Abstract
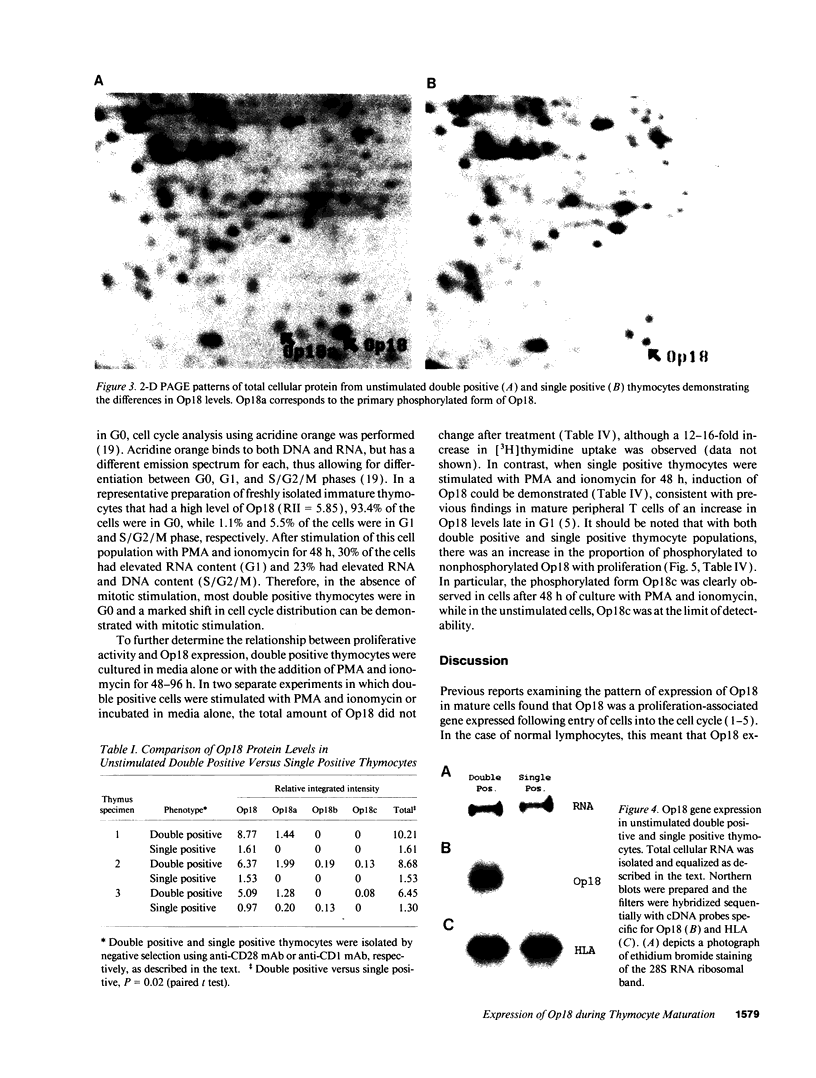
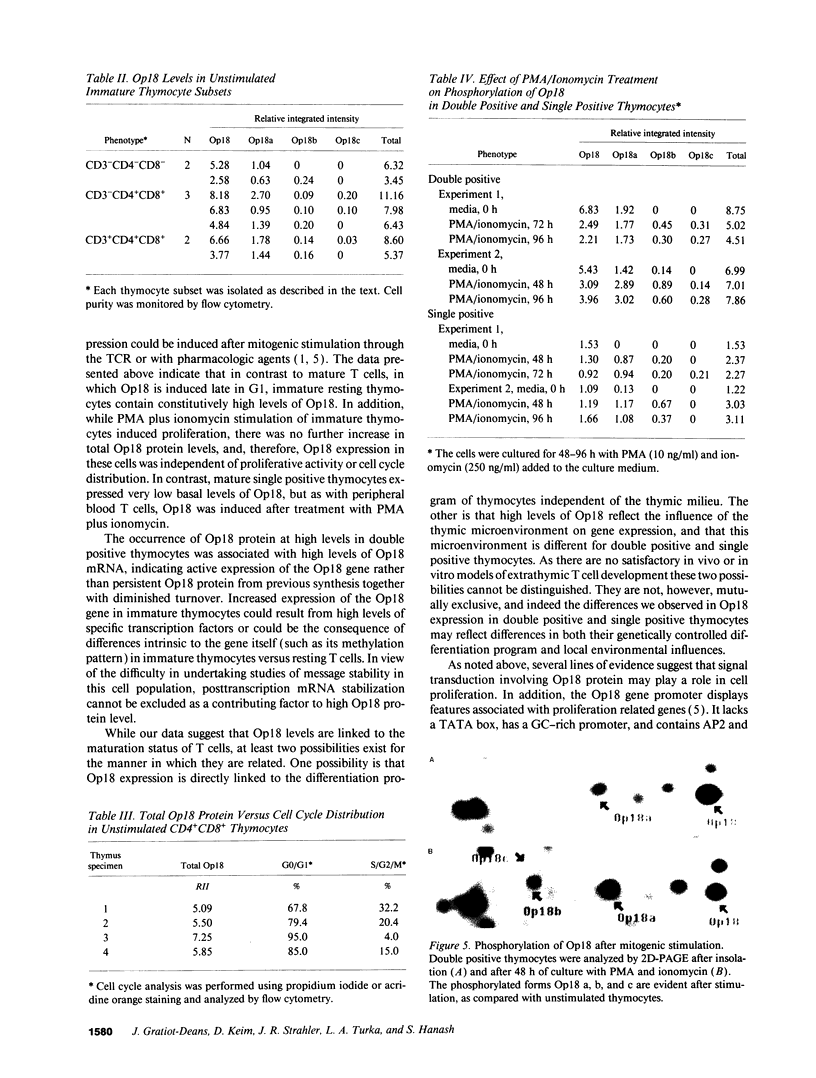
Op18 (also termed prosolin/stathmin) is a highly conserved 18-kD cytosolic phosphoprotein expressed in low levels in mature resting G0 lymphocytes, but induced in late G1 and S phases after entry into the cell cycle. In addition to its induction in normal proliferating lymphocytes, Op18 has been found to occur at high levels in acute leukemias and in neuroendocrine tissue. The presence and rapid phosphorylation of Op18 after stimulation of proliferating cells correlates with subsequent functional responses of the cells, and, therefore, Op18 has been suggested to play a key role in signal transduction. The pattern of expression of Op18 during lymphoid development is of interest in view of its high levels of expression in acute leukemias, representing cells arrested at an immature stage, thus raising the possibility that Op18 may be regulated differently in mature and immature lymphoid cells. We report here that immature human thymocytes bearing the cortical double positive phenotype (CD4+CD8+) constitutively express high levels of Op18 protein. In contrast, in mature single positive thymocytes (CD3+CD4+ or CD3+CD8+), Op18 protein is expressed at a lower level, comparable to that seen in peripheral blood T cells. Cell cycle analysis demonstrated that most of the cells in the double positive thymocyte population expressing high levels of Op18 were noncycling and arrested in G0. Furthermore, there was no correlation between Op18 levels and the proportion of cycling cells in double positive thymocyte populations isolated from different thymuses. Interestingly, although Op18 protein levels did not increase any further after mitogenic stimulation of double positive thymocytes, an increase in Op18 phosphorylation was observed, thus coupling of Op18 phosphorylation to cell activation remained intact. Our results show that during lymphoid maturation Op18 expression is uncoupled from cell proliferation. These data also suggest that the ordered expression of proliferation-associated genes seen in mature T cells may be disrupted during T cell maturation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackman M., Kappler J., Marrack P. The role of the T cell receptor in positive and negative selection of developing T cells. Science. 1990 Jun 15;248(4961):1335–1341. doi: 10.1126/science.1972592. [DOI] [PubMed] [Google Scholar]
- Cooper H. L., Fuldner R., McDuffie E., Braverman R. A specific defect of prosolin phosphorylation in T cell leukemic lymphoblasts is associated with impaired down-regulation of DNA synthesis. J Immunol. 1990 Aug 15;145(4):1205–1213. [PubMed] [Google Scholar]
- Cooper H. L., Fuldner R., McDuffie E., Braverman R. T cell receptor activation induces rapid phosphorylation of prosolin, which mediates down-regulation of DNA synthesis in proliferating peripheral lymphocytes. J Immunol. 1991 Jun 1;146(11):3689–3696. [PubMed] [Google Scholar]
- Cooper H. L., McDuffie E., Braverman R. Human peripheral lymphocyte growth regulation and response to phorbol esters is linked to synthesis and phosphorylation of the cytosolic protein, prosolin. J Immunol. 1989 Aug 1;143(3):956–963. [PubMed] [Google Scholar]
- Darzynkiewicz Z. Differential staining of DNA and RNA in intact cells and isolated cell nuclei with acridine orange. Methods Cell Biol. 1990;33:285–298. doi: 10.1016/s0091-679x(08)60532-4. [DOI] [PubMed] [Google Scholar]
- Doye V., Soubrier F., Bauw G., Boutterin M. C., Beretta L., Koppel J., Vandekerckhove J., Sobel A. A single cDNA encodes two isoforms of stathmin, a developmentally regulated neuron-enriched phosphoprotein. J Biol Chem. 1989 Jul 25;264(21):12134–12137. [PubMed] [Google Scholar]
- Furukawa Y., Piwnica-Worms H., Ernst T. J., Kanakura Y., Griffin J. D. cdc2 gene expression at the G1 to S transition in human T lymphocytes. Science. 1990 Nov 9;250(4982):805–808. doi: 10.1126/science.2237430. [DOI] [PubMed] [Google Scholar]
- Hailat N., Strahler J., Melhem R., Zhu X. X., Brodeur G., Seeger R. C., Reynolds C. P., Hanash S. N-myc gene amplification in neuroblastoma is associated with altered phosphorylation of a proliferation related polypeptide (Op18). Oncogene. 1990 Nov;5(11):1615–1618. [PubMed] [Google Scholar]
- Hanash S. M., Strahler J. R., Kuick R., Chu E. H., Nichols D. Identification of a polypeptide associated with the malignant phenotype in acute leukemia. J Biol Chem. 1988 Sep 15;263(26):12813–12815. [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Rigby P. W., Ziff E. B. Trans-acting protein factors and the regulation of eukaryotic transcription: lessons from studies on DNA tumor viruses. Genes Dev. 1988 Mar;2(3):267–281. doi: 10.1101/gad.2.3.267. [DOI] [PubMed] [Google Scholar]
- Melhem R. F., Strahler J. R., Hailat N., Zhu X. X., Hanash S. M. Involvement of OP18 in cell proliferation. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1649–1655. doi: 10.1016/0006-291x(91)91764-4. [DOI] [PubMed] [Google Scholar]
- Melhem R. F., Zhu X. X., Hailat N., Strahler J. R., Hanash S. M. Characterization of the gene for a proliferation-related phosphoprotein (oncoprotein 18) expressed in high amounts in acute leukemia. J Biol Chem. 1991 Sep 25;266(27):17747–17753. [PubMed] [Google Scholar]
- Mudryj M., Hiebert S. W., Nevins J. R. A role for the adenovirus inducible E2F transcription factor in a proliferation dependent signal transduction pathway. EMBO J. 1990 Jul;9(7):2179–2184. doi: 10.1002/j.1460-2075.1990.tb07387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J., Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell. 1989 Sep 8;58(5):833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Sobel A., Boutterin M. C., Beretta L., Chneiweiss H., Doye V., Peyro-Saint-Paul H. Intracellular substrates for extracellular signaling. Characterization of a ubiquitous, neuron-enriched phosphoprotein (stathmin). J Biol Chem. 1989 Mar 5;264(7):3765–3772. [PubMed] [Google Scholar]
- Sood A. K., Pereira D., Weissman S. M. Isolation and partial nucleotide sequence of a cDNA clone for human histocompatibility antigen HLA-B by use of an oligodeoxynucleotide primer. Proc Natl Acad Sci U S A. 1981 Jan;78(1):616–620. doi: 10.1073/pnas.78.1.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl G., Gunter K. C., Tschachler E., Yamada H., Lechler R. I., Yokoyama W. M., Steiner G., Germain R. N., Shevach E. M. Thy-1+ dendritic epidermal cells belong to the T-cell lineage. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2430–2434. doi: 10.1073/pnas.84.8.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahler J. R., Lamb B. J., Ungar D. R., Fox D. A., Hanash S. M. Cell cycle progression is associated with distinct patterns of phosphorylation of Op18. Biochem Biophys Res Commun. 1992 May 29;185(1):197–203. doi: 10.1016/s0006-291x(05)80975-1. [DOI] [PubMed] [Google Scholar]
- Strominger J. L. Developmental biology of T cell receptors. Science. 1989 May 26;244(4907):943–950. doi: 10.1126/science.2658058. [DOI] [PubMed] [Google Scholar]
- Turka L. A., Ledbetter J. A., Lee K., June C. H., Thompson C. B. CD28 is an inducible T cell surface antigen that transduces a proliferative signal in CD3+ mature thymocytes. J Immunol. 1990 Mar 1;144(5):1646–1653. [PubMed] [Google Scholar]
- Turka L. A., Linsley P. S., Paine R., 3rd, Schieven G. L., Thompson G. B., Ledbetter J. A. Signal transduction via CD4, CD8, and CD28 in mature and immature thymocytes. Implications for thymic selection. J Immunol. 1991 Mar 1;146(5):1428–1436. [PubMed] [Google Scholar]