Abstract
Genotyping and characterization of bacterial isolates are essential steps in the identification and control of antibiotic-resistant bacterial infections. Recently, one novel genotyping method using three genomic guided Escherichia coli markers (GIG-EM), dinG, tonB, and dipeptide permease (DPP), was reported. Because GIG-EM has not been fully evaluated using clinical isolates, we assessed this typing method with 72 E. coli collection of reference (ECOR) environmental E. coli reference strains and 63 E. coli isolates of various genetic backgrounds. In this study, we designated 768 bp of dinG, 745 bp of tonB, and 655 bp of DPP target sequences for use in the typing method. Concatenations of the processed marker sequences were used to draw GIG-EM phylogenetic trees. E. coli isolates with identical sequence types as identified by the conventional multilocus sequence typing (MLST) method were localized to the same branch of the GIG-EM phylogenetic tree. Sixteen clinical E. coli isolates were utilized as test isolates without prior characterization by conventional MLST and phylogenetic grouping before GIG-EM typing. Of these, 14 clinical isolates were assigned to a branch including only isolates of a pandemic clone, E. coli B2-ST131-O25b, and these results were confirmed by conventional typing methods. Our results suggested that the GIG-EM typing method and its application to phylogenetic trees might be useful tools for the molecular characterization and determination of the genetic relationships among E. coli isolates.
INTRODUCTION
Several species of antibiotic-resistant bacteria have been found to be causative agents in frequent nosocomial infections. To understand and contain nosocomial infections, the genetic relationships between the causative bacterial isolates need to be identified. To this end, many analytical methods have been developed, including pulsed-field gel electrophoresis (PFGE) (1), ribotyping (2), arbitrarily primed PCRs, such as enterobacterial repetitive intergenic consensus-PCR (ERIC-PCR) and repetitive element sequence-based PCR (rep-PCR) (3), and several multilocus sequence typing (MLST) schemes (4–7).
PFGE and ribotyping are principally based on restriction fragment length polymorphisms (RFLP). PFGE in particular has been utilized as one of the gold standard methods, because standard protocols for certain pathogens, such as Escherichia coli serotype O157, have been established and are available online at the PulseNet website (Centers for Disease Control and Prevention, USA) (8). It is relatively simple to obtain reproducible results, i.e., PFGE banding patterns, and perform comparisons of the consequent PFGE banding patterns among test isolates. However, it may be difficult to detect intrachromosomal rearrangements and recombination. PFGE is applicable for an assessment of clonality among test isolates but may not be suitable for considerations of genetic ancestor-descendant relationships.
Both ERIC-PCR and rep-PCR target repetitive DNA sequence elements, which are sporadically located throughout the bacterial genome. These PCR methods are convenient and appropriate for testing isolates using larger sample sizes. However, the number, size, and thickness of the amplified DNA fragments tend to vary among test isolates. Unclear results from ERIC-PCR or rep-PCR may make an assessment of the genetic relationships among test isolates difficult.
Generally, the results obtained by analytical methods based on PCR can be affected by factors such as the purity and complexity of template DNA and type of thermal cycler. In contrast, MLST is a nucleotide sequence-based method and is therefore regarded as one of the most reliable analytical methods for the comparison of genetic backgrounds among bacterial isolates (9). Sequences of seven or eight well-conserved housekeeping genes, selected as genetic markers, are utilized to determine the sequence type (ST) of each isolate. MLST is able to correctly classify bacterial isolates. Because of the reliability of the method, MLST has been well utilized in the analyses of bacterial isolates causing nosocomial infections, despite the labor and cost involved. The obtained sequences are applicable to phylogenetic analysis for the determination of the genetic relationships among test isolates and for drawing phylogenetic trees using several phylogenetic methods.
With the rapid advancement of next-generation sequencing technology and its application to microbiology research, whole-genome sequence information from various bacterial species has become widely accessible. Consequently, phylogenetic analysis using whole-genome sequence information can identify genetic ancestor-descendant relationships among isolates. Sahl, Matalka, and Rasko (10) used E. coli as a model to perform phylogenetic analysis using whole-genome sequences and designated three potential genetic markers (genomic guided E. coli markers [GIG-EM]) to classify the test isolates. They reported that the classification by the phylogenetic tree with whole-genome sequences was consistent with the phylogenetic grouping of E. coli, which mainly consists of A, B1, B2, and D groups (11, 12). However, classification by the phylogenetic tree drawn with concatenated sequences obtained using current MLST schemes was not completely concordant with the phylogenetic grouping of the test isolates (10). Sahl, Matalka, and Rasko (10) also reported that the phylogenetic group classifications of the reference isolates were matched with those of the reference E. coli strains by the phylogenetic tree drawn with concatenated sequences of the three GIG-EM. However, further evaluation of the usefulness of applying the GIG-EM method to analyses of clinical E. coli isolates from patients with nosocomial infections is important. Therefore, we evaluated the GIG-EM classification method using various E. coli isolates, including clinical isolates and isolates from asymptomatic healthy individuals.
MATERIALS AND METHODS
Isolates.
A total of 63 E. coli isolates possessing blaCTX-M were examined in this study. Thirty-two KC series E. coli isolates were isolated from fecal specimens from asymptomatic healthy Thai individuals in 2008 (see Table S1 in the supplemental material) (13, 14). Fifteen JO series E. coli isolates were obtained from fecal specimens from nursing home residents in the Kinki region of Japan in 2010 (15). All the JO isolates were E. coli B2-ST131-O25b, except for strain JO120, which was E. coli B2-ST131 without the O25b gene. The 16 N isolates were randomly selected from 97 E. coli isolates producing extended-spectrum β-lactamase (ESBL), which were obtained from the Okinawa Prefectural Nambu Medical Center between June 2013 and July 2014 (Table 1). The bacterial species of the N isolates were determined by the Vitek 2 system (bioMérieux, Marcy l'Etoile, France). ESBL production of the N isolates was confirmed according to CLSI guideline M100-S23 (16) and PCR with Ex Taq (TaKaRa Bio, Inc., Ōtsu, Japan) with specific primers targeting pan-blaCTX-M (13).
TABLE 1.
Test isolates used in this study
| IDa | ST | Ph.Gb | Specimen type | Patient type | O25 gene |
|---|---|---|---|---|---|
| N0021 | 131 | B2 | Urine | Inpatient | + |
| N0055 | 131 | B2 | Urine | Outpatientc | + |
| N0057 | 131 | B2 | Urine | Inpatient | + |
| N0058 | 131 | B2 | Urine | Outpatientc | + |
| N0127 | 131 | B2 | Urine | Outpatientc | + |
| N0211 | 131 | B2 | Vaginal discharge | Inpatient | + |
| N0214 | 131 | B2 | Sputum | Outpatientc | + |
| N0222 | 131 | B2 | Urine | Outpatientc | + |
| N0223 | 131 | B2 | Urine | Outpatientc | + |
| N0226 | 405 | D | Feces | Outpatientc | NDd |
| N0269 | 131 | B2 | Urine | Inpatient | + |
| N0327 | 131 | B2 | Urine | Outpatientc | + |
| N0349 | 131 | B2 | Urine | Inpatient | + |
| N0995 | 131 | B2 | Urine | Inpatient | + |
| N1011 | 95 | B2 | Urine | Outpatient | + |
| N1032 | 131 | B2 | Urine | Outpatientc | + |
ID, identification.
Ph.G, phylogenetic group.
Outpatient with past hospitalization history.
ND, not detected.
Phylogenetic analyses.
The STs of the isolates were determined by the MLST method described by Wirth et al. (4). Phylogenetic grouping of E. coli isolates using three genetic markers, such as chuA, yjaA, and TspE4C2, and O25b PCR confirmation of the E. coli-ST131 isolates were performed according to previously described protocols (12, 17).
Genomic guided E. coli marker phylogenetic typing.
The three selected markers dinG, tonB, and DPP were amplified with PrimeSTAR Max DNA polymerase (TaKaRa Bio, Inc.) using the primers described by Sahl, Matalka, and Rasko (10). The amplified DNA fragments were purified with the QIAquick gel extraction kit (Qiagen K.K., Tokyo, Japan), according to the manufacturer's product manual. The purified DNA fragments were subjected to sequence analysis using the BigDye Terminator version 3.1 cycle sequencing kit (Life Technologies Japan, Tokyo, Japan). The obtained sequences of the three genetic markers of the 63 E. coli isolates (dinG, GenBank accession no. LC032292 to LC032354; tonB, GenBank accession no. LC032229 to LC032291; and DPP, GenBank accession no. LC032166 to LC032228) were processed and concatenated using the MEGA software version 6.06 (18). The reference sequences of dinG (GenBank accession no. JQ283606 to JQ283677), tonB (GenBank accession no. JQ283534 to JQ283605), and DPP (GenBank accession no. JQ283462 to JQ283533) of the E. coli collection of reference (ECOR) environmental E. coli reference strains (19), which were previously submitted by Sahl, Matalka, and Rasko (10), were verified using GenBank (20). The concatenated sequences were utilized to construct phylogenetic trees by the neighbor-joining method (21) using the MEGA software.
RESULTS
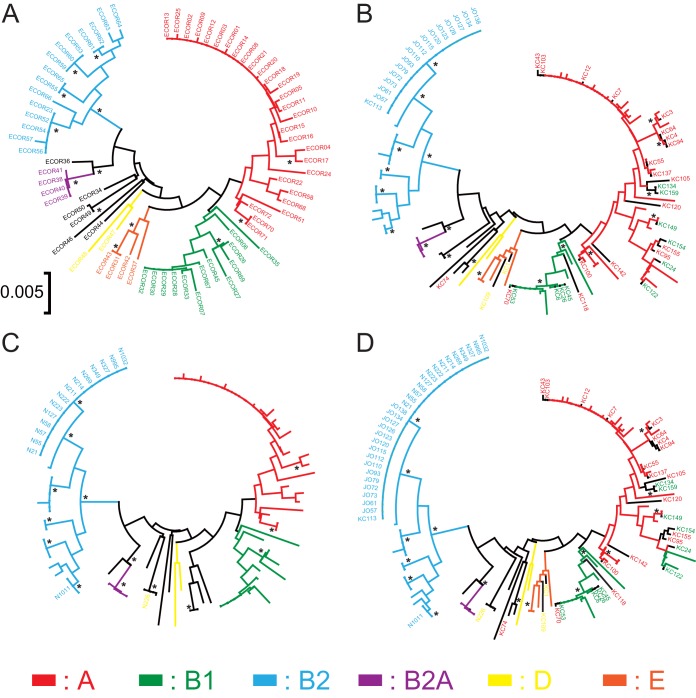
After several preliminary considerations, the 72 retrieved reference sequences of the ECOR strains were processed using the MEGA software. Consequently, 768 bp of dinG, 745 bp of tonB, and 655 bp of DPP were chosen (Table 2). Using the concatenated sequences, phylogenetic trees were drawn using the MEGA software (Fig. 1A). Bootstrap confidence levels of >95 were observed at some branches, particularly those including whole phylogenetic group B2 isolates. This demonstrated that the B2 branches in the phylogenetic trees were well supported. As shown in Fig. 1A, the reference ECOR isolates were assigned to their respective phylogenetic groups by following the original GIG-EM phylogenetic tree (10).
TABLE 2.
Length and location of the GIG-EM genetic markers
| Marker | Length (bp) | Locationa |
|---|---|---|
| dinG | 768 | 2861569–2862336 |
| tonB | 745 | 2853210–2853954 |
| DPP | 655 | 2955610–2956264 |
Regions of the GIG-EM genetic markers are indicated corresponding to the genome sequence of E. coli strain K-12 substrain MG1655 (GenBank accession no. CP009685).
FIG 1.
GIG-EM phylogenetic trees. Phylogenetic trees are drawn based on concatenations of the genetic markers dinG, tonB, and DPP of the ECOR E. coli reference strains (A), of the ECOR reference strains with KC series and JO series isolates (B), of the ECOR reference strains with N series isolates, which were utilized as test isolates (C), and of the ECOR reference strains with all E. coli isolates (D). *, branches with bootstrap confidence levels of >95.
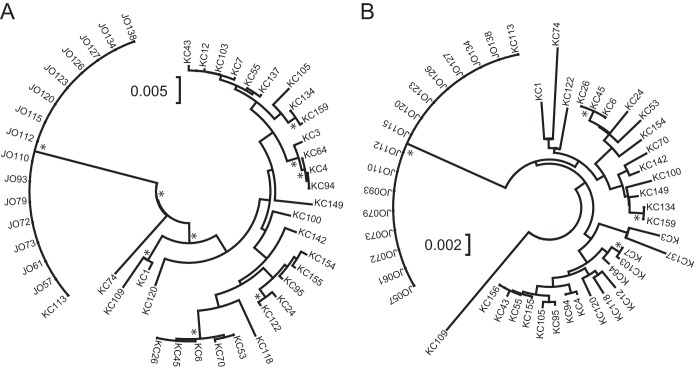
Forty-seven E. coli isolates (32 KC and 15 JO) were utilized to compare the distributions of the examined E. coli isolates on the phylogenetic trees based on sequences obtained using the GIG-EM typing method and conventional MLST (Fig. 2). The KC group consisted of isolates having 27 STs and belonging to four phylogenetic groups. In contrast, all JO isolates were identified as E. coli B2-ST131. The KC isolates were sporadically distributed, and the JO isolates localized to one branch on both the GIG-EM and MLST trees. With the exception of phylogenetic group B2 isolates, no clear relationship was evident between phylogenetic group and classification by GIG-EM and MLST.
FIG 2.
Comparison of GIG-EM and MLST phylogenetic trees. Phylogenetic trees are drawn based on concatenations of the genetic markers of the GIG-EM typing method (A) and conventional MLST typing method (B) of KC series and JO series isolates. *, branches with bootstrap confidence levels of >95.
Generally, the shapes of the phylogenetic trees and the bootstrap confidence levels of individual branches were easily altered by the number and character of input subjects. Therefore, the 47 E. coli isolates were subjected to the already established GIG-EM phylogenetic trees drawn using the ECOR reference strains (Fig. 1B). A similar trend was observed in the GIG-EM phylogenetic tree compared with the phylogenetic tree including the 72 ECOR reference strains. Isolates with the same ST, such as KC4 and KC94, were properly assigned to the same branch. However, isolates from phylogenetic groups A and B1 were not effectively separated and were localized together on the tree. Interestingly, E. coli B2-ST131 isolates were still located within a clade with a higher bootstrap confidence level.
To determine the suitability of using the GIG-EM typing method for analyzing clinical isolates, we examined 16 randomly selected N series E. coli isolates producing CTX-M-type ESBL obtained from a Japanese prefectural hospital. Except for strain N0226, 15 of the 16 E. coli isolates localized to the phylogenetic B2 branch. Among them, 14 isolates were distributed in the same clade as the E. coli B2-ST131 isolates (Fig. 1B to D). To confirm the classification obtained from the GIG-EM phylogenetic tree, the phylogenetic group and ST of each N isolate were determined. In addition, PCR was used to detect the gene encoding O25b serotypes of the N series isolates, because an association between O25b serotype and E. coli B2-ST131 strains was previously reported (22, 23). The 14 E. coli isolates were subsequently confirmed to be E. coli B2-ST131-O25b. In addition, a single group B2 isolate was identified as E. coli B2-ST95, and one non-B2 group isolate was identified as E. coli D-ST405. Taken together, our results indicate that the GIG-EM typing method consistently classified the test E. coli clinical isolates according to their STs. In particular, the discrimination of phylogenetic group B2 isolates in the GIG-EM phylogenetic tree was well supported by a high bootstrap confidence level.
DISCUSSION
E. coli isolates producing ESBL have frequently been detected in various clinical specimens, regardless of whether the infection was nosocomial or community acquired. These ESBL-producing E. coli isolates have been detected even in asymptomatic healthy individuals, especially in Asian countries (13, 14, 24). Along with epidemiological analysis to determine the factors contributing to the widespread distribution of ESBL-producing bacteria, effective phylogenetic typing methods are essential to understanding the distribution of ESBL-producing bacteria. In this study, we evaluated the currently proposed GIG-EM typing method with 63 ESBL-producing E. coli isolates of different origins and varied genetic backgrounds.
First, we considered the sequence length of each genetic marker included in the GIG-EM typing method using ECOR reference isolates as a model. The optimal sequence length was determined to be long enough to include maximum variation among the reference sequences, yet short enough to be analyzed by a single sequence reading. While a shorter sequence length of the genetic markers might be advantageous in sequencing, it may be disadvantageous in discriminatory power. In our preliminary consideration of the sequence length, shorter sequences distorted phylogenetic trees in comparison with the phylogenetic tree drawn using the whole-genome sequences reported by Sahl, Matalka, and Rasko (10).
In molecular epidemiology, the ST and phylogenetic group were important indices for indicating genetic relationships among the target bacterial isolates. As shown in Fig. 1A, the phylogenetic group classification of the ECOR reference isolates, i.e., A, B1, B2, B2A, D, and E, was relatively consistent with the topology of the GIG-EM phylogenetic tree (10). However, in our results, the phylogenetic groups of the test isolates were not always associated with locations in the GIG-EM phylogenetic tree (Fig. 1B). Because the chuA gene is absent from strains in groups B1 and A, phylogenetic grouping was based on the presence (B1) or absence (A) of an anonymous DNA region, TSPE4.C2 (17). It is possible that mutation in primer-targeting sequences of the TSPE4.C2 region could affect assignment to the correct phylogenetic group of certain E. coli isolates. This discrepancy between STs and phylogenetic groups in our test isolates was observed in both the GIG-EM and MLST trees (Fig. 2). Considering genetic distances and the number of branches with a bootstrap confidence level of >95, the classification of the isolates by the GIG-EM typing method was more accurate than that with the MLST tree. In contrast to phylogenetic groups B1 and A, the classification of phylogenetic group B2 requires triple-positive detection of chuA, yjaA, and TSPE4.C2 (17). Therefore, the assignment of an isolate to phylogenetic group B2 can be done with relative certainty. Both GIG-EM- and MLST-based phylogenetic trees effectively separated B2 isolates in accordance with their classification as phylogenetic group B2, supported by higher bootstrap confidence levels (Fig. 2).
We then evaluated the GIG-EM typing method, using clinical isolates, before performing the genetic characterization of these isolates. E. coli B2-ST131-O25b has been recognized as one of the pandemic ESBL-producing E. coli clones (25, 26). Therefore, the detection and confirmation of E. coli B2-ST131-O25b are important, especially in nosocomial infection. In addition to the ST131 strain, an E. coli D-ST405 isolate and a B2-ST95 isolate were also identified in this study. E. coli D-ST405 has frequently been reported as one of the ESBL-producing E. coli clones in many countries, particularly in Japan (27–35). Another E. coli isolate, B2-ST95, was reported as an extraintestinal pathogenic E. coli strain exhibiting high virulence, which potentially originated from poultry (36). These isolates were located separately from E. coli B2-ST131 isolates in the phylogenetic tree (Fig. 1C). In our results, all strains of E. coli isolates with the same ST, such as ST131, were classified in one branch in the GIG-EM phylogenetic tree. This meant that those isolates could be typed in the GIG-EM phylogenetic tree and confirmed with conventional phylogenetic grouping and O-antigen determination by PCR or serological methods (Table 1 and Fig. 1C and D).
The GIG-EM typing method, which utilizes only three genetic marker sequences, is a less laborious analytical method than conventional MLST schemes. Therefore, using GIG-EM to acquire information about E. coli isolates, including pandemic nosocomial clones, may facilitate the identification of STs of clinical isolates from patients with nosocomial and community-acquired infections. Taken together, our results indicate that typing and classification of E coli isolates with the GIG-EM method can be a useful tool to determine molecular and genetic relationships among E. coli isolates and could replace conventional MLST methods.
Supplementary Material
ACKNOWLEDGMENTS
We thank A. Shingaki, R. Nakama, and Y. Tohma for their technical assistance.
This work was supported in part by Technology Agency/Japan International Cooperation Agency, Science, and Technology Research Partnership for Sustainable Development (JST/JICA, SATREPS).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00227-15.
REFERENCES
- 1.Sader HS, Pfaller MA, Jones RN. 1994. Prevalence of important pathogens and the antimicrobial activity of parenteral drugs at numerous medical centers in the United States. II. Study of the intra- and interlaboratory dissemination of extended-spectrum beta-lactamase-producing Enterobacteriaceae. Diagn Microbiol Infect Dis 20:203–208. [DOI] [PubMed] [Google Scholar]
- 2.Grimont F, Grimont PA. 1986. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol 137B:165–175. doi: 10.1016/S0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- 3.Versalovic J, Koeuth T, Lupski JR. 1991. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res 19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, Karch H, Reeves PR, Maiden MC, Ochman H, Achtman M. 2006. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol 60:1136–1151. doi: 10.1111/j.1365-2958.2006.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reid SD, Herbelin CJ, Bumbaugh AC, Selander RK, Whittam TS. 2000. Parallel evolution of virulence in pathogenic Escherichia coli. Nature 406:64–67. doi: 10.1038/35017546. [DOI] [PubMed] [Google Scholar]
- 6.Escobar-Páramo P, Sabbagh A, Darlu P, Pradillon O, Vaury C, Denamur E, Lecointre G. 2004. Decreasing the effects of horizontal gene transfer on bacterial phylogeny: the Escherichia coli case study. Mol Phylogenet Evol 30:243–250. doi: 10.1016/S1055-7903(03)00181-7. [DOI] [PubMed] [Google Scholar]
- 7.Konstantinidis KT, Ramette A, Tiedje JM. 2006. Toward a more robust assessment of intraspecies diversity, using fewer genetic markers. Appl Environ Microbiol 72:7286–7293. doi: 10.1128/AEM.01398-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swaminathan B, Barrett TJ, Hunter SB, Tauxe RV, CDC PulseNet Task Force. 2001. PulseNet: the molecular subtyping network for foodborne bacterial disease surveillance, United States. Emerg Infect Dis 7:382–389. doi: 10.3201/eid0703.010303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pérez-Losada M, Cabezas P, Castro-Nallar E, Crandall KA. 2013. Pathogen typing in the genomics era: MLST and the future of molecular epidemiology. Infect Genet Evol 16:38–53. doi: 10.1016/j.meegid.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Sahl JW, Matalka MN, Rasko DA. 2012. Phylomark, a tool to identify conserved phylogenetic markers from whole-genome alignments. Appl Environ Microbiol 78:4884–4892. doi: 10.1128/AEM.00929-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herzer PJ, Inouye S, Inouye M, Whittam TS. 1990. Phylogenetic distribution of branched RNA-linked multicopy single-stranded DNA among natural isolates of Escherichia coli. J Bacteriol 172:6175–6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clermont O, Johnson JR, Menard M, Denamur E. 2007. Determination of Escherichia coli O types by allele-specific polymerase chain reaction: application to the O types involved in human septicemia. Diagn Microbiol Infect Dis 57:129–136. doi: 10.1016/j.diagmicrobio.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki T, Hirai I, Niki M, Nakamura T, Komalamisra C, Maipanich W, Kusolsuk T, Sa-Nguankiat S, Pubampen S, Yamamoto Y. 2010. High prevalence of CTX-M beta-lactamase-producing Enterobacteriaceae in stool specimens obtained from healthy individuals in Thailand. J Antimicrob Chemother 65:666–668. doi: 10.1093/jac/dkq008. [DOI] [PubMed] [Google Scholar]
- 14.Luvsansharav UO, Hirai I, Nakata A, Imura K, Yamauchi K, Niki M, Komalamisra C, Kusolsuk T, Yamamoto Y. 2012. Prevalence of and risk factors associated with faecal carriage of CTX-M β-lactamase-producing Enterobacteriaceae in rural Thai communities. J Antimicrob Chemother 67:1769–1774. doi: 10.1093/jac/dks118. [DOI] [PubMed] [Google Scholar]
- 15.Luvsansharav UO, Hirai I, Niki M, Nakata A, Yoshinaga A, Yamamoto A, Yamamoto M, Toyoshima H, Kawakami F, Matsuura N, Yamamoto Y. 2013. Fecal carriage of CTX-M beta-lactamase-producing Enterobacteriaceae in nursing homes in the Kinki region of Japan. Infect Drug Resist 6:67–70. doi: 10.2147/IDR.S43868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 23rd informational supplement. CLSI document M100-S23 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 17.Clermont O, Bonacorsi S, Bingen E. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol 66:4555–4558. doi: 10.1128/AEM.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riley MA, Gordon DM. 1992. A survey of Col plasmids in natural isolates of Escherichia coli and an investigation into the stability of Col-plasmid lineages. J Gen Microbiol 138:1345–1352. doi: 10.1099/00221287-138-7-1345. [DOI] [PubMed] [Google Scholar]
- 20.Benson DA, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. 2014. GenBank. Nucleic Acids Res 42:D32–D37. doi: 10.1093/nar/gku1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. [DOI] [PubMed] [Google Scholar]
- 22.Clermont O, Lavollay M, Vimont S, Deschamps C, Forestier C, Branger C, Denamur E, Arlet G. 2008. The CTX-M-15-producing Escherichia coli diffusing clone belongs to a highly virulent B2 phylogenetic subgroup. J Antimicrob Chemother 61:1024–1028. doi: 10.1093/jac/dkn084. [DOI] [PubMed] [Google Scholar]
- 23.Rogers BA, Sidjabat HE, Paterson DL. 2011. Escherichia coli O25b-ST131: a pandemic, multiresistant, community-associated strain. J Antimicrob Chemother 66:1–14. doi: 10.1093/jac/dkq415. [DOI] [PubMed] [Google Scholar]
- 24.Li B, Sun JY, Liu QZ, Han LZ, Huang XH, Ni YX. 2011. High prevalence of CTX-M β-lactamases in faecal Escherichia coli strains from healthy humans in Fuzhou, China. Scand J Infect Dis 43:170–174. doi: 10.3109/00365548.2010.538856. [DOI] [PubMed] [Google Scholar]
- 25.Qureshi ZA, Doi Y. 2014. Escherichia coli sequence type 131: epidemiology and challenges in treatment. Expert Rev Anti Infect Ther 12:597–609. doi: 10.1586/14787210.2014.899901. [DOI] [PubMed] [Google Scholar]
- 26.Peirano G, Pitout JD. 2010. Molecular epidemiology of Escherichia coli producing CTX-M beta-lactamases: the worldwide emergence of clone ST131 O25:H4. Int J Antimicrob Agents 35:316–321. doi: 10.1016/j.ijantimicag.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Fam N, Leflon-Guibout V, Fouad S, Aboul-Fadl L, Marcon E, Desouky D, El-Defrawy I, Abou-Aitta A, Klena J, Nicolas-Chanoine MH. 2011. CTX-M-15-producing Escherichia coli clinical isolates in Cairo (Egypt), including isolates of clonal complex ST10 and clones ST131, ST73, and ST405 in both community and hospital settings. Microb Drug Resist 17:67–73. doi: 10.1089/mdr.2010.0063. [DOI] [PubMed] [Google Scholar]
- 28.Smet A, Martel A, Persoons D, Dewulf J, Heyndrickx M, Claeys G, Lontie M, Van Meensel B, Herman L, Haesebrouck F, Butaye P. 2010. Characterization of extended-spectrum beta-lactamases produced by Escherichia coli isolated from hospitalized and nonhospitalized patients: emergence of CTX-M-15-producing strains causing urinary tract infections. Microb Drug Resist 16:129–134. doi: 10.1089/mdr.2009.0132. [DOI] [PubMed] [Google Scholar]
- 29.van der Bij AK, Peirano G, Goessens WH, van der Vorm ER, van Westreenen M, Pitout JDD. 2011. Clinical and molecular characteristics of extended-spectrum-β-lactamase-producing Escherichia coli causing bacteremia in the Rotterdam Area, Netherlands. Antimicrob Agents Chemother 55:3576–3578. doi: 10.1128/AAC.00074-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mshana SE, Imirzalioglu C, Hain T, Domann E, Lyamuya EF, Chakraborty T. 2011. Multiple ST clonal complexes, with a predominance of ST131, of Escherichia coli harbouring blaCTX-M-15 in a tertiary hospital in Tanzania. Clin Microbiol Infect 17:1279–1282. doi: 10.1111/j.1469-0691.2011.03518.x. [DOI] [PubMed] [Google Scholar]
- 31.Peirano G, Sang JH, Pitondo-Silva A, Laupland KB, Pitout JD. 2012. Molecular epidemiology of extended-spectrum-β-lactamase-producing Klebsiella pneumoniae over a 10 year period in Calgary, Canada. J Antimicrob Chemother 67:1114–1120. doi: 10.1093/jac/dks026. [DOI] [PubMed] [Google Scholar]
- 32.Park SH, Byun JH, Choi SM, Lee DG, Kim SH, Kwon JC, Park C, Choi JH, Yoo JH. 2012. Molecular epidemiology of extended-spectrum β-lactamase-producing Escherichia coli in the community and hospital in Korea: emergence of ST131 producing CTX-M-15. BMC Infect Dis 12:149. doi: 10.1186/1471-2334-12-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsumura Y, Yamamoto M, Nagao M, Hotta G, Matsushima A, Ito Y, Takakura S, Ichiyama S, Kyoto-Shiga Clinical Microbiology Study Group . 2012. Emergence and spread of B2-ST131-O25b, B2-ST131-O16 and D-ST405 clonal groups among extended-spectrum-β-lactamase-producing Escherichia coli in Japan. J Antimicrob Chemother 67:2612–2620. doi: 10.1093/jac/dks278. [DOI] [PubMed] [Google Scholar]
- 34.Brolund A, Edquist PJ, Makitalo B, Olsson-Liljequist B, Soderblom T, Wisell KT, Giske CG. 2014. Epidemiology of extended-spectrum β-lactamase-producing Escherichia coli in Sweden 2007–2011. Clin Microbiol Infect 20:O344–352. doi: 10.1111/1469-0691.12413. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Zheng B, Zhao L, Wei Z, Ji J, Li L, Xiao Y. 2014. Nationwide high prevalence of CTX-M and an increase of CTX-M-55 in Escherichia coli isolated from patients with community-onset infections in Chinese county hospitals. BMC Infect Dis 14:659. doi: 10.1186/s12879-014-0659-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mora A, Viso S, Lopez C, Alonso MP, Garcia-Garrote F, Dabhi G, Mamani R, Herrera A, Marzoa J, Blanco M, Blanco JE, Moulin-Schouleur M, Schouler C, Blanco J. 2013. Poultry as reservoir for extraintestinal pathogenic Escherichia coli O45:K1:H7-B2-ST95 in humans. Vet Microbiol 167:506–512. doi: 10.1016/j.vetmic.2013.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.