Abstract
We describe a 22-year-old soldier with 19% total body surface area burns, polytrauma, and sequence- and culture-confirmed Pythium aphanidermatum wound infection. Antemortem histopathology suggested disseminated Pythium infection, including brain involvement; however, postmortem PCR revealed Cunninghamella elegans, Lichtheimia corymbifera, and Saksenaea vasiformis coinfection. The utility of molecular diagnostics in invasive fungal infections is discussed.
CASE REPORT
In August 2013, a 22-year-old U.S. soldier was injured by an improvised explosive device (IED) while on foot patrol in Bamyan Province, central Afghanistan, which propelled him into a nearby stream. He sustained 19% total body surface area (TBSA) partial- and full-thickness burns, including superficial burns to the penis and multiple skin and soft tissue injuries. These injuries extended from the distal right medial thigh to the right midcalf and from the left gluteus to the left thigh, involving portions of the scrotum. Open fractures of the right tibia/fibula and left proximal femur were noted in addition to comminuted, minimally displaced fractures of the left pelvic girdle complicated by intra- and retroperitoneal hematomas. Soft tissue wounds from IED fragments without bony injury were observed in the left posterior upper arm. He was evacuated to Craig Joint Theater Hospital (CJTH) in Bagram in north-central Afghanistan.
Upon arrival at CJTH, he received a massive transfusion of packed red blood cells (PRBC; 18 units within the first 24 h from injury), fresh frozen plasma (20 units), platelets (3 units apheresis platelets), and cryoprecipitate (20 units) and underwent irrigation and debridement of all soft tissue wounds with external fixation of the right tibia/fibula and left femoral fractures. The minimally displaced fractures of the left pelvic girdle were treated nonoperatively. The patient was medically evacuated to Landstuhl Regional Medical Center (LRMC) in Germany. Despite serial irrigation and debridement of each of these injuries (except the pelvic girdle injuries) over the next 48 h, progressive wound infection in the proximal open left femur fracture necessitated a left hip disarticulation on day 4 after injury (PDOI 4). Intraoperative cultures of the left lower extremity (the site was not further specified) revealed 2 isolates of multidrug-resistant (MDR) Escherichia coli. A prior fungal culture from the left lower extremity (PDOI 2) grew three separate molds (Aspergillus species, Geotrichum species, and Alternaria species) and an unspecified yeast, whereas a fungal culture from this site on PDOI 4 became overgrown with bacteria and could not be further evaluated. No additional operative cultures were obtained at LRMC. To minimize fecal contamination of the left hip wound, an open laparotomy was performed, with placement of a diverting colostomy and evacuation of a retroperitoneal hematoma.
On PDOI 12, the patient was transferred to the Burn Intensive Care Unit at San Antonio Military Medical Center (SAMMC) for continued treatment. He arrived intubated with the following vital signs: temperature of 100.2°F, blood pressure of 129/64 mm Hg, and heart rate of 130 bpm. The patient had been receiving meropenem for a MDR E. coli bacteremia prior to his arrival at SAMMC. On initial intraoperative evaluation at SAMMC (PDOI 13), necrotic muscle, dirt, and IED fragments were observed within the left posterior upper arm and the deep fascial planes of the proximal right thigh and right calf. Concurrent evaluation of the site of the prior left hip disarticulation revealed a viable skin and muscle flap without necrotic material, dirt, or fragments. The finding of nonviable scrotal tissue required resection of the anterior scrotum and placement of 5% sulfamylon–amphotericin B (2 μg/ml; SMAT) dressings. The left posterior upper arm and right thigh/calf wounds were also covered with SMAT. Intravenous liposomal amphotericin B (5 mg/kg of body weight daily) and voriconazole (4 mg/kg every 12 h) were initiated due to the presence of plant material and necrotic tissue within wounds and a concern of invasive mold infection.
On PDOI 17, the patient had an acute clinical decompensation and physical examination findings which were concerning for an intra-abdominal catastrophe. Emergent exploratory laparotomy revealed a jejunal perforation with an adjacent area of ischemic bowel. Due to leakage of enteric contents into the abdomen, resection of the affected bowel and temporary intestinal discontinuity was required. This was the first intra-abdominal procedure performed since the diverting colostomy placement at LRMC, and the colostomy appeared viable on inspection. Surgical re-exploration of the abdomen on PDOI 18 revealed additional punctate, serosal lesions on the ileum and jejunum that were excised. Fungal histopathology was unrevealing, but growth was noted on fungal culture of the excised small bowel tissue (Table 1). No additional lesions were noted on re-evaluation at PDOI 19 and PDOI 21; intestinal reanastomosis was performed, and a negative-pressure wound dressing was placed over the open abdomen.
TABLE 1.
Fungal culture, histopathology, and PCR results for the case patienta
| Site | PDOI and wound cultureb | PDOI and histopathology (specific morphology, if available) |
PDOI and PCR result (specimen) | |
|---|---|---|---|---|
| Vascular invasion | No vascular invasion | |||
| Left lower extremity | 2 (single sample)—yeast, Aspergillus species, Geotrichum species, Alternaria species | |||
| Left triceps | 18—P. aphanidermatum; 14—P. aphanidermatum (UTHSCSCA DI14-349) | 18—fungal elements | 23—medium caliber fungal hyphae with inconspicuous branchings; 22—medium caliber fungal hyphae with branchings and a few spores; 20—superficial fungal elements | 42—C. elegans (FFPE autopsy); 14—P. aphanidermatum (culture) (UTHSCSCA DI14-349) |
| Left gluteus | 16—P. aphanidermatum; 15—P. aphanidermatum | 15—predominantly septate fungal elements | 33—medium to broad nonseptate hyphae with direct angular branchings | |
| Left acetabular tissue | 17—P. aphanidermatum | 19—fungal elements; 17—fungal elements; 15—fungal elements; 14—fungal elements | 18—Fungal elements in muscle | 17—S. vasiformis (culture) |
| Small bowel | 18—P. aphanidermatum | 18—no fungal elements identified | ||
| Right leg tissue | 18—P. aphanidermatum | 32—broad nonseptate and direct angular hyphae | 18—fungal elements with and without vascular invasion | 18—C. elegans (culture) |
| 15; fungal elements | ||||
| Right posterior thigh | 25—P. aphanidermatum | 28—broad nonseptate and ribbon-shaped fungal elements with direct angular hyphae | 25—deep colonization of fungus. Broad nonseptate hyphae with direct angular branchings | |
| 18—P. aphanidermatum | 20—focal fungal vascular invasion; 19—focal fungal vascular invasion; 18—focal fungal vascular invasion | 14—broad nonseptate hyphae; 12—broad nonseptate fungal forms | ||
| Right gluteus maximus | 32—broad nonseptate and ribbon-shaped fungal elements with direct angular hyphae | |||
| Right hip disarticulation | 29—P. aphanidermatum; 25—P. aphanidermatum | 32—broad nonseptate and ribbon-shaped fungal elements with direct angular hyphae | ||
| Sacrum | 35—broad nonseptate and ribbon-shaped fungal elements with direct angular hyphae | |||
| Brain | 42—fungal elements (autopsy) | 42—L. corymbifera (FFPE autopsy) | ||
| Testis | 42—fungal elements (autopsy) | |||
PDOI, day after date of injury.
Specimen identification of Pythium aphanidermatum was confirmed via PCR on culture from PDOI 14 only; all other “culture positives” were identified via in vitro morphology similar to that of this specimen.
On PDOI 18, the left upper extremity/right lower extremity and left pelvic wounds were also evaluated intraoperatively. Despite initial improvement in the appearance on gross exam, follow-up evaluation revealed visible fungal growth and necrotic tissue of the right calf, requiring a right above-knee amputation three centimeters proximal to the knee joint. Bone fragments of the left pelvic rim appeared devascularized, and the surrounding skeletal muscle appeared dead. Thus, a left hemipelvectomy was performed, leading to exposure of the distal rectum.
With each surgical intervention, tissue was sent for bacterial and fungal cultures and histopathology. Bacterial cultures grew MDR extended-spectrum-beta-lactamase (ESBL)-producing E. coli, MDR ESBL-producing Klebsiella pneumoniae, Stenotrophomonas maltophilia, MDR Morganella morganii, Enterococcus faecium (ampicillin resistant, vancomycin susceptible), and Bacteroides species. Histopathology of tissue obtained from the left posterior upper arm, right hip/thigh/calf, and pelvic region (including the left gluteal, left acetabular, and sacral regions) revealed nonseptate hyphae suggestive of mucormycosis. Several specimens revealed evidence of deep fungal infection with vascular invasion (Table 1).
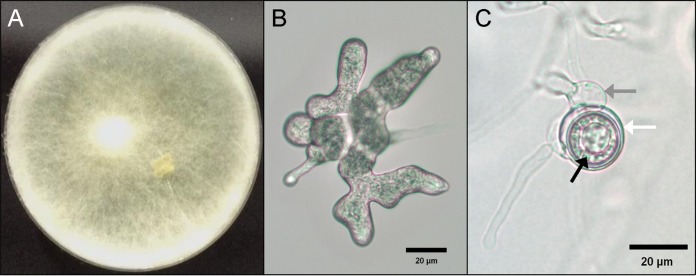
Due to difficulty with isolation and identification by conventional methods, a culture specimen obtained from the left triceps on PDOI 14 was sent to the Fungus Testing Laboratory at the University of Texas Health Science Center at San Antonio (UTHSCA FTL) for identification by morphology and DNA sequence analysis and for antifungal susceptibility testing (Table 1). The isolate was added to the culture collection with accession number UTHSCSA DI14-349 and was studied phenotypically using a combination of media, including potato flakes agar (PFA), irradiated carnation leaf agar (CLA) (1), and cornmeal/irradiated carnation leaf agar (CMACLA), all prepared in-house on 60-mm plates; no blood agar was used. Plates were overlaid with several drops of sterile water and incubated at 25°C. Colonies demonstrated rapid, white woolly growth after 48 h on all media (Fig. 1A). The CLA plate was most informative for production of characteristic microscopic features examined after 5 days of incubation in lactophenol cotton blue preparations. Figure 1B shows toruloid sporangia; Fig. 1C shows an oogonium (white arrow) containing oospores (black arrow), attached to the antheridium (gray arrow) (2). Temperature studies on PFA indicated growth at 37°C and 40°C and no growth at 45°C or 50°C.
FIG 1.
(A) Pythium aphanidermatum UTHSC DI 14-349 colony on cornmeal/irradiated carnation leaf agar at 48 h in ambient air at 25°C; (B) toruloid sporangium; (C) oogonium (white arrow), oospores (black arrow), and antheridium (gray arrow).
For sequence analysis, template DNA was prepared from cultures as previously described (3). Isolates were subcultured onto PFA (Difco, Detroit, MI) and grown for 24 h at 30°C. A section of hyphae and agar was removed with a disposable 10-μl loop and gently rolled onto a separate area of the agar plate to remove residual agar. Hyphal material was then added to 200 μl Prepman Ultra reagent (Life Technologies, Grand Island, NY) in a 2.0-ml screw-cap microcentrifuge tube (Biospec, Inc., Bartlesville, OK, USA) that also contained 100 μl (vol/vol) 0.5-mm glass beads (Biospec, Inc.) and heated to 99°C for 15 min. The suspension was agitated for 30 s in a mini-bead beater (Biospec, Inc.) set at the highest speed, cooled in an ice bath for 2 min, and heated again at 99°C for 10 min. The suspension was pelleted at 13,200 × g for 1 min in a microcentrifuge (Eppendorf, Inc., Westbury, NY, USA) and immediately used to set up PCRs. Internal transcribed spacer (ITS) and D1/D2 sequences were obtained as described using the primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and NL4 (5′-GGTCCGTGTTTCAAGACGG-3′) to amplify the ITS-D1/D2 region, followed by sequencing with these primers and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) and NL1 (5′-GCATATCAATAAGCGGAGGAAAAG-3′) (3). The ITS sequence showed 100% identity to Pythium aphanidermatum accession number AJ628984 (791/791 bp matched), Pythium aphanidermatum accession number AY151180 (783/783 bp matched), and Pythium aphanidermatum accession number KF840479 (781/781 bp matched) in a BLAST search of GenBank (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The three top GenBank hits for the D1/D2 search showed 100% (accession number AB544448; 701/701 bp matched), 99.86% (accession number JF412452; 705/706 bp matched), and 99.73% (accession number AY598622; 705/706 bp matched) identities to the same species. The next closest species, Pythium deliense, showed 97.5% identity. These phenotypic features combined with the ITS and D1/D2 sequence data confirmed the identification of the isolate as Pythium aphanidermatum on PDOI 30. With the pathogen identified, prospective and retrospective fungal culture results were again reviewed by the SAMMC Mycology lab and identified as P. aphanidermatum based on morphological similarity with this isolate.
Given this finding, minocycline was added (due to reports of in vitro susceptibility of Pythium species) (4), and liposomal amphotericin B, voriconazole, and trimethoprim-sulfamethoxazole (20 mg/kg trimethoprim daily, divided every 8 h for S. maltophilia and MDR Chryseobacterium indologenes ventilator-associated pneumonia) were continued. Susceptibility testing of the P. aphanidermatum isolate was performed by broth macrodilution testing following the method described in the Clinical and Laboratory Standards Institute M38-A2 standard (5). Briefly, a stock inoculum suspension was prepared using a spectrophotometer and diluted 1:100 to obtain the test inoculum of 0.4 × 104 to 5 × 104 CFU/ml. Then the test inoculum was added to tubes containing serial 2-fold dilutions of amphotericin B (final concentration range, 0.03 to 16 μg/ml), the echinocandins anidulafungin, caspofungin, and micafungin (final concentration range, 0.015 to 8 μg/ml), and the azoles fluconazole (final concentration range, 0.125 to 64 μg/ml), itraconazole, posaconazole, and voriconazole (final concentration range, 0.03 to 16 μg/ml). The assay was conducted in RPMI 1640 medium (with l-glutamine and without bicarbonate) buffered with 0.165 M morpholinepropanesulfonic acid (MOPS). The tubes were incubated at 35°C for 48 h, and the MIC was measured as the lowest concentration that resulted in ∼100% inhibition of growth compared to the drug-free control for amphotericin B and the azoles. For the echinocandins, the minimum effective concentration (MEC) was measured as the lowest concentration that resulted in abnormally branched, stubby hyphae. These methods are consistent with those reported in the literature to evaluate the activity of antifungal agents against Pythium species (6–8).
The following MICs were reported: amphotericin B, >16 μg/ml; anidulafungin, >8 μg/ml; caspofungin, >8 μg/ml; micafungin, >8 μg/ml; fluconazole, >64 μg/ml; itraconazole, >16 μg/ml; posaconazole, >16 μg/ml; voriconazole, >16 μg/ml. Although no clinical breakpoints have been established for antifungals against Pythium species, these MICs indicate a lack of activity for these agents at clinically relevant concentrations. As terbinafine has shown in vitro synergistic activity with azoles and echinocandins, microdilution checkerboard testing was performed with several combinations (9). Susceptibility testing demonstrated no activity for terbinafine alone, and no synergistic activity was observed when this agent was combined with posaconazole, itraconazole, caspofungin, or micafungin at the maximum concentrations tested (2 μg/ml for terbinafine and 4 μg/ml for the other agents). Minocycline susceptibility testing was not performed.
Gross fungal contamination in the right above-knee amputation stump led to resection of an additional four centimeters of femur on PDOI 23 followed by a right hip disarticulation on PDOI 32. Serial evaluations of the left posterior upper arm did not show evidence of gross fungal growth, and this wound was treated conservatively with serial irrigation, debridement and placement of antimicrobial beads containing amphotericin B, voriconazole, and tobramycin.
Further complicating management of the polymicrobial wound infections, the patient sustained recurrent bacteremia, ventilator-associated pneumonias, and line-associated infections with MDR pathogens, requiring frequent courses of broad-spectrum antibiotics. Due to these repeated infections (including wound infections threatening peritoneal extension due to rectal and sacral tissue exposure), multiple system organ failure requiring frequent blood transfusions, coagulopathy, dialysis, and prolonged ventilator assistance, the patient was transitioned to comfort care and died on PDOI 40.
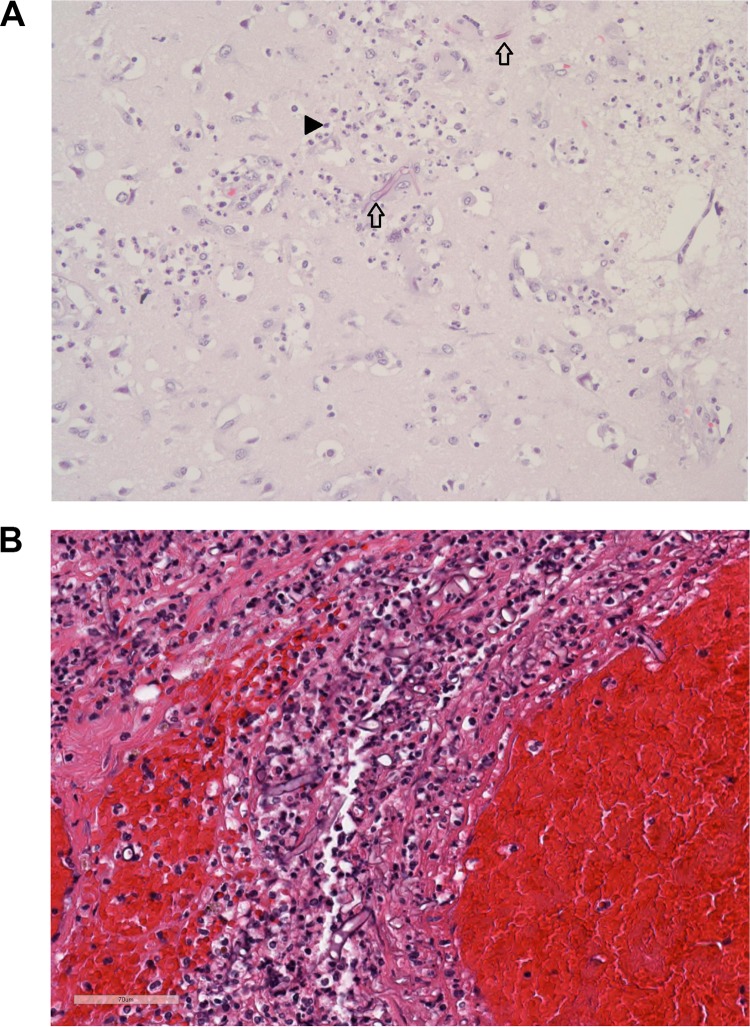
On autopsy, cause of death was determined to be sepsis and multiple system organ failure with invasive fungal infection (IFI) from blast injuries. Additional autopsy findings included residual soft tissue defects of the left gluteal and left upper arm regions, evidence of renal insufficiency, splenic infarction, diffuse alveolar damage with histopathologic evidence of cytomegalovirus (CMV) pneumonia of the right lung, and hepatomegaly with confluent hepatic necrosis. Figure 2 shows tissue from the brain (Fig. 2A) and from the right testis (Fig. 2B). Postmortem histopathology specimens (in formalin-fixed, paraffin-embedded [FFPE] blocks) showing fungal elements in left arm muscle and brain tissue were sent for pathogen extraction and molecular identification. In addition, three culture samples (two morphologically identified as P. aphanidermatum: the left acetabular tissue on PDOI 17 and the right leg tissue on PDOI 18) were also sent for molecular identification. The previously PCR-confirmed P. aphanidermatum specimen from cultures of tissue samples from the left arm (PDOI 14) was sent for comparison via morphology and sequencing with new postmortem samples.
FIG 2.
(A) Tissue taken from the cortex of the right occipital lobe reveals brain parenchyma with patchy infiltration by broad, aseptate fungal hyphae (arrows) and robust acute encephalitis, as noted by the large number of neutrophils (arrowhead). No other foci of necrosis or inflammation were noted in the brain. (B) Hematoxylin-and-eosin-stained right testis and soft tissue (PDOI 42/autopsy) with acute and chronic inflammation with hemorrhage. Broad, aseptate hyphae are seen interspersed with the inflammatory cells.
With PCR sequencing, the specimens from the FFPE autopsy specimens of the left posterior arm and brain were identified as Cunninghamella elegans and Lichtheimia corymbifera (formerly Absidia corymbifera), respectively. The cultures from the left acetabular tissue and right leg (PDOI 17 and 18), which were initially identified via morphological similarity as P. aphanidermatum, were identified by sequencing as Saksenaea vasiformis and Cunninghamella elegans. No additional P. aphanidermatum was isolated at autopsy.
Historically, combat-related injuries have most often been complicated by bacterial wound and pulmonary infections. During the recent combat operations in Afghanistan, IFIs have emerged as a significant cause of morbidity and mortality among U.S. military personnel (10–12). Blast injuries from IEDs have the potential for severe disruption of skin and bone and contamination of wounds with organic material such as soil and plant matter (13). Risk factors associated with IFIs include dismounted injuries while on foot patrol and large-volume blood transfusions (11, 14). Notably, large-volume PRBC transfusion (>20 units) may represent a marker for severe disease rather than an independent risk factor (14).
Early identification, aggressive surgical debridement, and systemic antifungals are key measures in the management of these infections. Current diagnosis often depends on histopathologic appearance and culture, which can lead to delays in accurate diagnosis due to low sensitivity and specificity of these methods (15–20). Increasingly, PCR-based methods have been evaluated for use in IFI identification, with improved sensitivity and specificity relative to standard culture techniques (21). Advantages of PCR-based methods include their utility on small amounts of tissue and paraffin-embedded tissue, which would preclude diagnosis by fungal culture.
As described for the case patient, PCR testing has also enabled the identification of unusual pathogens such as P. aphanidermatum. Diagnosis of Pythium infection via conventional histopathology and culture is difficult because the appearance of broad, aseptate hyphae can resemble members of the Mucorales on histopathology. Furthermore, Pythium species demonstrate poor growth on standard culture media, making definitive identification difficult (22). Despite similarities in morphology with the Mucorales, Pythium species are genetically and physiologically distinct organisms (23–25) of the oomycetes (class Oomycota; parafungal organisms closely related to green algae) and may require alternative treatment strategies.
Prior to 2011, the only reported human pathogen in this genus was Pythium insidiosum. This organism, primarily reported in Thailand, has been shown to cause invasive cutaneous, ocular, vascular, and disseminated infections and is often associated with aquatic exposure in tropical and subtropical environments (26–29). The case patient was found in a stream near the blast site, which might have contributed to acquisition. In 2011, the first reported case of P. aphanidermatum infection occurred in a soldier who sustained a lower-extremity wound from an IED explosion in Afghanistan. That patient received aggressive debridement, leading to bilateral lower extremity disarticulation, and systemic antifungals but died 16 weeks postinjury from multiple system organ failure (related to a complex hospital course including recurrent bacteremias, ventilator-associated pneumonias, and multiple surgeries for gastric necrosis and perforation). No residual Pythium infection was noted on autopsy (30).
Several similarities are noted between these two patients with P. aphanidermatum infections: both were injured during foot patrol in Afghanistan (although the exact geographic location of the first case was not reported), both required large-volume blood transfusions and extensive surgical interventions, and both had evidence of multiple environmental fungal isolates on wound cultures. In both cases, susceptibility testing for P. aphanidermatum revealed panresistance to available agents. This is not unexpected for these pseudofungi, as the cell wall is composed of cellulose and polymerized beta-1,3-glucan but lacks ergosterol, the target of most available antifungal agents (29–31). Several antibacterial agents and the squalene oxidase inhibitor terbinafine have been shown to have some in vitro synergistic activity, but these have been published only for P. insidiosum. In these studies, a wide range of synergistic effects was noted among the 17 isolates tested, suggesting that different strains may have different susceptibilities (4, 9). In an in vivo rabbit model of P. insidiosum infection, a combination of terbinafine, itraconazole, and fluvastatin was associated with the smallest amount of hyphae relative to other combinations (8). In this patient, resistance to terbinafine was identified in vitro, alone and in combination with posaconazole, itraconazole, caspofungin, and micafungin.
Given the limitations of medical therapy for Pythium infections, aggressive surgical debridement (often requiring amputation) is the mainstay of therapy (27, 32). PCR testing in this patient, although limited to a small sample of available cultures, revealed P. aphanidermatum only in the left triceps from one culture. A second tissue FFPE sample from the same area on autopsy was PCR positive for Cunninghamella elegans, suggesting that repeated debridements might have cleared the P. aphanidermatum infection.
The presence of several ubiquitous environmental molds (S. vasiformis, C. elegans, and L. corymbifera) identified from wound cultures is not unexpected, as polymicrobial wound contamination in combat-related injuries has been well described (11, 33). However, the presence of a fungal organism (L. corymbifera) in FFPE brain tissue on autopsy is unusual in combat-related injuries. In an autopsy study of causes of mortality in combat casualties in a burn unit, there was no evidence of fungal central nervous system (CNS) involvement in the 45 patients who died from infection (34). In addition, in a review of trauma-associated IFIs, none of the 31 patients was reported to have CNS involvement (11). Similarly, the recent Apophysomyces trapeziformis (a member of the Mucorales) outbreak associated with the Joplin, MO, tornado found no cases of disseminated infection in 13 patients; however, no autopsies were performed on the five who died (35). Thus, CNS infection cannot be ruled out conclusively. Cerebral infection in immunocompetent patients is rare and primarily described in two patient populations: injection drug users (thought to be from fungal inoculation at the time of drug injection) and near-drowning patients (related to aspiration of large volumes of contaminated water with subsequent dissemination from the lungs or direct invasion through the sinuses) (36–38). In nonimmunocompromised, nondiabetic patients, cutaneous infection is the most common site of mucormycosis, whereas CNS infection is more often associated with diabetic patients with sinus involvement and contiguous spread (39). In bone marrow transplant patients, disseminated mucormycosis from a pulmonary source can also lead to CNS involvement due to the angioinvasive nature of these organisms (40). For the case patient, there was no evidence of neuroinvasive fungal disease on intracranial imaging.
In summary, this report provides further evidence for the benefits of molecular diagnosis over standard histopathologic and culture methods for the diagnosis of IFIs. In combat-related injuries, wound infection with multiple isolates is not unexpected, and definitive diagnosis using PCR might be a useful adjunct to guide antifungal therapy. Molecular tests might also prove valuable in identifying unusual pathogens that may not be susceptible to standard antimicrobial agents. Based on limited human data, Pythium species appear to be particularly resistant and invasive, necessitating early identification to improve mortality through prompt, aggressive surgical interventions (27, 30, 32). Given the extensive debridement often required, additional research into alternative antimicrobial agents may allow decreased morbidity associated with these infections.
Nucleotide sequence accession numbers.
The D1/D2 and ITS sequences were deposited in GenBank under accession numbers KP331544 and KP331545.
ACKNOWLEDGMENTS
The view(s) expressed herein are those of the authors and do not reflect the official policy or position of Brooke Army Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of the Army and Department of Defense, or the U.S. Government.
A.R.F., C.K.M., I.R.D., E.A.R., and T.J.V. are employees of the U.S. Government. B.L.W. is supported by grant W81XWH-13-C-0103 from the U.S. Army Medical Research and Materiel Command, Office of Congressionally Directed Medical Research Programs, Joint Warfighter Medical Research Program, and the Army Research Office of the Department of Defense under contract no. W911NF-11-1-0136. A.G. was supported by the Department of the Navy under the Wounded, Ill, and Injured Program and the military infectious disease research program D_MIDCTA_I_12_J2_29.
REFERENCES
- 1.Nelson PE, Toussoun TA, Marasas WFO. 1983. Fusarium species: an illustrated manual for identifications. Pennsylvania State University Press, University Park, PA. [Google Scholar]
- 2.Watanabe T. 2002. Pictorial atlas of soil and seed fungi: morphologies of cultured fungi and key to species, 2nd ed CRC Press, Boca Raton, FL. [Google Scholar]
- 3.Romanelli AM, Sutton DA, Thompson EH, Rinaldi MG, Wickes BL. 2010. Sequence-based identification of filamentous basidiomycetous fungi from clinical specimens: a cautionary note. J Clin Microbiol 48:741–752. doi: 10.1128/JCM.01948-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loreto ES, Mario DA, Denardi LB, Alves SH, Santurio JM. 2011. In vitro susceptibility of Pythium insidiosum to macrolides and tetracycline antibiotics. Antimicrob Agents Chemother 55:3588–3590. doi: 10.1128/AAC.01586-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi: approved standard, 2nd ed CLSI document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 6.Pereira DI, Santurio JM, Alves SH, Argenta JS, Potter L, Spanamberg A, Ferreiro L. 2007. Caspofungin in vitro and in vivo activity against Brazilian Pythium insidiosum strains isolated from animals. J Antimicrob Chemother 60:1168–1171. doi: 10.1093/jac/dkm332. [DOI] [PubMed] [Google Scholar]
- 7.Fonseca AO, Pereira DI, Maia Filho FS, Osorio LG, Maroneze BP, Valente JS, Potter L, Meireles MC. 2014. In vitro susceptibility of zoospores and hyphae of Pythium insidiosum to antifungals. J Antimicrob Chemother 69:1564–1567. doi: 10.1093/jac/dku021. [DOI] [PubMed] [Google Scholar]
- 8.Argenta JS, Alves SH, Silveira F, Maboni G, Zanette RA, Cavalheiro AS, Pereira PL, Pereira DI, Sallis ES, Potter L, Santurio JM, Ferreiro L. 2012. In vitro and in vivo susceptibility of two-drug and three-drug combinations of terbinafine, itraconazole, caspofungin, ibuprofen and fluvastatin against Pythium insidiosum. Vet Microbiol 157:137–142. doi: 10.1016/j.vetmic.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Cavalheiro AS, Maboni G, de Azevedo MI, Argenta JS, Pereira DI, Spader TB, Alves SH, Santurio JM. 2009. In vitro activity of terbinafine combined with caspofungin and azoles against Pythium insidiosum. Antimicrob Agents Chemother 53:2136–2138. doi: 10.1128/AAC.01506-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray CK, Wilkins K, Molter NC, Li F, Yu L, Spott MA, Eastridge B, Blackbourne LH, Hospenthal DR. 2011. Infections complicating the care of combat casualties during operations Iraqi Freedom and Enduring Freedom. J Trauma 71:S62–S73. doi: 10.1097/TA.0b013e3182218c99. [DOI] [PubMed] [Google Scholar]
- 11.Warkentien T, Rodriguez C, Lloyd B, Wells J, Weintrob A, Dunne JR, Ganesan A, Li P, Bradley W, Gaskins LJ, Seillier-Moiseiwitsch F, Murray CK, Millar EV, Keenan B, Paolino K, Fleming M, Hospenthal DR, Wortmann GW, Landrum ML, Kortepeter MG, Tribble DR, Infectious Disease Clinical Research Program Trauma Infectious Disease Outcomes Study Group. 2012. Invasive mold infections following combat-related injuries. Clin Infect Dis 55:1441–1449. doi: 10.1093/cid/cis749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tribble DR, Conger NG, Fraser S, Gleeson TD, Wilkins K, Antonille T, Weintrob A, Ganesan A, Gaskins LJ, Li P, Grandits G, Landrum ML, Hospenthal DR, Millar EV, Blackbourne LH, Dunne JR, Craft D, Mende K, Wortmann GW, Herlihy R, McDonald J, Murray CK. 2011. Infection-associated clinical outcomes in hospitalized medical evacuees after traumatic injury: trauma infectious disease outcome study. J Trauma 71:S33–42. doi: 10.1097/TA.0b013e318221162e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hajdu S, Obradovic A, Presterl E, Vecsei V. 2009. Invasive mycoses following trauma. Injury 40:548–554. doi: 10.1016/j.injury.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez CJ, Weintrob AC, Shah J, Malone D, Dunne JR, Weisbrod AB, Lloyd BA, Warkentien TE, Murray CK, Wilkins K, Shaikh F, Carson ML, Aggarwal D, Tribble DR, Infectious Disease Clinical Research Program Trauma Infectious Disease Outcomes Study Group. 2014. Risk factors associated with invasive fungal infections in combat trauma. Surg Infect (Larchmt) 15:521–526. doi: 10.1089/sur.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guarner J, Brandt ME. 2011. Histopathologic diagnosis of fungal infections in the 21st century Clin Microbiol Rev 24:247–280. doi: 10.1128/CMR.00053-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rickerts V, Mousset S, Lambrecht E, Tintelnot K, Schwerdtfeger R, Presterl E, Jacobi V, Just-Nubling G, Bialek R. 2007. Comparison of histopathological analysis, culture, and polymerase chain reaction assays to detect invasive mold infections from biopsy specimens. Clin Infect Dis 44:1078–1083. doi: 10.1086/512812. [DOI] [PubMed] [Google Scholar]
- 17.Alexander BD, Pfaller MA. 2006. Contemporary tools for the diagnosis and management of invasive mycoses. Clin Infect Dis 43:S15–S27. doi: 10.1086/504491. [DOI] [Google Scholar]
- 18.Schofield CM, Murray CK, Horvath EE, Cancio LC, Kim SH, Wolf SE, Hospenthal DR. 2007. Correlation of culture with histopathology in fungal burn wound colonization and infection. Burns 33:341–346. doi: 10.1016/j.burns.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 19.Sangoi AR, Rogers WM, Longacre TA, Montoya JG, Baron EJ, Banaei N. 2009. Challenges and pitfalls of morphologic identification of fungal infections in histologic and cytologic specimens: a ten-year retrospective review at a single institution. Am J Clin Pathol 131:364–375. doi: 10.1309/AJCP99OOOZSNISCZ. [DOI] [PubMed] [Google Scholar]
- 20.Tarrand JJ, Lichterfeld M, Warraich I, Luna M, Han XY, May GS, Kontoyiannis DP. 2003. Diagnosis of invasive septate mold infections. A correlation of microbiological culture and histologic or cytologic examination. Am J Clin Pathol 119:854–858. doi: 10.1309/EXBVYAUPENBM285Y. [DOI] [PubMed] [Google Scholar]
- 21.Lau A, Chen S, Sorrell T, Carter D, Malik R, Martin P, Halliday C. 2007. Development and clinical application of a panfungal PCR assay to detect and identify fungal DNA in tissue specimens. J Clin Microbiol 45:380–385. doi: 10.1128/JCM.01862-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Botton SA, Pereira DI, Costa MM, Azevedo MI, Argenta JS, Jesus FP, Alves SH, Santurio JM. 2011. Identification of Pythium insidiosum by nested PCR in cutaneous lesions of Brazilian horses and rabbits. Curr Microbiol 62:1225–1229. doi: 10.1007/s00284-010-9781-4. [DOI] [PubMed] [Google Scholar]
- 23.Mendoza L, Hernandez F, Ajello L. 1993. Life cycle of the human and animal oomycete pathogen Pythium insidiosum. J Clin Microbiol 31:2967–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schurko AM, Mendoza L, Levesque CA, Desaulniers NL, de Cock AW, Klassen GR. 2003. A molecular phylogeny of Pythium insidiosum. Mycol Res 107:537–544. doi: 10.1017/S0953756203007718. [DOI] [PubMed] [Google Scholar]
- 25.Mendoza L, Vilela R. 2013. The mammalian pathogenic oomycetes. Curr Fungal Infect Rep 7:198–208. doi: 10.1007/s12281-013-0144-z. [DOI] [Google Scholar]
- 26.Krajaejun T, Kunakorn M, Pracharktam R, Chongtrakool P, Sathapatayavongs B, Chaiprasert A, Vanittanakom N, Chindamporn A, Mootsikapun P. 2006. Identification of a novel 74-kilodalton immunodominant antigen of Pythium insidiosum recognized by sera from human patients with pythiosis. J Clin Microbiol 44:1674–1680. doi: 10.1128/JCM.44.5.1674-1680.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krajaejun T, Sathapatayavongs B, Pracharktam R, Nitiyanant P, Leelachaikul P, Wanachiwanawin W, Chaiprasert A, Assanasen P, Saipetch M, Mootsikapun P, Chetchotisakd P, Lekhakula A, Mitarnun W, Kalnauwakul S, Supparatpinyo K, Chaiwarith R, Chiewchanvit S, Tananuvat N, Srisiri S, Suankratay C, Kulwichit W, Wongsaisuwan M, Somkaew S. 2006. Clinical and epidemiological analyses of human pythiosis in Thailand. Clin Infect Dis 43:569–576. doi: 10.1086/506353. [DOI] [PubMed] [Google Scholar]
- 28.Mendoza L, Prasla SH, Ajello L. 2004. Orbital pythiosis: a non-fungal disease mimicking orbital mycotic infections, with a retrospective review of the literature. Mycoses 47:14–23. doi: 10.1046/j.1439-0507.2003.00950.x. [DOI] [PubMed] [Google Scholar]
- 29.Shenep JL, English BK, Kaufman L, Pearson TA, Thompson JW, Kaufman RA, Frisch G, Rinaldi MG. 1998. Successful medical therapy for deeply invasive facial infection due to Pythium insidiosum in a child. Clin Infect Dis 27:1388–1393. doi: 10.1086/515042. [DOI] [PubMed] [Google Scholar]
- 30.Calvano TP, Blatz PJ, Vento TJ, Wickes BL, Sutton DA, Thompson EH, White CE, Renz EM, Hospenthal DR. 2011. Pythium aphanidermatum infection following combat trauma. J Clin Microbiol 49:3710–3713. doi: 10.1128/JCM.01209-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sekhon AS, Padhye AA, Garg AK. 1992. In vitro sensitivity of Penicillium marneffei and Pythium insidiosum to various antifungal agents. Eur J Epidemiol 8:427–432. doi: 10.1007/BF00158578. [DOI] [PubMed] [Google Scholar]
- 32.Imwidthaya P. 1994. Human pythiosis in Thailand. Postgrad Med J 70:558–560. doi: 10.1136/pgmj.70.826.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hospenthal DR, Chung KK, Lairet K, Thompson EH, Guarro J, Renz EM, Sutton DA. 2011. Saksenaea erythrospora infection following combat trauma. J Clin Microbiol 49:3707–3709. doi: 10.1128/JCM.05095-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gomez R, Murray CK, Hospenthal DR, Cancio LC, Renz EM, Holcomb JB, Wade CE, Wolf SE. 2009. Causes of mortality by autopsy findings of combat casualties and civilian patients admitted to a burn unit. J Am Coll Surg 208:348–354. doi: 10.1016/j.jamcollsurg.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Neblett Fanfair RBK, Bos J, Bennett SD, Lo YC, Adebanjo T, Etienne K, Deak E, Derado G, Shieh WJ, Drew C, Zaki S, Sugerman D, Gade L, Thompson EH, Sutton DA, Engelthaler DM, Schupp JM, Brandt ME, Harris JR, Lockhart SR, Turabelidze G, Park BJ. 2012. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med 367:2214–2225. doi: 10.1056/NEJMoa1204781. [DOI] [PubMed] [Google Scholar]
- 36.Carpenter M, Polk C, Castellani R, Mochoruk K, Sanche S, Stern B, Donnenberg MS. 2007. Encephalitis of the basal ganglia in an injection drug user. Clin Infect Dis 45:1479, 1522–1524. doi: 10.1086/522994. [DOI] [PubMed] [Google Scholar]
- 37.Stave GM, Heimberger T, Kerkering TM. 1989. Zygomycosis of the basal ganglia in intravenous drug users. Am J Med 86:115–117. doi: 10.1016/0002-9343(89)90242-8. [DOI] [PubMed] [Google Scholar]
- 38.Kantarcioglu AS, Guarro J, de Hoog GS. 2008. Central nervous system infections by members of the Pseudallescheria boydii species complex in healthy and immunocompromised hosts: epidemiology, clinical characteristics and outcome. Mycoses 51:275–290. doi: 10.1111/j.1439-0507.2008.01489.x. [DOI] [PubMed] [Google Scholar]
- 39.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ. 2005. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 40.McCarthy M, Rosengart A, Schuetz AN, Kontoyiannis DP, Walsh TJ. 2014. Mold infections of the central nervous system. N Engl J Med 371:150–160. doi: 10.1056/NEJMra1216008. [DOI] [PMC free article] [PubMed] [Google Scholar]