Quibrera PM, Smith RL, Catellier DJ, Liao D, Henley AC, Heiss G. Accuracy of commercial geocoding: assessment and implications. Epidemiol Perspect Innov. 2006;3:8. doi: 10.1186/1742-5573-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsel EA, Rose KM, Wood JL, Henley AC, Liao D, Heiss G. Accuracy and repeatability of commercial geocoding. Am J Epidemiol. 2004;160(10):1023–1029. doi: 10.1093/aje/kwh310. [DOI] [PubMed] [Google Scholar]
- Women’s Health Initiative. Design of the Women’s Health Initiative Clinical Trial andObservational Study. Controlled Clinical Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- Wyshak G. Height, Socioeconomic and Subjective Well-Being Factors among U.S. Women, Ages 49–79. PLoS One. doi: 10.1371/journal.pone.0096061. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


