Abstract
Background
Studies of symptomatic gastroparetics consistently find poor correlation with gastric emptying. We hypothesized that concomitant small bowel dysmotility may play a role in symptom causation in gastroparesis and sought to test this hypothesis by using wireless motility capsule (WMC) testing to simultaneously measure antral and duodenal area under pressure curve (AUC) in patients with delayed gastric emptying.
Methods
Using a cohort from a multicenter clinical trial and a separate tertiary clinical database, we identified gastroparetics that underwent concurrent WMC testing and completed the Gastroparesis Cardinal Symptom Index, a validated questionnaire. Our study included 35 gastroparetics defined by a Gastric Emptying Time (GET) >5 hrs. Antral and duodenal AUC were assessed at 1-hour windows pre-GET and post-GET, respectively.
Key Results
We found moderate correlations between duodenal AUC and symptom severity in the combined cohort (n=35; R=−0.42; p=0.01; 95% CI −0.7, −0.1). Removing patients with colonic delay resulted in a stronger correlation of duodenal AUC to symptom severity (n=21; R=−0.63; p<0.01; 95% CI −0.81, −0.31). The multicenter trial (n=20) and clinical practice cohorts (n=15) had significantly-different symptom severity and exclusion criteria. When analyzed separately, significant correlations between duodenal AUC and symptom severity were observed (R=−0.71; p<0.01; 95% CI −0.9, −0.4 and R=−0.72; p<0.01; 95% CI −0.9, −0.3, respectively). Symptom severity and antral motility showed no correlation.
Conclusions & Inferences
We found significant correlations between duodenal AUC and symptom severity in two cohorts of gastroparetics. Small bowel motility may contribute to symptom generation in gastroparetic patients and this may inform therapeutic considerations.
Keywords: Gastroparesis, motility, duodenal, wireless motility capsule, symptoms, small bowel
INTRODUCTION
Functional dyspepsia and gastroparesis are the two most common sensorimotor disorders of the upper gastrointestinal tract. According to the Rome III criteria, functional dyspepsia is defined as the presence of one or more of the following: postprandial fullness, early satiation, or epigastric pain/ burning with no evidence of structural disease or mechanical obstruction (1). Gastroparesis is a syndrome of objectively-delayed gastric emptying in the absence of mechanical obstruction that has many overlapping symptom features. Clinically, the two entities can be difficult to distinguish and there have been suggestions that mild gastroparesis, particularly of idiopathic origin, is the same as functional dyspepsia (2).
Largely due to the similarity of symptoms with which these patients present, treating these disorders based on symptoms alone is difficult for physicians because assessment is often empiric without any pathophysiologic basis. Although altered gastric motor function is clearly associated with gastroparesis, the correlation between gastric emptying time alone and symptom severity is not well established (3,4).
Because the GI tract has limited ways of expressing symptoms of dysfunction through both motor and sensory components, it has proven difficult to determine the physiological mechanisms responsible for GI symptoms and thus, clinicians are often unable to discern which patients among this population will respond to pro-motility agents and which will be refractory to treatment.
Pressure profiles in the stomach and duodenum are traditionally obtained via antroduodenal manometry, which is cumbersome, rarely performed, and usually not simultaneous with gastric and small bowel transit measurement. Another means of assessment is by Wireless motility capsule (WMC) testing, which continuously samples intraluminal pH, temperature, pressure, and motility parameters both regionally and throughout the gastrointestinal tract for five days after ingestion. Unlike antroduodenal manometry, WMC testing allows simultaneous antroduodenal measurement of contractility and transit (5). Contractile parameters measured by WMC include area under the pressure curve (AUC), contraction frequency (Ct), and motility index (MI). AUC is calculated as a summation of pressure amplitudes over time, an integral of contraction frequency and amplitude. Motility index is a composite parameter that incorporates both contraction frequency and amplitude (6). In contrast to Ct and MI, AUC directly measures both the strength and frequency of contractions in an integrated fashion, consistent with previously reported work (3).
While previous studies have failed to demonstrate a correlation between symptom severity and gastric emptying in gastroparesis, we hypothesize that concomitant small bowel contractile dysmotility may play a role in symptom causation in gastroparesis. We therefore sought to use WMC technology to determine whether AUC (a measure of contractile fitness) is a potential biomarker of disease severity (as measured by a validated gastroparesis questionnaire) in gastroparetic patients. Evidence linking duodenal motility and symptom severity could inform therapeutic options and identify an important new therapeutic target in this population.
METHODS
Subject Enrollment
Using a cohort from a multicenter clinical trial and a separate clinical practice database of a tertiary GI motility clinic, we identified gastroparesis patients that had undergone both wireless motility capsule testing and completed the Patient Assessment of Upper Gastrointestinal Symptom Severity Index (PAGI-SYM), a validated measure of symptom severity in patients with upper GI disorders, concurrently (7). All available patients that met these criteria were considered for analysis.
The multicenter trial was conducted at seven medical centers from March 2005 to November 2005 and enrolled both healthy subjects and subjects with a history of gastroparesis as described by Kuo et al (6). The study protocol was approved by the Institutional Review Board of each center, and each subject gave informed consent before enrollment. The eligibility criteria for the gastroparesis subjects included males and females between ages 18 and 65 with a history of upper GI symptoms for at least 6 months and a delayed gastric scintigraphy test documented within 2 years (6). Patients were asked to discontinue all laxatives and drugs that affect gut motility at least 48 hours before the study; however, stable doses (≥ 6 mo) of antidepressants, oral contraceptives, and lipid-lowering drugs were allowed. Narcotic drugs, proton pump inhibitors, and non-steroidal anti-inflammatory drugs were not allowed in the week that preceded the study. All over-the-counter medications were suspended 3 days prior to WMC ingestion. Subjects that had undergone previous abdominal surgery, bowel movement frequencies >72 h, had severe weight loss (>4.5 kg in last 2 months), or experienced severe dysphagia, vomiting, or lower abdominal pain were excluded (6).
The second, clinical practice cohort consisted of patients that underwent WMC testing as part of clinical diagnostic evaluation from August 2009 to February 2013 at the GI motility clinic at Massachusetts General Hospital, Boston, MA—a tertiary referral center. These patients consisted of males and females between ages 18 and 65 years with history of upper GI symptoms for at least 6 months (4). Exclusion criteria consistent with the medical device indications for use were applied including previous bowel surgery, evidence of bezoars, known strictures, dysphagia and metabolic disease. Patient medications that affect gut motility were discontinued on a case-by-case basis.
In both cohorts, the analysis was limited to patients with gastroparesis objectively defined by a gastric emptying time of greater than 5 hours by WMC. The use of the 5 hour timepoint to mark delayed gastric emptying was based on previous analysis from Kuo and colleagues that provided normal WMC values for gastric emptying, with a reported sensitivity and specificity of 0.65 and 0.87, respectively (6).
Materials
The SmartPill system consists of an ingestible single-use WMC, a receiver, and display software (8). The indigestible capsule houses sensors for pH (range, 0.05–9.0 pH units), pressure (range, 0–350 mm Hg), and temperature (range, 25°C–49°C). Transit parameters assessed by the device include gastric emptying time (GET), small bowel transit time (SBTT), and colonic transit time (CTT). The WMC defines gastric emptying and ileocecal transit based on changes in pH profile (6). Contractile parameters measured by WMC include contraction frequency (Ct), area under pressure curve (AUC), and motility index (MI), which were assessed using GIMS Viewer (Ver 3.0, SmartPill, Covidien, Dublin, Ireland). Ct is determined by the number of contractions, AUC by the integral of contraction amplitude over time, and MI is calculated as the natural log of (sum of amplitudes × number of contractions + 1) over an established one-hour timeframe for this study.
In addition to physiologic data collected from the SmartPill system, patient symptoms were assessed with standardized, validated questionnaires querying upper GI symptoms and stool consistency during WMC digestion. The PAGI-SYM questionnaire is widely used, well-validated measure assessing self-reported symptom severity in patients with dyspepsia, GERD, or gastroparesis (9). It consists of six sub-scales: Nausea/Vomiting, Post-prandial Fullness, Bloating, Upper abdominal pain, Lower abdominal pain, and Heartburn (10). The questionnaire uses a six-point Likert response scale, ranging from 0–5, with higher scores reflecting greater symptom severity over the previous two weeks (11). The Gastroparesis Cardinal Symptoms Index (GCSI) is a subset of PAGI-SYM that includes the sub-scales related to gastroparesis: Nausea/Vomiting, Post-prandial Fullness, and Bloating. The GCSI total score represents an average of the sub-scales. Questionnaire data were collected and managed using REDCap secure electronic data capture (12).
Protocol
Following an overnight fast, subjects reported to the study center and completed the PAGI-SYM questionnaire. The WMC was administered both clinically and during the trial in the typical fashion reported in Kuo et al (6). Subjects first ingested a SmartBar (260-kcal, 17% protein, 66% carbohydrates, 2% fat, 3% fiber) or nutritionally-identical radiolabelled eggbeater meal along with 50 mL of water as a standardized meal. After swallowing an activated and calibrated WMC (SmartPill), subjects in the quaternary clinic were allowed to leave the center with instructions to fast for six hours to prevent ingestion of another meal that could compromise determination of GET, while subjects in the multicenter trial were observed in the study center until that time. Six hours after capsule ingestion, subjects consumed 250 mL Ensure (Abbott Laboratories, Abbott Park, IL, USA) and water was taken ad libitum (6). All subjects were instructed to wear the portable receiver (SmartPill) until capsule evacuation. Subjects were instructed to resume a normal diet eight hours after pill ingestion. After completion of the study, the data were downloaded to a secure computer for analysis.
Outcomes
The primary outcome of this study was to determine if gastroparesis symptom severity correlated with antral or duodenal AUC. The secondary outcome was an exploratory analysis of the correlation between symptom severity and other WMC antroduodenal contractile (Ct, MI) and transit (GET, SBTT, CTT) measurements.
Data Analysis
Transit times for gastric emptying (GET), small bowel transit (SBTT), and colonic transit (CTT), were assessed by WMC in addition to contractile motility parameters AUC, Ct, and MI. These parameters were determined at 1 hour pre-GET and 1 hour post-GET based on previous studies that established these windows as reliable estimates of antral and duodenal motility, respectively (6,13). Because symptoms of colonic dysmotility can confound GCSI-measured symptom severity, we calculated correlations for all patients and those without concomitant colonic transit delay defined by CTT>59 hrs (WMC standard definition) (14). Capsule distance from monitor can lead to data loss compromising data fidelity. Patients with >70% of data recorded by the device within each one-hour window were considered for analysis.
Student’s t tests were used to assess any differences between the two independent cohorts for both contractile motility data and symptom indices. We determined data normality by assessing kurtosis and skewness. For our primary endpoint, we calculated Pearson correlation statistics to evaluate the relationship between GCSI score and AUC. Bonferroni corrections for multiple comparisons were used to adjust Pearson coefficients for the secondary outcomes of the study. Statistical analyses were performed using Excel Version 14.4 (Microsoft, WA, USA). P < 0.05 was considered statistically significant.
RESULTS
Study cohorts and demographics
In both the multicenter trial and clinical practice cohorts, gastroparesis patients defined by GET>5 hrs represented approximately 20% (31/48, 25/125) of the total study population. Of this gastroparesis population, loss of data availability due to capsule distance from monitor excluded an additional 35% of subjects in each cohort (21/31, 18/25). All remaining subjects were included for analysis; 32 total patients with antral contractile parameters, and 35 total patients with duodenal contractile parameters (Supplemental Table 1).
The etiology of gastroparesis was 12 idiopathic (57%), 9 diabetic (43%) in the multicenter trial cohort and 14 idiopathic (77%), 4 diabetic (23%) in the clinical practice cohort. For antral contractile parameters, the multicenter trial cohort consisted of 18 subjects (13 female, 5 male; 15 Caucasian, 3 Black), the clinical practice cohort consisted of 14 subjects (10 female, 4 male; 12 Caucasian, 2 Black). For duodenal contractile parameters, the multicenter trial cohort consisted of 20 subjects (15 female, 5 male; 17 Caucasian, 3 Black), the clinical practice cohort consisted of 15 subjects (11 female, 4 male; 13 Caucasian, 2 Black).
Cohorts were analyzed both together and independently; all data was normally-distributed via kurtosis and skewness. Inclusion/exclusion criteria (see Methods) differed slightly between the two groups including evidence of prior gastric emptying delay was not required for the clinical practice cohort. There were significant differences (p ≤ 0.01) between the two cohorts GCSI total score, antral contractile parameters (Ct, AUC, MI), and small bowel contractile parameters (CT, MI)(Table 1).
Table 1.
Two-tailed t tests comparing two independent cohorts
| Clinical Practice Average | Multicenter Trial Average | p values | ||
|---|---|---|---|---|
|
| ||||
| Transit Times | GET | 12:10 | 32:40 | 0.08 |
| SBTT | 5:30 | 4:50 | 0.08 | |
| CTT | 52:10 | 43:10 | 0.4 | |
|
| ||||
| Antral Contractility | Ct | 115 | 36 | <0.001 |
| AUC | 8351 | 3454 | 0.01 | |
| MI | 12.5 | 10 | <0.001 | |
|
| ||||
| Duodenal Contractility | Ct | 237 | 116 | 0.007 |
| AUC | 8700 | 5600 | 0.09 | |
| MI | 13.2 | 12.1 | 0.04 | |
|
| ||||
| Symptom Severity | GCSI Total Score | 3.2 | 2.3 | 0.002 |
Correlation of symptoms to contractile motility
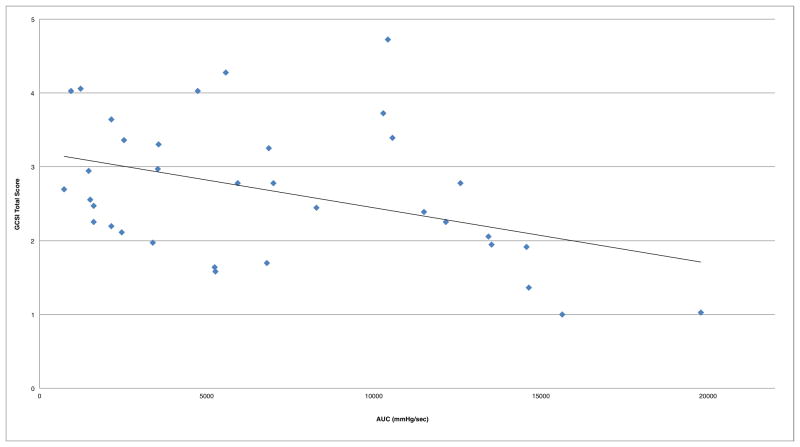
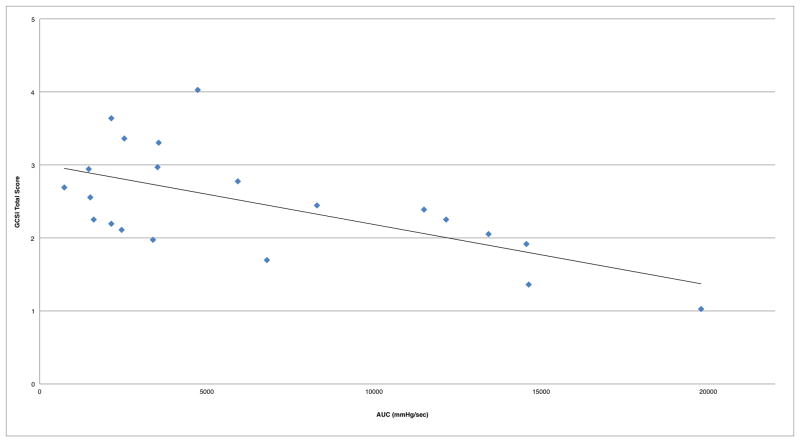
Combined analysis of both cohorts (N=35) demonstrated moderate correlation of duodenal AUC to symptom severity, and no significant correlation with antral AUC (Table 2, Figure 1). Removing patients with colonic delay (CTT>59 hrs) resulted in a stronger correlation of duodenal AUC to symptom severity (n=21; R=−0.63; p<0.01; 95%CI −0.81, −0.31)(Figure 2).
Table 2.
Significant correlations from analysis of combined and independent cohorts
| Cohort | Motility Parameter | Pearson Correlation to GCSI Total Score | p value | 95% confidence interval |
|---|---|---|---|---|
| Combined (n=35) | Duodenal AUC | -0.42 | 0.01 | (−0.65, −0.10) |
| Clinical Practice (n=15) | Duodenal AUC | −0.71 | 0.005 | (−0.88, −0.39) |
| Multicenter Trial (n=20) | Duodenal AUC | −0.72 | 0.002 | (−0.90, −0.33) |
Figure 1.
Duodenal AUC correlation to symptom severity in combined cohorts (R=−0.42)
Figure 2.
Duodenal AUC correlation to symptom severity in combined cohorts without subjects that have delayed colonic transit (R=−0.63)
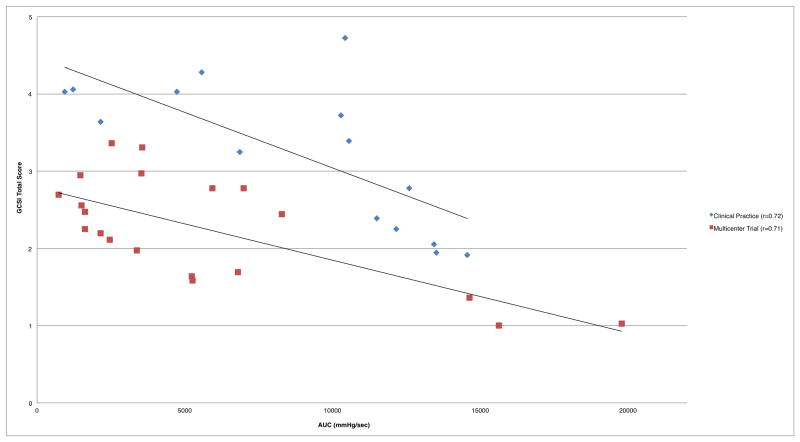
Analyzing each cohort independently showed stronger correlations between duodenal AUC to symptom severity (Table 2, Figure 3). There were no significant correlations between antral AUC and symptom severity in the independently-analyzed cohorts.
Figure 3.
Duodenal AUC correlation to symptom severity in independent cohorts
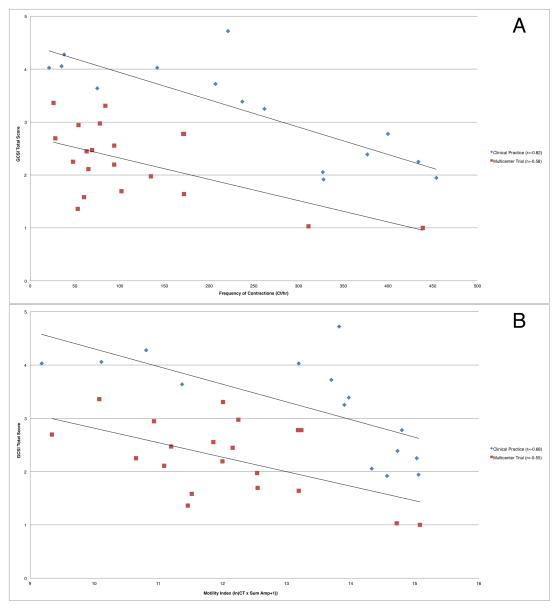
Tests of the secondary outcome variables Ct, MI, GET, and SBTT were conducted using Bonferroni adjusted alpha levels of .0125 per test (.05/4). For this exploratory analysis, there were no significant correlations between duodenal motility parameters Ct and MI with symptom severity in the combined cohorts. When analyzed separately, the individual cohorts demonstrated significant correlations between duodenal contractility parameters and symptom severity (Table 3, Figure 4A–B). There were no significant correlations between symptom severity and gastric contractile motility parameters, GET, or SBTT (Supplemental Table 2)
Table 3.
Summary of significant results from exploratory analysis shared by both cohorts with Bonferroni correction
| Cohort | Duodenal Motility Parameter | Pearson Correlation to GCSI Total Score | p value* | 95% confidence interval |
|---|---|---|---|---|
|
| ||||
| Multicenter Trial (n=20) | Ct | −0.58 | 0.007 | (−0.81, −0.19) |
| MI | −0.55 | 0.01 | (−0.91, −0.26) | |
|
| ||||
| Clinical Practice (n=15) | Ct | −0.82 | <0.001 | (−0.94, −0.52) |
| MI | −0.68 | 0.005 | (−0.89, −0.25) | |
Bolding denotes statistical significance via Bonferroni Correction with an adjusted level of significance of <0.0125
Figure 4.
Duodenal (a) Ct and (b) MI correlation to symptom severity in independent cohorts
DISCUSSION
In this study of two independent cohorts of gastroparetic patients, we found a significant negative correlation between symptom severity as assessed by a validated questionnaire and duodenal motility as determined by area under the pressure curve (AUC) from wireless motility capsule studies. Our literature review suggests this is the first study to demonstrate a strong correlation between symptoms and a motility parameter in gastroparesis using a duodenal—rather than gastric—motility measurement. Past studies have been limited to analysis of transit times such as gastric emptying by GES and have shown poor correlation with upper GI symptoms (15–17). In this study, we observed significant negative correlations, such that decreased contractile motility corresponded to increased symptom severity.
In addition to the duodenal AUC symptom-motility correlation observed, 18% of gastroparetics from the multicenter trial cohort and 27% from the clinical practice cohort showed delayed colonic transit. Because symptoms of colonic dysmotility can overlap with gastroparesis symptom severity as measured by GCSI, we calculated correlations for patients with delayed CTT (defined by CTT>59 hrs) and those without colonic transit delay (14). When removing the delayed colonic transit patients from analysis, correlations improved from moderate (R=−0.42) to strong (R=−0.63). As such, some patients diagnosed with gastroparesis based on traditional methods such as gastric emptying scintigraphy may in fact have pan-enteric dysmotility (18), with symptoms of colonic transit delay contributing to symptom severity. These findings suggest that there is additional clinically-useful information obtained by assessing both small bowel and colonic motility in patients with suspected gastroparesis.
Strong correlations between duodenal AUC and gastroparesis symptom severity were present when cohorts (clinical practice and multicenter trial) with different patient populations were independently assessed, with similarly-sloped trend lines displayed for each (Figure 3). Despite this heterogeneity, combining these two populations into a single cohort showed a more modest yet statistically-significant correlation (Figure 1). There were no significant correlations in any stratification of either cohort for antral motility or transit times, while multiple moderate to strong correlations were observed for duodenal motility and symptoms.
In addition to statistically-significant differences in symptom severity, there were dissimilar exclusion criteria between the two cohorts that added to the heterogeneity of these two populations. The clinical practice cohort consisted of a patient population representing a wide spectrum of functional and motility GI disorders referred to a tertiary motility clinic. In contrast, the strict exclusion criteria for the multicenter trial cohort excluded subjects with lower GI symptoms—a frequent comorbidity of patients presenting with upper GI symptoms—and required evidence of gastric delay by gastric emptying scintigraphy within the past two years. Given these potentially confounding differences, we would argue that these cohorts could be validly analyzed independently and may account for the more modest correlation when the populations were combined. The trend lines in Figures 3 and 4 highlight the parallel nature of this relationship.
Using antroduodenal manometry (ADM), other groups have demonstrated the use of duodenal motility patterns to make diagnoses that could not be made empirically from the clinical history alone (19). To date, the link between symptom severity and physiologic abnormalities in gastroparesis has proven elusive, with poor correlation continually observed between symptoms and gastric transit and pressure parameters (15–17). This, along with the accumulating evidence suggesting the diffuse nature of abnormal motility observed in other studies prompted us to look a motility parameters measured beyond the stomach using WMC technology (14,18). Although WMC does not measure propagated peristaltic contractions, it does measure physiologically-relevant motility parameters including AUC. These pressure parameters could be altered in GI motility disorders and could contribute to patient symptoms (20), as demonstrated by our results suggesting duodenal motility as a correlate for gastroparetics symptoms.
This study intentionally excluded patients without delayed gastric emptying that may have had the same constellation of symptoms as patients with WMC-proven gastroparesis but were likely suffering from chronic pain or visceral hypersensitivity. By refining each cohort to patients with objective dysmotility, we sought to identify a relationship between transit and pressure motility parameters with symptom generation in cases where motility was impaired. There were no significant correlations between symptoms and WMC measured pressure or transit parameters observed in patients without objective dysmotility, which we attribute to visceral hypersensitivity inherent in functional dyspepsia and other functional GI disorders that may obscure this relationship. We believe this isolation of gastroparetics from functional dyspeptics likely contributes to the strength of the correlations observed.
Findings of the secondary, exploratory analysis demonstrated the correlation of additional interrelated duodenal contractile motility parameters with symptom severity. From a conceptual level, we expect related indices to show consistent correlations with AUC, the primary outcome measured. As hypothesized, AUC —the only directly measured parameter that integrates both contraction strength and frequency— showed the strongest correlation to symptom severity. While correlations between symptom severity and duodenal motility were observed, no significant correlations with antral motility were observed. Additionally, no significant correlation was observed between symptom severity and small bowel transit time.
Our study had several limitations. Notably, although correlations were statistically significant in both the combined and independent analyses, the more modest correlations in the combined analysis suggest that if a true correlation between symptoms and motility exists, it is sensitive to cohort composition. We suspect that these differences reflect patient selection in each cohort. Additional important exclusion criteria for the multicenter trial included mandatory discontinuation of a wide spectrum of medications prior to capsule ingestion versus the clinical practice cohort in which medications that did not directly affect motility were limited on a per-patient basis. Consequently, these medications could directly influence both symptom severity and motility parameters as well as their interdependent relationship.
The study is limited by a moderate sample size, which was mostly due to the small number of gastroparesis patients assessed with WMC at advanced care centers possessing WMC technology. Although the sample size may be too small to generate estimates that apply to the entire gastroparesis population or draw conclusions about how the observed correlations may differ with respect to gastroparesis etiology, the sample size of the two cohorts with gastroparesis in this study is larger than any single reported study with ADM symptom correlation (16,22). Importantly, a statistically-significant correlation was still observed after combining heterogeneous cohorts, suggesting that the effect of AUC on symptom severity is not subtle. We suspect that correlation between AUC and symptom severity would be stronger with a larger sample size where the effects of individual outliers play less of a role.
Although symptom severity and motility measurements were assessed on the same day, it is important to note that GCSI measures symptoms over the preceding two weeks. Thus, a potential limitation of the study is that symptom severity in the two weeks preceding motility testing may not reflect symptom severity during testing. GCSI is currently the most widely-validated measure of gastroparesis symptom severity to date, and this study sought to correlate motility to the most accurate assessment of gastroparesis symptom severity available (9).
An additional limitation of WMC testing is the fidelity of data capture, which excluded 19 gastroparetic patients from our analysis. The most common reason for data loss is patient non-compliance related to maintaining receiver on the body. We reasoned a minimum of 70% of available pressure data from the capsule must be captured in the two one-hour windows of interest before and after gastric emptying to be representative of the pressure profile in those regions.
Our finding that the severity of gastroparesis symptoms is correlated with small bowel rather than gastric motility parameters via WMC represents a potential shift in our thinking about gastroparesis. Until now, physiologic testing has served as a poor biomarker for symptom severity and patients with more severe symptoms were thought to require interventions that further targeted gastric motility. To our knowledge, this study is unique in correlating symptoms to expanded upper GI motility parameters by WMC in gastroparesis. However, pharmacologic agents that target small bowel motility are limited at best. In this study, we demonstrated a simpler and more clinically-palatable alternative to measuring complex upper GI physiology by traditional antroduodenal manometry. Consideration of small bowel delay in patients with gastroparesis has practical application in clinical medicine, particularly in the consideration of therapeutic pro-motility agents. We conclude that gastroparetic patients with altered small bowel motility could have a more severe symptom profile and may require special therapeutic consideration, with novel small bowel motility agents sorely needed.
Supplementary Material
KEY MESSAGES.
While previous studies have failed to demonstrate a correlation between symptom severity and gastric emptying in gastroparesis, assessing antroduodenal area under pressure curve (AUC) by wireless motility capsule (WMC) showed duodenal rather than antral motility correlation to gastroparesis symptom severity in two independent cohorts.
We hypothesized that concomitant small bowel dysmotility may play a role in symptom causation in gastroparesis, and sought to test this hypothesis by using WMC technology to simultaneously measure duodenal AUC in patients with delayed gastric emptying.
Using a cohort from a multicenter clinical trial as well as a separate clinical practice database of a tertiary GI motility clinic, gastroparesis patients with delayed gastric emptying (≥ 5 hrs via WMC) who completed a validated measure of gastroparesis symptom severity on the same day were analyzed.
Differences in the exclusion criteria and resultant symptom severity of the two independent cohorts warranted independent and combined analysis. When combining cohorts, moderate correlation was observed for duodenal AUC and symptom severity, however, independent analysis of both groups showed strong correlations.
Acknowledgments
We thank Shahar Castel, M.A.M.S. and Sonia Yoon, M.D. for help with data collection and processing.
FUNDING
The trial was sponsored by The SmartPill Corporation and partly supported by NYSTAR grant C020118, NIH grant DK069614, and the International Foundation for Functional GI Disorders.
Footnotes
DISCLOSURES
KB, KS have no competing interests. JRS has received consulting support from Given Imaging. BK has received consulting support from Genova Diagnostics, Given Imaging, Prostrakan; clinical trial support from Furiex, Vibrant, Given Imaging, Glaxo Smith Kline.
KB helped with data collection, analysis and interpretation, and drafting of the manuscript. KS helped with data interpretation and manuscript editing. JRS helped with data interpretation and manuscript editing. BK helped with study planning and design, data interpretation, and manuscript editing.
References
- 1.Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, et al. Functional Gastroduodenal Disorders. Gastroenterology. 2006;130:1466–79. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 2.Lacy BE. Functional dyspepsia and gastroparesis: one disease or two? Am J Gastroenterol [Internet] 2012 Nov;107(11):1615–20. doi: 10.1038/ajg.2012.104. cited 2014 May 7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23160285. [DOI] [PubMed] [Google Scholar]
- 3.Tran K, Brun R, Kuo B. Evaluation of regional and whole gut motility using the wireless motility capsule: relevance in clinical practice. Therapeutic Advances in Gastroenterology. 2012:249–60. doi: 10.1177/1756283X12437874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee a, Wilding G, Kuo B. Variable abnormal physiological motility in the proximal upper gastrointestinal tract in gastroparesis. Neurogastroenterol Motil [Internet] 2012;24:652–7. e276. doi: 10.1111/j.1365-2982.2012.01905.x. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3376693&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao SSC. Advances in diagnostic assessment of fecal incontinence and dyssynergic defecation. Clinical Gastroenterology and Hepatology. 2010 doi: 10.1016/j.cgh.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuo B, McCallum RW, Koch KL, Sitrin MD, Wo JM, Chey WD, et al. Comparison of gastric emptying of a nondigestible capsule to a radio-labelled meal in healthy and gastroparetic subjects. Aliment Pharmacol Ther [Internet] 2008 Jan 15;27(2):186–96. doi: 10.1111/j.1365-2036.2007.03564.x. cited 2014 May 29. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17973643. [DOI] [PubMed] [Google Scholar]
- 7.Rentz AM, Kahrilas P, Stanghellini V, Tack J, Talley NJ, de la Loge C, et al. Development and psychometric evaluation of the patient assessment of upper gastrointestinal symptom severity index (PAGI-SYM) in patients with upper gastrointestinal disorders. Qual Life Res [Internet] 2004 Dec;13(10):1737–49. doi: 10.1007/s11136-004-9567-x. cited 2014 May 19. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15651544. [DOI] [PubMed] [Google Scholar]
- 8.FDA. Market approval notification 30 October 2009. Silver Spring, MD: Food and Drug Administration, Department of Health and Human Services; 2009. Smartpill GI Monitoring System, version 2.0. [Google Scholar]
- 9.Revicki Da, Camilleri M, Kuo B, Szarka La, McCormack J, Parkman HP. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index-Daily Diary (GCSI-DD) Neurogastroenterol Motil [Internet] 2012:1–10. doi: 10.1111/j.1365-2982.2012.01879.x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22284754. [DOI] [PubMed]
- 10.Wyrwich KW, Mody R, Larsen LM, Lee M, Harnam N, Revicki DA. Validation of the PAGI-SYM and PAGI-QOL among healing and maintenance of erosive esophagitis clinical trial participants. Qual Life Res. 2010;19:551–64. doi: 10.1007/s11136-010-9620-x. [DOI] [PubMed] [Google Scholar]
- 11.Revicki DA, Rentz AM, Dubois D, Kahrilas P, Stanghellini V, Talley NJ, et al. Gastroparesis Cardinal Symptom Index (GCSI): development and validation of a patient reported assessment of severity of gastroparesis symptoms. Qual Life Res [Internet] 2004 May;13(4):833–44. doi: 10.1023/B:QURE.0000021689.86296.e4. cited 2014 May 7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15129893. [DOI] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. J Biomed Inform [Internet] 2. Vol. 42. Elsevier; 2009. Apr 1, Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support; pp. 377–81. cited 2014 Apr 30. Available from: http://www.j-biomed-inform.com/article/S1532-0464(08)00122-6/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brun R, Michalek W, Surjanhata BC, Parkman HP, Semler JR, Kuo B. Comparative analysis of phase III migrating motor complexes in stomach and small bowel using wireless motility capsule and antroduodenal manometry. Neurogastroenterol Motil [Internet] 2012 Apr;24(4):332–e165. doi: 10.1111/j.1365-2982.2011.01862.x. cited 2014 May 7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22292793. [DOI] [PubMed] [Google Scholar]
- 14.Camilleri M, Thorne NK, Ringel Y, Hasler WL, Kuo B, Esfandyari T, et al. Wireless pH-motility capsule for colonic transit: prospective comparison with radiopaque markers in chronic constipation. Neurogastroenterol Motil [Internet] 2010 Aug;22(8):874–82. e233. doi: 10.1111/j.1365-2982.2010.01517.x. cited 2014 Aug 5. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2911492&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talley NJ, Verlinden M, Jones M. Can symptoms discriminate among those with delayed or normal gastric emptying in dysmotility-like dyspepsia? Am J Gastroenterol. 2001;96:1422–8. doi: 10.1111/j.1572-0241.2001.03683.x. [DOI] [PubMed] [Google Scholar]
- 16.Ardila-Hani A, Arabyan M, Waxman A, Ih G, Berel D, Pimentel M, et al. Severity of dyspeptic symptoms correlates with delayed and early variables of gastric emptying. Dig Dis Sci [Internet] 2013 Feb;58(2):478–87. doi: 10.1007/s10620-012-2355-5. cited 2014 May 22. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22918685. [DOI] [PubMed] [Google Scholar]
- 17.Guo W-J, Yao S-K, Zhang Y-L, Yan J, Yin L-J, Li H-L. Relationship between symptoms and gastric emptying of solids in functional dyspepsia. J Int Med Res [Internet] 2012 Jan;40(5):1725–34. doi: 10.1177/030006051204000511. cited 2014 May 7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23206454. [DOI] [PubMed] [Google Scholar]
- 18.Sarosiek I, Selover KH, Katz LA, Semler JR, Wilding GE, Lackner JM, et al. The assessment of regional gut transit times in healthy controls and patients with gastroparesis using wireless motility technology. Aliment Pharmacol Ther [Internet] 2010 Jan 15;31(2):313–22. doi: 10.1111/j.1365-2036.2009.04162.x. cited 2014 May 19. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19814743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patcharatrakul T, Gonlachanvit S. Technique of functional and motility test: how to perform antroduodenal manometry. J Neurogastroenterol Motil [Internet] 2013 Jul;19(3):395–404. doi: 10.5056/jnm.2013.19.3.395. cited 2014 Jun 3. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3714419&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kloetzer L, Chey WD, McCallum RW, Koch KL, Wo JM, Sitrin M, et al. Motility of the antroduodenum in healthy and gastroparetics characterized by wireless motility capsule. Neurogastroenterol Motil [Internet] 2010 May;22(5):527–33. e117. doi: 10.1111/j.1365-2982.2010.01468.x. cited 2014 May 22. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20122128. [DOI] [PubMed] [Google Scholar]
- 21.Rao SSC, Mysore K, Attaluri A, Valestin J. Diagnostic utility of wireless motility capsule in gastrointestinal dysmotility. J Clin Gastroenterol [Internet] 2011 Sep;45(8):684–90. doi: 10.1097/MCG.0b013e3181ff0122. cited 2014 Nov 9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21135705. [DOI] [PubMed] [Google Scholar]
- 22.Sha W, Pasricha PJ, Chen JDZ. Correlations among electrogastrogram, gastric dysmotility, and duodenal dysmotility in patients with functional dyspepsia. J Clin Gastroenterol [Internet] 2009 Sep;43(8):716–22. doi: 10.1097/MCG.0b013e31818b8ed9. cited 2014 May 22. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19247205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.