Abstract
We evaluated quantitative real-time PCR to establish the diagnosis of rotavirus gastroenteritis in a high-disease-burden population in Malawi using enzyme immunoassay as the gold standard diagnostic test. In 146 children with acute gastroenteritis and 65 asymptomatic children, we defined a cutoff point in the threshold cycle value (26.7) that predicts rotavirus-attributable gastroenteritis in this population. These data will inform the evaluation of direct and indirect rotavirus vaccine effects in Africa.
TEXT
Rotavirus gastroenteritis is a major cause of infant morbidity and mortality with 90% of rotavirus-associated deaths occurring in low-income settings (1–3). Two live, oral vaccines (Rotarix [GlaxoSmithKline Biologicals, Belgium] and RotaTeq [Merck & Co. Inc., USA]) are entering childhood immunization programs worldwide. These have greatly reduced rotavirus-attributable hospitalizations in high-income and middle-income countries (4, 5), and a recently published study from Malawi was the first report of vaccine effectiveness from a low-income country (6).
Laboratory diagnosis of rotavirus gastroenteritis is typically established by detection of rotavirus antigen in stool using enzyme-immunoassay (EIA). This correlates well with clinical disease in developed settings but is less sensitive than real-time reverse transcription-quantitative PCR (qRT-PCR) (7, 8). However, as qRT-PCR can detect rotavirus shedding in the stool in up to 30% of asymptomatic young children in developed settings, the clinical implications of a positive result is uncertain (9, 10). A positive relationship between fecal viral load and EIA-positive rotavirus disease has been demonstrated in children from the United Kingdom and the United States (7, 11) and has been used to define a quantitative cycle threshold (CT) cutoff point in qRT-PCR corresponding to clinical disease (7, 11).
Much of our understanding of the performance of rotavirus diagnostic tests comes from well-resourced settings (11–13). However, rotavirus disease patterns in the poorest settings differ from those in the developed world, with higher force of infection, earlier disease onset, and delayed acquisition of immunity, which may impact diagnosis (14, 15). This study in Blantyre, southern Malawi, aimed to evaluate the performance of qRT-PCR and EIA in order to define a cutoff point in the qRT-PCR CT value to define clinical disease in a high-burden, resource-poor African country and was undertaken as part of an assessment of monovalent rotavirus vaccine effectiveness following its introduction into Malawi's national immunization program in October 2012 (6). Children <5 years of age were recruited on presentation to Queen Elizabeth Central Hospital (QECH) with acute diarrhea (the passage of three or more loose stools within a 24-h time period within 14 days of presentation). Age- and neighborhood-matched community controls were enrolled in Blantyre District (6). A single stool sample was collected from each child and tested for rotavirus antigen by EIA (Premier Rotaclone; Meridian Biosciences, Cincinnati, OH) without knowledge of clinical status.
Following nucleic acid extraction from 10% to 20% stool suspensions, cDNA was synthesized using random primers. VP6 qRT-PCR was performed using VP6 gene-specific primers and probes as previously described, using 2× TaqMan Universal Master Mix II (Invitrogen, Paisley, United Kingdom), and a Rotor-Gene Q 5-plex system (Qiagen, Manchester, United Kingdom) (13, 16). A rotavirus qRT-PCR result was deemed positive if the CT value was <40 and negative when the CT value was ≥40.
For amounts of cDNA, >30 copies intra-assay reproducibility was high (Table 1). Samples positive by qRT-PCR that had a CT value below 11 (n = 2) could not be quantified.
TABLE 1.
VP6 qPCR inter-assay reproducibility in eight separate runs
| Standard curve input copy no. | CT value |
Calculated copy no. |
|||
|---|---|---|---|---|---|
| Mean | Minimum | Maximum | Mean | %CVa | |
| 3,000,000 | 15.08 | 14.86 | 16.35 | 2,929,261 | 0.13 |
| 300,000 | 18.91 | 18.14 | 19.25 | 335,457 | 0.12 |
| 30,000 | 23.34 | 22.29 | 24.99 | 27,236 | 0.14 |
| 3,000 | 26.85 | 25.90 | 27.73 | 3,320 | 0.20 |
| 300 | 31.47 | 30.51 | 32.88 | 306 | 0.20 |
| 30 | 34.94 | 33.45 | 35.98 | 30 | 0.15 |
| 3 | 38.23 | 37.27 | >40 (neg) | 4b | 0.25b |
CV, coefficient of variation.
Calculated only for assays in which the result was positive for this sample (<40).
Frequency of detection of rotavirus using EIA and qRT-PCR was described and tested for discordance using McNemar's test. Median CT values were compared between EIA-positive (diarrhea), EIA-negative (diarrhea), and asymptomatic samples using Wilcoxon rank sum tests. Receiver operating characteristics (ROC) analysis was used to evaluate the ability of qRT-PCR CT values to discriminate between the presence and absence of symptomatic gastroenteritis, using EIA as a gold standard (17, 18) and defining the absence of disease as EIA negative without diarrhea. A cutoff point that optimized sensitivity and specificity was identified using Youden's index [(sensitivity + specificity) − 1] (19, 20). Analysis was conducted using Stata 12.1 (StataCorp, USA) and GraphPad Prism 6 (GraphPad Software, Inc., USA). Ethical approval was provided by the National Health Sciences Research Committee, Lilongwe, Malawi (867) and by the Research Ethics Committee of the University of Liverpool, Liverpool, United Kingdom (000490).
A total of 238 fecal samples were collected. Of these, clinical data were available for 225. Fourteen community samples were excluded for intercurrent diarrhea, resulting in 211 samples available for analysis: 77 EIA-positive diarrheal cases, 69-EIA negative diarrheal cases, and 65 asymptomatic children. Of the 211 samples with corresponding clinical data, 77 of the 77 (100%) children with EIA-positive diarrhea were also qRT-PCR positive and 34 (49.3%) of 69 children with EIA-negative diarrhea were qRT-PCR positive. Of the 65 asymptomatic community controls, 1 (1.5%) was EIA positive and 20/65 (30.8%) had virus detected using qRT-PCR.
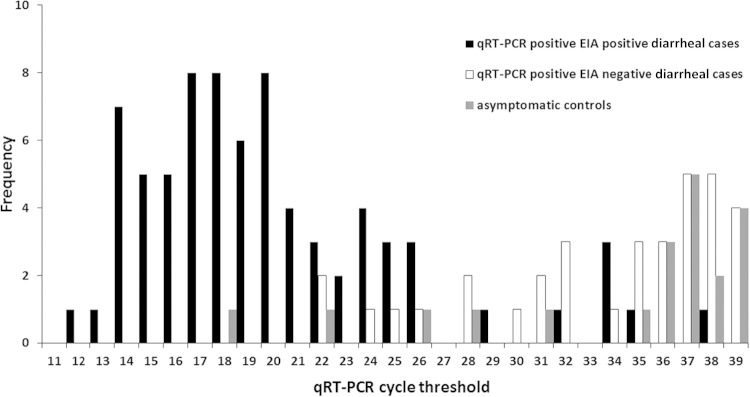
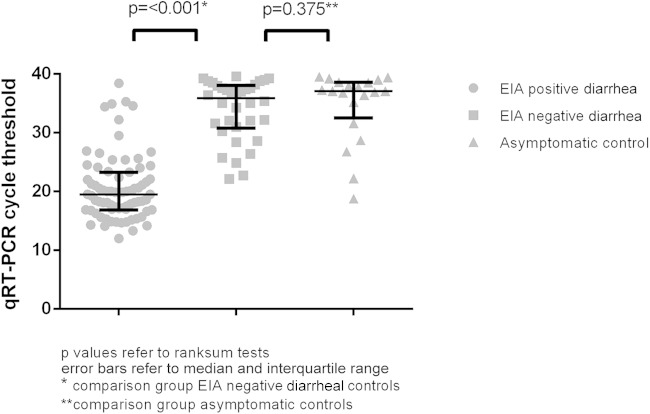
There was a significant difference in median qRT-PCR CT values between EIA-negative diarrheal cases (median, 35.9; interquartile range [IQR], 31.0 to 38.0) and those of EIA-positive diarrheal cases (median, 19.5; IQR, 16.9 to 23.3; P = <0.001), but there was no significant difference in CT values between EIA-negative diarrheal cases and those of asymptomatic community controls (median, 37.1; IQR, 33.4 to 38.6; P = 0.375) (Fig. 1 and 2). Quantitative real-time PCR CT values discriminated well between the presence and absence of rotavirus gastroenteritis as defined using EIA, with an area under the curve of 0.96 (95% confidence interval [CI], 0.93 to 1.00). The point that maximized the Youden index was at a CT value of 26.7, at which point sensitivity and specificity were 89% and 95%, respectively (corresponding with an average cDNA copy number per reaction of 3,000 [95% CI, 2,657 to 3,514]; Table 1).
FIG 1.
Distribution of qRT-PCR CT values between diarrheal cases and asymptomatic controls.
FIG 2.
qRT-PCR CT values according to EIA result and clinical category.
We have demonstrated differences in CT values between rotavirus EIA-positive and EIA-negative diarrheal children in a high-burden, low-resource setting and defined an optimal CT cutoff point, which may be utilized to diagnose clinical disease as molecular diagnostics are increasingly rolled out in this region. We found that, consistent with data from the United Kingdom and the United States, a positive rotavirus EIA result was strongly associated with the presence of diarrhea, with only one asymptomatic, EIA-positive child identified (7, 11). However, qRT-PCR was more sensitive at detecting rotavirus in stool than EIA (12), with a high frequency (31%) of low-level viral shedding detected by qRT-PCR in samples from the asymptomatic group. This confirms that detection of rotavirus using qRT-PCR without consideration of viral load is insufficient to attribute disease causation (11). The prevalence of viral shedding in the asymptomatic group is high (31%) but is consistent with that found in young children in the United Kingdom (9).
There were significant differences in the rotavirus CT values between diarrheal children who were EIA positive and those who were EIA negative, with substantially higher CT values (lower viral load) in children who were EIA negative, and no significant difference in qRT-PCR CT values between EIA-negative diarrheal children and those of asymptomatic children. ROC analysis confirmed the discriminatory value of qRT-PCR CT values. The cutoff point in our study is similar to that defined in a United Kingdom population using the same assay (11), suggesting that any difference in frequency of infection or in disease severity between the two settings does not impact distribution of viral loads and that EIA and qRT-PCR perform similarly in the detection of rotavirus gastroenteritis in each environment.
Children were recruited from a referral hospital, and a large number had severe disease. However, the cutoff we identified correlates well with that identified using a cohort of children recruited from primary care in the United Kingdom, in whom less severe disease should be better represented (11). Moreover, since reaction sensitivities may vary between PCR assays conducted in different laboratories, we have provided cDNA copy numbers corresponding to the qRT-PCR CT values to facilitate comparison.
Assessment of rotavirus vaccine effectiveness requires accurate laboratory diagnostics. In high-burden, low-income settings where direct vaccine effectiveness is lower (6), demonstrating indirect (herd) benefits becomes more important. Should rotavirus vaccine reduce asymptomatic shedding, its impact on community rotavirus transmission may be substantial. Future evaluation of vaccine indirect effects will require measurement of impact on asymptomatic shedding and community transmission using the cutoffs we have defined in this paper. Our findings, therefore, inform the evaluation of direct and indirect rotavirus vaccine effects in Africa.
ACKNOWLEDGMENTS
This work was supported by a Wellcome Trust program grant (no. 091909/Z/10/Z) and the Malawi-Liverpool Wellcome Trust clinical research program core award. A.B. was supported by a Wellcome Trust clinical Ph.D. fellowship (grant no. 102466/Z/13/A).
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
N.B.-Z. and N.F. have received research grant support from GlaxoSmithKline Biologicals for work on rotavirus vaccines. M.I.-G. has received research grant support from GlaxoSmithKline Biologicals and Sanofi Pasteur MSD for work on rotavirus. O.N. has received research grant support and honoraria from Japan Vaccine and Merck Sharp & Dohme for lectures on rotavirus vaccines. N.A.C. has received research grant support and honoraria for participation in rotavirus vaccine advisory board meetings from GlaxoSmithKline Biologicals. The other authors declare no conflicts of interest.
REFERENCES
- 1.Parashar UD, Nelson EA, Kang G. 2013. Diagnosis, management, and prevention of rotavirus gastroenteritis in children. BMJ 347:f7204. doi: 10.1136/bmj.f7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE. 2013. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One 8:e72788. doi: 10.1371/journal.pone.0072788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2012. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 12:136–141. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- 4.Patel MM, Patzi M, Pastor D, Nina A, Roca Y, Alvarez L, Iniguez V, Rivera R, Tam KI, Quaye O, Bowen M, Parashar U, De Oliveira LH. 2013. Effectiveness of monovalent rotavirus vaccine in Bolivia: case-control study. BMJ 346:f3726. doi: 10.1136/bmj.f3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tate JE, Haynes A, Payne DC, Cortese MM, Lopman BA, Patel MM, Parashar UD. 2013. Trends in national rotavirus activity before and after introduction of rotavirus vaccine into the national immunization program in the United States, 2000 to 2012. Pediatr Infect Dis J 32:741–744. doi: 10.1097/INF.0b013e31828d639c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bar-Zeev N, Kapanda L, Tate JE, Jere KC, Iturriza-Gomara M, Nakagomi O, Mwansambo C, Costello A, Parashar UD, Heyderman RS, French N, Cunliffe NA. 2015. Effectiveness of a monovalent rotavirus vaccine in infants in Malawi after programmatic roll-out: an observational and case-control study. Lancet Infect Dis 15:422–428. doi: 10.1016/S1473-3099(14)71060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tate JE, Mijatovic-Rustempasic S, Tam KI, Lyde FC, Payne DC, Szilagyi P, Edwards K, Staat MA, Weinberg GA, Hall CB, Chappell J, McNeal M, Gentsch JR, Bowen MD, Parashar UD. 2013. Comparison of 2 assays for diagnosing rotavirus and evaluating vaccine effectiveness in children with gastroenteritis. Emerg Infect Dis 19:1245–1252. doi: 10.3201/eid1908.130461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amar CF, East CL, Gray J, Iturriza-Gomara M, Maclure EA, McLauchlin J. 2007. Detection by PCR of eight groups of enteric pathogens in 4,627 faecal samples: re-examination of the English case-control Infectious Intestinal Disease Study (1993-1996). Eur J Clin Microbiol Infect Dis 26:311–323. doi: 10.1007/s10096-007-0290-8. [DOI] [PubMed] [Google Scholar]
- 9.Phillips G, Lopman B, Rodrigues LC, Tam CC. 2010. Asymptomatic rotavirus infections in England: prevalence, characteristics, and risk factors. Am J Epidemiol 171:1023–1030. doi: 10.1093/aje/kwq050. [DOI] [PubMed] [Google Scholar]
- 10.Corcoran MS, van Well GT, van Loo IH. 2014. Diagnosis of viral gastroenteritis in children: interpretation of real-time PCR results and relation to clinical symptoms. Eur J Clin Microbiol Infect Dis 33:1663–1673. doi: 10.1007/s10096-014-2135-6. [DOI] [PubMed] [Google Scholar]
- 11.Phillips G, Lopman B, Tam CC, Iturriza-Gomara M, Brown D, Gray J. 2009. Diagnosing rotavirus A associated IID: using ELISA to identify a cut-off for real time RT-PCR. J Clin Virol 44:242–245. doi: 10.1016/j.jcv.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Wilde J, Yolken R, Willoughby R, Eiden J. 1991. Improved detection of rotavirus shedding by polymerase chain reaction. Lancet 337:323–326. doi: 10.1016/0140-6736(91)90945-L. [DOI] [PubMed] [Google Scholar]
- 13.Iturriza-Gomara M, Elliot AJ, Dockery C, Fleming DM, Gray JJ. 2009. Structured surveillance of infectious intestinal disease in pre-school children in the community: ‘The Nappy Study'. Epidemiol Infect 137:922–931. doi: 10.1017/S0950268808001556. [DOI] [PubMed] [Google Scholar]
- 14.Gladstone BP, Ramani S, Mukhopadhya I, Muliyil J, Sarkar R, Rehman AM, Jaffar S, Gomara MI, Gray JJ, Brown DW, Desselberger U, Crawford SE, John J, Babji S, Estes MK, Kang G. 2011. Protective effect of natural rotavirus infection in an Indian birth cohort. N Engl J Med 365:337–346. doi: 10.1056/NEJMoa1006261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunliffe NA, Ngwira BM, Dove W, Thindwa BD, Turner AM, Broadhead RL, Molyneux ME, Hart CA. 2010. Epidemiology of rotavirus infection in children in Blantyre, Malawi, 1997-2007. J Infect Dis 202(Suppl):S168–S174. doi: 10.1086/653577. [DOI] [PubMed] [Google Scholar]
- 16.Eurorota.net: European Rotavirus network. 2011. Rotavirus detection and typing. Nucleic acid extraction and reverse transcription virus detection by PCR. Rotavirus VP7, VP4, VP6 and NSP4 genotyping. http://www.eurorota.net/docs.php Accessed 15 February 2015.
- 17.Altman DG, Bland JM. 1994. Diagnostic tests 3: receiver operating characteristic plots. BMJ 309:188. doi: 10.1136/bmj.309.6948.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zweig MH, Campbell G. 1993. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 39:561–577. [PubMed] [Google Scholar]
- 19.Youden WJ. 1950. Index for rating diagnostic tests. Cancer 3:32–35. doi:. [DOI] [PubMed] [Google Scholar]
- 20.Fluss R, Faraggi D, Reiser B. 2005. Estimation of the Youden index and its associated cutoff point. Biom J 47:458–472. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]