Abstract
We report a case in which fecal microbiota transplantation (FMT) utilized for relapsing Clostridium difficile colitis successfully eradicated colonization with several multidrug-resistant organisms (MDROs). FMT may have an additive benefit of reducing MDRO carriage and should be further investigated as a potential measure to eradicate additional potentially virulent organisms beyond C. difficile.
CASE REPORT
A 66-year-old male was admitted to Scripps Mercy Hospital in June of 2012 for debridement of a large sacral wound. His past medical history was significant for a spinal epidural abscess in March of 2011 that resulted in C4-level spinal cord injury (quadriplegia). His condition required residence in a skilled nursing facility and placement of a tracheostomy and a feeding tube and chronic Foley catheterization. While at the facility, he was found to be colonized by multiple multidrug-resistant (MDR) organisms (MDROs), including carbapenem-resistant Enterobacteriaceae (CRE), methicillin-resistant Staphylococcus aureus (MRSA), and MDR Acinetobacter baumannii, and developed sepsis on a monthly basis during the 6 months preceding admission. Three days prior to admission, he was diagnosed with a CRE Klebsiella pneumoniae urinary tract infection (UTI).
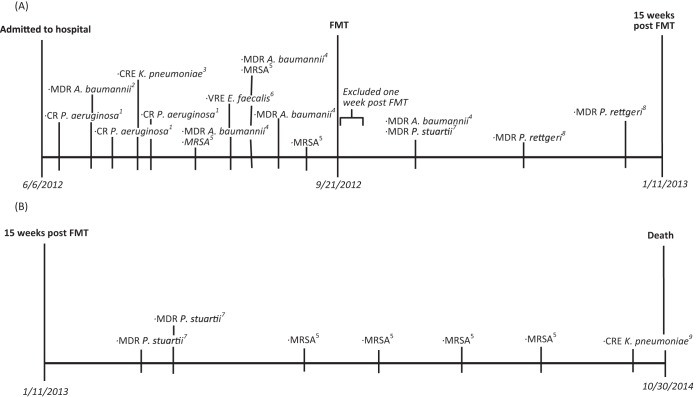
As a result of his ventilator-dependent condition and complex medical needs, he was admitted to the intensive care unit (ICU) at our facility. The sacral wound was treated with debridement and flap placement as well as a diverting colostomy. He was diagnosed with Clostridium difficile colitis during the first week of admission and treated with oral vancomycin (250 mg orally [p.o.] via a feeding tube 4 times/day). In addition, during the first 15 weeks of hospitalization, a variety of MDROs were isolated, including carbapenem-resistant (CR) Pseudomonas aeruginosa (respiratory tract), MDR Acinetobacter baumannii (wound and respiratory tract), CRE Klebsiella pneumoniae (wound), vancomycin-resistant Enterococcus faecalis (VRE) (wound), and MRSA (respiratory tract, urine, and abdominal fluid) (Fig. 1A). The patient received antibiotic courses for these infections, but these were limited to short courses due to concurrent C. difficile colitis and MDROs that were resistant to all or the majority of antibiotics.
FIG 1.
(A) MDR pathogens isolated during 15 weeks pre-FMT and post-FMT. (B) MDR pathogens isolated subsequently during the remainder of the hospitalization period. CR, carbapenem resistant; CRE, carbapenem-resistant Enterobacteriaceae; MDR, multidrug resistant; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococcus faecalis. 1, Pseudomonas aeruginosa (respiratory tract). Resistant to gentamicin, imipenem, levofloxacin, meropenem, and tobramycin; intermediate to piperacillin-tazobactam; susceptible to amikacin, cefepime, ceftazidime, and ciprofloxacin. 2, Acinetobacter baumannii (Jackson-Pratt [JP] drain in wound). Resistant to amikacin, cefepime, ciprofloxacin, gentamicin, levofloxacin, tobramycin, and trimethoprim-sulfamethoxazole; susceptible to ceftazidime, imipenem, and meropenem. 3, Klebsiella pneumoniae (ulcer). Resistant to amikacin, ampicillin-sulbactam, cefepime, ceftazidime, ciprofloxacin, gentamicin, imipenem, levofloxacin, meropenem, and piperacillin-tazobactam; susceptible to trimethoprim-sulfamethoxazole. 4, Acinetobacter baumannii (respiratory tract). Resistant to amikacin, cefepime, ceftazidime, ciprofloxacin, gentamicin, imipenem, levofloxacin, meropenem, and tobramycin. 5, methicillin-resistant Staphylococcus aureus (respiratory tract/urethral meatus/urine/abdominal fluid). Resistant to clindamycin and oxacillin; susceptible to doxycycline, linezolid, rifampin, and vancomycin (MIC = 1 per Phoenix method). 6, vancomycin-resistant Enterococcus faecalis (neck wound). Resistant to vancomycin; susceptible to ampicillin, daptomycin, and linezolid. 7, Providencia stuartii (respiratory tract). Resistant to ampicillin-sulbactam, cefazolin, ceftazidime, ceftriaxone, ciprofloxacin, gentamicin, tobramycin, and trimethoprim-sulfamethoxazole; intermediate to imipenem and piperacillin-tazobactam; susceptible to amikacin, cefepime, and meropenem. 8, Providencia rettgeri (urine). Resistant to ampicillin-sulbactam, cefazolin, ceftazidime, ceftriaxone, imipenem, and trimethoprim-sulfamethoxazole; intermediate to gentamicin; susceptible to amikacin, ciprofloxacin, levofloxacin, meropenem, piperacillin-tazobactam, and tobramycin. 9, Klebsiella pneumoniae (respiratory tract). Resistant to ampicillin-sulbactam, cefazolin, cefepime, ceftazidime, ceftriaxone, ciprofloxacin, imipenem, levofloxacin, meropenem, piperacillin-tazobactam, and tobramycin; intermediate to amikacin; susceptible to gentamicin and trimethoprim-sulfamethoxazole.
Despite continual oral vancomycin therapy for C. difficile colitis over the first 3.5 months of hospitalization, multiple episodes of relapsing C. difficile colitis occurred when vancomycin therapy was tapered. Hence, in September 2012, voluntary informed consent was obtained for a FMT. A stool donation was obtained from his sister, who underwent predonation blood and stool screening that excluded a variety of bacterial, parasitic, and viral pathogens (i.e., HIV, hepatitis A, B, and C virus, and Treponema pallidum [syphilis], plus stool examination for ova and parasites [O&P], C. difficile, and enteric pathogens). Vancomycin treatment was discontinued on the day prior to FMT, and the donor stool (480 ml) was transferred to the patient via a colonoscope. He tolerated the procedure without complication, and all signs and symptoms of C. difficile colitis resolved. After the FMT, he received no further antibiotic therapy for C. difficile colitis and all results of follow-up testing (nine C. difficile stool toxin tests over the next 2 years) were negative.
In addition to the resolution of the C. difficile infection post-FMT, a marked reduction in MDRO colonization and in episodes of sepsis, infections, and antibiotic use was noted. During the period of the 15 weeks from hospital admission to the FMT (6 June 2012 to 21 September 2012), a total of 12 MDROs were isolated from the total of 24 cultures obtained (23 for clinical and 1 for surveillance purposes). During the period from 1 week after FMT and for the next 15 weeks (28 September 2012 to 11 January 2013 [a period chosen to match the pre-FMT time range]), only 4 MDROs (from the total of 11 cultures) were noted; the lower number of cultures was due to fewer clinical indicators of infection during this period (Fig. 1A). In the pre-FMT period, there were two episodes of sepsis, four infections, and five antibiotic courses utilized. In the post-FMT period, there were no episodes of sepsis and only one infection, which was treated with a single antibiotic course (5 days for a UTI due to a newly acquired pathogen, Providencia rettgeri, not previously isolated from the patient). Due to the patient's debilitated condition, he received continuous treatment in the intensive care unit (ICU) and thus remained at risk for potential acquisition of new MDROs; despite this, the number of MDROs detected post-FMT was markedly reduced. Additionally, during the post-FMT period, there was an absence of more-virulent MDROs, including CREs, CR Pseudomonas spp., VRE, or MRSA.
The patient continued to reside in the ICU during the 2 years following FMT secondary to placement issues. During this period, he never had detected colonization or infection with a CRE, an extended-spectrum-beta-lactamase (ESBL)-producing organism, or VRE. He did become recolonized with MRSA in his urine, which occurred several months after the FMT (Fig. 1B). The patient was clinically stable throughout his 2-year stay post-FMT, with the exception of one septic episode secondary to Streptococcus pyogenes bacteremia and cellulitis treated with cefazolin. In October of 2014, over 2 years after the FMT, he developed bacteremia with Escherichia coli and Pseudomonas aeruginosa (non-MDROs). A respiratory tract culture found CRE Klebsiella pneumoniae which was deemed a colonizer and which had a susceptibility pattern slightly different from that seen with the prior CRE in 2012. Due to his debilitated state, he was made comfort care and expired.
Fecal microbiota transplantation (FMT) is a strategy which utilizes the fecal microbiota of a healthy donor to provide exogenous bacterial flora as a therapeutic agent. Since the infusion of human feces (described as a “yellow soup”) for treatment of intestinal infections reportedly 1,700 years ago (1) and its sporadic use for treatment of pseudomembranous colitis since the 1950s (2), FMT has recently become an area of intense scientific investigation for the eradication of recurrent or relapsing Clostridium difficile colitis. FMT has demonstrable efficacy in curing C. difficile infections based on case series (3, 4) and, more recently, on a randomized clinical trial (5). The current case report demonstrates not only the successful treatment of relapsing C. difficile colitis using FMT but also the concurrent resolution of MDRO colonization at multiple body sites. Since MDRO colonization may lead to subsequent infections with few antibiotic treatment options, the elimination of such carriage is of clinical interest.
The intact human microbiome is a primary host defense for the prevention of the colonization, dominance, and infection of pathogens and pathobionts (6). For example, C. difficile recurrences are associated with both the loss of intestinal microbiota biodiversity and persistence of C. difficile spores. FMT reintroduces a complex and diverse luminal microbiota that inhibits colonization by pathogenic organisms (such as C. difficile) through various mechanisms, including out-competition for nutrients and binding sites, alteration of environmental conditions (e.g., pH), and release of metabolic waste products. Additionally, genetic signalizing and host-microbiome interactions may play a role in inhibiting pathogens (6).
The mechanism by which FMT may clear MDRO colonization remains unknown but is likely similar to that ascribed to eradicating C. difficile—repopulation with normal intestinal microbiota together with subsequent out-competition of MDROs (7). Since the gastrointestinal tract serves as a reservoir for MDROs, eradication from the intestine may also result in their disappearance from other body sites. In our case, VRE, MDR Enterobacteriaceae, and CR Pseudomonas spp., which often have their primary residence in the gastrointestinal tract, were no longer observed after FMT. Prior studies have shown that the dominance of pathogenic Gram-negative bacteria and enterococcus in the gastrointestinal tract often precedes colonization at other body sites and subsequent infections, especially among critically ill patients (8, 9).
We are aware of only two other reports of FMT resulting in successful MDRO decolonization. The first case described a 14-year-old immunosuppressed girl (with hemophagocytic lymphohistiocytosis treated with chemotherapy and steroids) who persistently carried CRE Klebsiella pneumoniae in her gastrointestinal system (10). After FMT, 3 stools over the next 8 months were negative for the pathogenic organism and no further CRE infections occurred. The second case involved a 60-year-old male with end-stage renal disease with recurrent ESBL E. coli pyelonephritis and persistent stool carriage. After FMT, ESBL E. coli infection was found in the stool at 1 week but was resolved for up to 12 weeks with no further infections noted (11). Our case is unique as it demonstrates the concurrent resolution of C. difficile colitis and eradication of MDROs at nongastrointestinal body sites (e.g., urine, wound, and respiratory tract). While FMT is not currently indicated as a MDRO decolonization strategy, these cases demonstrate its potential additive benefit among patients requiring FMT for other purposes (i.e., C. difficile colitis), especially among critically ill patients at risk for MDROs.
After FMT, our patient experienced not only the resolution of MDRO colonization but also a marked reduction in episodes of sepsis, infections, and antibiotic use. Since our patient received standard clinical care and treatment, it is difficult to estimate the precise effect of FMT; however, there was a notable temporal decline in MDRO colonization and infections, and studies have shown that decolonization often prevents future infections (9). Not all MDRO infections resolved in our patient after FMT; however, their appearance typically occurred many months later and the MDROs were often “new” pathogens not previously isolated. Further, it is important to consider potential alterations in the fitness of MDROs in causing persistent colonization or infection. Although mutations that lead to antibiotic resistance may result in reduced fitness, studies have shown that additional mutations over time may compensate for these fitness costs and that MDROs can subsequently become even more virulent even in settings free of antimicrobials (12–15).
Overall, the impact of FMT on MDRO carriage and infections should be investigated among future recipients. Specifically, future randomized clinical trials investigating FMT for recurrent or relapsing C. difficile infections, or for other novel indications, should evaluate pre- and post-FMT surveillance cultures for MDROs. Further randomized studies evaluating the efficacy of FMT among patients with recalcitrant MDRO colonization and infections should also now be considered.
FMT is currently not a commonly used treatment entity; however, this may rapidly change. Efficacy data for FMT are rapidly emerging, especially in regard to C. difficile colitis (3–5). Further, FMT has been suggested for conditions that include inflammatory bowel disease, irritable bowel syndrome, and metabolic disorders, although efficacy data are currently lacking (3, 16). Advances to make FMT easier, less invasive, and more esthetically acceptable are under way. The reasons for reluctance to undergo FMT have been multifaceted, including the labor intensity of screening donors and collecting stool for donation, the associated costs for screening tests and stool installation, and the well-described “yuck” factor (17). However, the possibility of encapsulated cryopreserved, concentrated, fecally derived bacteria that may allay many of these concerns is on the horizon (18).
In summary, the results of the current case suggest that FMT may have additional benefits beyond its efficacy in treating C. difficile colitis, including eradicating MDRO colonization. Whether FMT could prevent future MDRO infections and reduce antibiotic use (an important goal to limit subsequent C. difficile infections) should be further investigated.
ACKNOWLEDGMENTS
We declare that we have no conflicts of interest related to this article.
We all contributed to the content of the manuscript and concurred with the decision to submit it for publication.
The content and views expressed in this publication are solely our responsibility.
This work is original and has not been published elsewhere.
REFERENCES
- 1.Zhang F, Luo W, Shi Y, Fan Z, Ji G. 2012. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol 107:1755. doi: 10.1038/ajg.2012.251. [DOI] [PubMed] [Google Scholar]
- 2.Eiseman B, Silen W, Bascom GS, Kauvar AJ. 1958. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 44:854–859. [PubMed] [Google Scholar]
- 3.Gough E, Shaikh H, Manges AR. 2011. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis 53:994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 4.Kassam Z, Lee CH, Yuan Y, Hunt RH. 2013. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol 108:500–508. doi: 10.1038/ajg.2013.59. [DOI] [PubMed] [Google Scholar]
- 5.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos VM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, Speelman P, Dijkgraaf MG, Keller JJ. 2013. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 6.Kamada N, Chen GY, Inohara N, Núñez G. 2013. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol 14:685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buffie CG, Pamer EG. 2013. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol 13:790–801. doi: 10.1038/nri3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taur Y, Xavier JB, Lipuma L, Ubeda C, Goldberg J, Gobourne A, Lee YJ, Dubin KA, Socci ND, Viale A, Perales MA, Jenq RR, van den Brink MR, Pamer EG. 2012. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 55:905–914. doi: 10.1093/cid/cis580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, Viale A, Socci ND, van den Brink MR, Kamboj M, Pamer EG. 2010. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest 120:4332–4341. doi: 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman A, Eppes S. 2014. Use of stool transplantation to clear fecal colonization with carbapenem-resistant Enterobacteriaceae (CRE): proof of concept, abstr 1805. Abstr Soc Healthcare Epidemiol AM (SHEA) IDWeek 2014, 11 October 2014, Philadelphia, PA. [Google Scholar]
- 11.Singh R, van Nood E, Nieuwdorp M, van Dam B, ten Berge IJ, Geerlings SE, Bemelman FJ. 2014. Donor feces infusion for eradication of extended spectrum beta-lactamase producing Escherichia coli in a patient with end stage renal disease. Clin Microbiol Infect 20:O977–O978. doi: 10.1111/1469-0691.12683. [DOI] [PubMed] [Google Scholar]
- 12.Hughes D. 2014. Selection and evolution of resistance to antimicrobial drugs. IUBMB Life 66:521–529. doi: 10.1002/iub.1278. [DOI] [PubMed] [Google Scholar]
- 13.Martinez JL, Baquero F. 2000. Mutation frequencies and antibiotic resistance. Antimicrob Agents Chemother 44:1771–1777. doi: 10.1128/AAC.44.7.1771-1777.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machuca J, Briales A, Labrador G, Díaz-de-Alba P, López-Rojas R, Docobo-Pérez F, Martínez-Martínez L, Rodríguez-Baño J, Pachón ME, Pascual A, Rodríguez-Martínez JM. 2014. Interplay between plasmid-mediated and chromosomal-mediated fluoroquinolone resistance and bacterial fitness in Escherichia coli. J Antimicrob Chemother 69:3203–3215. doi: 10.1093/jac/dku308. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan E, Bensman J, Lou M, Agnello M, Shriner K, Wong-Beringer A. 2014. Risk of developing pneumonia is enhanced by the combined traits of fluoroquinolone resistance and type III secretion virulence in respiratory isolates of Pseudomonas aeruginosa. Crit Care Med 42:48–56. doi: 10.1097/CCM.0b013e318298a86f. [DOI] [PubMed] [Google Scholar]
- 16.Singh R, Nieuwdorp MM, ten Berge IJ, Bemelman FJ, Geerlings SE. 2014. The potential beneficial role of fecal microbiota transplantation in diseases other than Clostridium difficile infection. Clin Microbiol Infect 20:1119–1125. doi: 10.1111/1469-0691.12799. [DOI] [PubMed] [Google Scholar]
- 17.Brandt LJ. 2012. Editorial commentary: fecal microbiota transplantation: patient and physician attitudes. Clin Infect Dis 55:1659–1660. doi: 10.1093/cid/cis812. [DOI] [PubMed] [Google Scholar]
- 18.Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. 2014. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 312:1772–1778. doi: 10.1001/jama.2014.13875. [DOI] [PubMed] [Google Scholar]