Abstract
The frequency of infection caused by the recently described pathogen Mycobacterium lepromatosis is unknown. Here, we describe the demographics, clinical characteristics, and therapeutic outcomes of five lepromatous leprosy patients suffering from M. lepromatosis infection in Nuevo Léon, Mexico. Diagnosis was facilitated by a new highly specific PCR procedure.
TEXT
Mycobacterium leprae causes Hansen's disease, or leprosy, a chronic infection transmitted from human to human by close contact and manifests clinically in several forms. Leprosy was recently declared to have been eliminated from most regions of the world (1). Since its original description by Armauer Hansen in 1873, the diagnosis of leprosy has relied on the detection of acid-fast bacilli (AFB) in clinical samples. In 2008, a new etiologic agent, Mycobacterium lepromatosis, was associated with leprosy in the United States (2). It was detected in autopsy specimens from two patients of Mexican ethnicity, using molecular biology techniques. On screening samples from patients attending our dermatology clinic who were diagnosed with Hansen's disease, we detected M. lepromatosis in a diffuse lepromatous leprosy (DLL) case (3) but found no evidence for M. leprae in the biopsy sample. Subsequently, cases of M. lepromatosis infection have been reported in Canada, Singapore, Brazil, and Myanmar (4, 5).
In Mexico, the largest study conducted was that of Han et al. (6), and this included 120 samples from patients with various clinical forms of leprosy; 63.2% of the cases harbored M. lepromatosis alone, and 16% were mixed infections in which both M. leprae and M. lepromatosis were present. In all these cases, the bacteria were identified using a PCR assay employing primers for the 16S rRNA gene. The genome sequence of M. lepromatosis was recently obtained (7), and loci present in M. lepromatosis, but absent from M. leprae, were identified, thus enabling a highly specific PCR procedure to be established.
In the present work, we screened biopsy specimens from patients currently receiving treatment at our clinic to determine the prevalence of M. lepromatosis, or of mixed infections, using a new specific PCR procedure. A diagnosis was initially made clinically and confirmed by the detection of AFB in Fite-Faraco-stained skin biopsy specimens. For molecular analysis, DNA was extracted from the biopsy specimen and used in PCR assays with primers designed to detect M. leprae (RLEP-7 and RLEP-8) or M. lepromatosis (LPM244-F [5′-GTTCCTCCACCGACAAACAC-3′] and LPM244-R [5′-TTCGTGAGGTACCGGTGAAA-3′]) (7). The pair for M. lepromatosis amplifies a 244-bp fragment from the hemN gene missing in M. leprae (7).
A total of 38 patients were analyzed; among them, we observed 5 cases positive for M. lepromatosis (Table 1), constituting 13% of the total cases. Four were from Nuevo León and one from the neighboring state of San Luis Potosi, and none were related. Four presented with lepromatous leprosy, of whom three were diagnosed with DLL and one with nodular lepromatous leprosy (NLL); none of them presented any immunological reaction, such as erythema nodosum leprosum or Lucio's phenomenon. Clinically, they were identical to patients infected with M. leprae (Fig. 1).
TABLE 1.
Demographic and clinical features of patients with M. lepromatosis infection
| Patient | Age (yr) | Gendera | Originb | Classificationc | Duration of infection | Treatment (mo) | Sequelae | Dietary risk factors |
|---|---|---|---|---|---|---|---|---|
| 1 | 49 | M | Monterrey, NL | DLL | 18 yr | 50 | Peripheral neuropathy | None declared |
| 2 | 46 | F | Juárez, NL | BL | 9 yr | 25 | None | None declared |
| 3 | 65 | F | Charcas, SLP | DLL | 25 yr | 24 | None | Rat meat consumption |
| 4 | 70 | M | San Nicolás, NL | DLL | 14 yr | 24 | None | None declared |
| 5 | 69 | M | Los Herrera, NL | NLL | 6 mo | 2 | None to date; on therapy | Armadillo and rat meat consumption |
M, male; F, female.
NL, Nuevo León State; SLP, San Luis Potosí State.
DLL, diffuse lepromatous leprosy; BL, borderline leprosy; NLL, nodular lepromatous leprosy.
FIG 1.
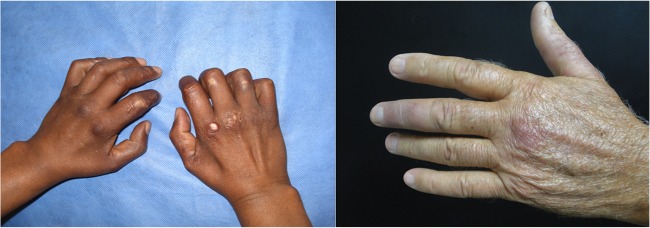
Patients with infection by M. lepromatosis. Left, smooth, thick, and shiny skin in the hands of a patient with diffuse lepromatous leprosy (DLL). Right, skin nodules (lepromas) in the left hand of a patient with nodular lepromatous leprosy (NLL).
All patients were treated with the WHO regimen for multibacillary leprosy, namely, 600 mg of rifampin, 300 mg of clofazimine, and 100 mg of dapsone once a month, and 50 mg of clofazimine and 100 mg of dapsone daily. The only variation was in the number of months used to treat the patients. Remission of the symptoms was observed in all cases, except in patient 5, who had only just begun therapy.
Since the first description of M. lepromatosis, there has been little information regarding its prevalence, clinical presentation, or therapeutic response of the patients. In 2010, Han et al. (6) identified the etiologic agent in 87 confirmed leprosy cases: 55 (63%) harbored M. lepromatosis, 14 were mixed infections, and 18 were M. leprae (6). In our study, we found a much lower frequency (13%) of M. lepromatosis infection and no mixed infections with M. leprae. There are different possible reasons for this discrepancy. These include the PCR procedures and the storage of specimens. Another possibility is the geographical origin, as in the earlier study, no samples from Nuevo León were analyzed, and most M. lepromatosis or mixed infection cases were observed in the West Coast states of Mexico (6).
Organisms similar to M. leprae and M. lepromatosis have been described very recently in diseased squirrels and bovines (8, 9), which raises the possibility of carriers in nature other than the well-known armadillo. In Mexico, where most of the M. lepromatosis cases have been described, it is a custom to eat meat from armadillos and field rats (Rattus rattus) (10). Consequently, since this might be a risk factor for M. lepromatosis infection, all five patients were asked whether they had consumed meat from armadillos or field rats. Two of the patients admitted having eaten field rat meat, and one of them had also eaten armadillo meat. One patient had not eaten such meat, and two other patients did not answer this question (Table 1).
Because of the inability to culture M. leprae, the diagnosis of leprosy has been based on a simple method, such as the visualization of AFB, in addition to clinical symptoms. The use of DNA-based techniques has revolutionized the taxonomy of human pathogens, particularly for organisms previously identified on the basis of simple morphological or biochemical tests (11, 12). More widespread use of molecular diagnostic techniques for Hansen's disease will provide us with better identification of the bacteria involved in this infection in places like Brazil or India, where a high prevalence still exists, or even in specimen banks in parts of the world where this infection has been eliminated.
ACKNOWLEDGMENTS
This work was supported in part by grants from the Fondation Raoul Follereau and the Swiss National Science Foundation (Brazilian Swiss Joint Research Program).
REFERENCES
- 1.World Health Organization. 2013. Global leprosy: update on the 2012 situation. Wkly Epidemiol Rec 88:365–380. [PubMed] [Google Scholar]
- 2.Han XY, Seo YH, Sizer KC, Schoberle T, May GS, Spencer JS, Li W, Nair RG. 2008. A new Mycobacterium species causing diffuse lepromatous leprosy. Am J Clin Pathol 130:856–864. doi: 10.1309/AJCPP72FJZZRRVMM. [DOI] [PubMed] [Google Scholar]
- 3.Vera-Cabrera L, Escalante-Fuentes WG, Gomez-Flores M, Ocampo-Candiani J, Busso P, Singh P, Cole ST. 2011. Case of diffuse lepromatous leprosy associated with “Mycobacterium lepromatosis”. J Clin Microbiol 49:4366–4368. doi: 10.1128/JCM.05634-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han XY, Sizer KC, Tan HH. 2012. Identification of the leprosy agent Mycobacterium lepromatosis in Singapore. J Drugs Dermatol 11:168–172. [PubMed] [Google Scholar]
- 5.Han XY, Aung FM, Choon SE, Werner B. 2014. Analysis of the leprosy agents Mycobacterium leprae and Mycobacterium lepromatosis in four countries. Am J Clin Pathol 142:524–532. doi: 10.1309/AJCP1GLCBE5CDZRM. [DOI] [PubMed] [Google Scholar]
- 6.Han XY, Sizer KC, Velarde-Félix JS, Frias-Castro LO, Vargas-Ocampo F. 2010. The leprosy agents Mycobacterium lepromatosis and Mycobacterium leprae in Mexico. Int J Dermatol 51:952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh P, Benjak A, Schuenemann VJ, Herbig A, Avanzi C, Busso P, Nieselt K, Krause J, Vera-Cabrera L, Cole ST. 23 March 2015. Insight into the evolution and origin of leprosy bacilli from the genome sequence of Mycobacterium lepromatosis. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1421504112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meredith A, Del Pozo J, Smith S, Milne E, Stevenson K, McLuckie J. 2014. Leprosy in red squirrels in Scotland. Vet Rec 175:285–286. doi: 10.1136/vr.g5680. [DOI] [PubMed] [Google Scholar]
- 9.Pin D, Guérin-Faublée V, Garreau V, Breysse F, Dumitrescu O, Flandrois JP, Lina G. 2014. Mycobacterium species related to M. leprae and M. lepromatosis from cows with bovine nodular thelitis. Emerg Infect Dis 20:2111–2114. doi: 10.3201/eid2012.140184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chacon R. 1999. In Mexico, field rats are secret delicacy. The Miami Herald, Miami, FL: http://www.latinamericanstudies.org/mexico/rats.htm. [Google Scholar]
- 11.López-Romero E, Reyes-Montes Mdel R, Pérez-Torres A, Ruiz-Baca E, Villagómez-Castro JC, Mora-Montes HM, Flores-Carreón A, Toriello C. 2011. Sporothrix schenckii complex and sporotrichosis, an emerging health problem. Future Microbiol 6:85–102. doi: 10.2217/fmb.10.157. [DOI] [PubMed] [Google Scholar]
- 12.Najafzadeh MJ, Gueidan C, Badali H, Van Den Ende AH, Xi L, De Hoog GS. 2009. Genetic diversity and species delimitation in the opportunistic genus Fonsecaea. Med Mycol 47:17–25. doi: 10.1080/13693780802527178. [DOI] [PubMed] [Google Scholar]


