Abstract
The performances of the AmpliVue, BD Max, and illumigene group B Streptococcus (GBS) nucleic acid amplification tests (NAATs) were compared to that of enriched culture for detection of GBS in antenatal screening specimens. Two hundred specimens were tested simultaneously with the NAATs, following 18 to 24 h of Lim broth enrichment; 15% of specimens were culture positive for GBS, whereas 31.5% were positive by at least one NAAT. All three NAATs were more sensitive (sensitivity, 90.9 to 100%) than culture (sensitivity, 53.6%).
TEXT
Early-onset group B Streptococcus (GBS) infection remains a leading cause of morbidity and death among newborns in the United States, despite nationwide adherence to preventive measures endorsed by the Centers for Disease Control and Prevention (CDC) in their guidelines for the prevention of GBS disease (1–4). These measures include universal culture-based screening for GBS colonization in all pregnant women between 35 and 37 weeks of gestation and intrapartum administration of antimicrobials to colonized patients, patients with GBS bacteruria in any trimester of current pregnancy, and patients with one of the defined intrapartum risk factors for whom the GBS status is unknown at the onset of labor (1). A critical component of the CDC strategy to prevent early-onset GBS disease is the identification of GBS colonization, which to date has relied on recovery of the organism from appropriately collected vaginal and rectal swabs (1). However, a national cohort study found that screening culture results were negative for 81% of mothers of babies who developed early-onset neonatal GBS disease. These data suggest that there may be missed opportunities for intrapartum antimicrobial prophylaxis, due to changes in colonization status at the time of birth, improper specimen collection techniques or timing, or suboptimal sensitivity of GBS cultures (5). The CDC guidelines include suggestions for improving the sensitivity of cultures, such as utilizing selective pigmented broths, DNA probes, or nucleic acid amplification tests (NAATs), but these remain optional (1).
In recent years, a number of Food and Drug Administration (FDA)-cleared NAATs for GBS detection in antenatal screening specimens have entered the market. Most of the currently available NAATs specify that testing is to be performed on 18- to 24-h enrichment broth cultures, as recommended by the CDC (1). An advantage of such tests is that results are available 1 day earlier than with traditional cultures. In addition, several studies have shown improved GBS detection rates using NAATs, compared to cultures (6–9). However, a limitation of NAATs is that an isolate may not be recoverable for antimicrobial susceptibility testing, which is required to guide the treatment of penicillin-allergic women at high risk for anaphylaxis (1).
This study evaluated the performance of three molecular GBS assays, namely, the AmpliVue GBS assay (Quidel Corp., San Diego, CA), the BD Max GBS assay (BD Diagnostics, Quebec, Canada), and the illumigene group B Streptococcus assay (Meridian Bioscience, Cincinnati, OH), in comparison with Lim-broth-enriched culture as the gold standard. The AmpliVue GBS assay uses helicase-dependent amplification technology for isothermal amplification of a conserved thiolase gene (atoB) and a proprietary detection cassette that allows for visualization of assay results (10). The BD Max system automates and integrates DNA extraction and concentration, reagent preparation, and nucleic acid amplification and detection of a region of the group B streptococcal CAMP factor gene (cfb) using real-time PCR (11). The illumigene GBS assay uses loop-mediated isothermal DNA amplification (LAMP) technology to target a highly conserved 213-bp sequence of the Streptococcus agalactiae genome (not specified by the manufacturer) (12).
Two hundred vaginal/rectal swabs (collected from 200 pregnant women) that were submitted to the laboratory for GBS culture between November 2013 and February 2014 were processed following CDC recommendations prior to inclusion in this study (1). Briefly, single swabs were removed from the nonnutritive liquid transport medium (ESwab system with liquid Amies transport medium; Copan Diagnostics, Murrieta, CA) and, with the use of a transfer pipette, 3 drops of the liquid Amies medium (approximately 150 μl) were inoculated into each medium, including BBL Trypticase soy agar with 5% sheep blood (BD Diagnostics, Sparks, MD), BBL Columbia colistin-nalidixic acid (CNA) agar with 5% sheep blood (BD), and BBL Lim broth (Todd-Hewitt broth with 10 μg/ml colistin and 15 μg/ml nalidixic acid; BD). Inoculated media were incubated at 37°C in 5% CO2 for 18 to 24 h, after which the enriched Lim broth was subcultured onto another plate of Trypticase soy agar with 5% sheep blood and incubated an additional 48 h prior to a negative result being signed out. Colonies suspected to be GBS (either beta-hemolytic or nonhemolytic) on either primary or subculture plates were confirmed by PathoDx latex (Remel, Lenexa, KS) agglutination. After subculture, each remnant Lim broth was assigned a unique study number and deidentified. Study numbers and culture results were recorded. Specimens were tested immediately or stored at 4°C for up to 7 days, according to the manufacturer's stability specifications. This study was approved by the University of California, Los Angeles, Institutional Review Board.
All NAATs were performed and interpreted according to the manufacturer's specifications. At the time of this study, only BD Max and illumigene assays were FDA approved; however, AmpliVue has since received FDA clearance. Fifty microliters of enriched Lim broth was tested with the AmpliVue and illumigene platforms, and 15 μl was tested with the BD Max platform. External controls were included daily when NAATs were performed. Each of the assays had an internal positive control (IPC) to account for amplification inhibition, and specimens for which the IPC result was negative underwent repeat testing; if the IPC tested negative a second time, then the results were considered invalid. Specimens were considered truly GBS positive if (i) GBS was recovered from the culture or (ii) at least two NAAT results were positive for a culture-negative specimen. To further evaluate specimens for which only one or two of the NAAT results were positive, the Lim broths were retested with all three NAATs simultaneously. To assess whether the performances of these tests were significantly different, a McNemar's chi-square test was performed in Excel.
Of 200 specimens tested, 30 (15%) were culture positive. Initial NAATs resulted in 60 positive results (30%) by AmpliVue, 62 (31%) by BD Max, and 53 (26.6%) by illumigene (Table 1). All culture-positive specimens were positive with the three NAATs, with the exception of one specimen that was repeatedly invalid by the illumigene method; that specimen was removed from the performance calculations for the illumigene assay, as a new specimen likely would have been requested for testing in real practice (Table 1). A second invalid result was obtained with the illumigene assay but the result was negative upon repeat testing, in accordance with the results of both culture and the other two NAATs (data not shown). No invalid results were obtained with the AmpliVue or BD Max assays.
TABLE 1.
Performance of NAATs versus culture results
| Assay and result | No. |
||
|---|---|---|---|
| Positive culture result | Negative culture result | Total | |
| AmpliVue | |||
| Positive | 30 | 30 | 60 |
| Negative | 0 | 140 | 140 |
| BD Max | |||
| Positive | 30 | 32 | 62 |
| Negative | 0 | 138 | 138 |
| illumigene | |||
| Positive | 29a | 24 | 53 |
| Negative | 0 | 146 | 146 |
| Total | 30 | 170 | 200 |
One result remained invalid in repeat testing.
Nineteen culture-negative specimens were positive by all three NAATs and were considered true-positive specimens in this analysis. Twenty-two specimens (11%) yielded discordant results with the three NAATs (Table 2). Using a definition of a true-positive specimen as one with at least 2 of the 3 initial NAAT results being positive, an additional 7 specimens were defined as positive, yielding 56 positive specimens overall and 144 negative specimens. Sensitivity was calculated for the illumigene assay as 90.9% (95% confidence interval [CI], 79.3 to 96.6%), for the BD Max GBS assay as 100% (95% CI, 91.9 to 100%), and for the AmpliVue assay as 96.4% (95% CI, 86.6 to 99.3%); specificities were 97.9% (95% CI, 93.6 to 99.5%), 95.8% (95% CI, 90.8 to 98.3%), and 95.8% (95% CI, 90.8 to 98.3%), respectively (Table 3). No significant difference in sensitivity or specificity between the three NAATs was found (McNemar's chi-square test, P > 0.5). In contrast, the NAATs were significantly more sensitive than the culture method (P < 0.0001). Agreement between the 3 NAATs in initial testing was as follows: 93.0% concordance between AmpliVue and BD Max (κ = 0.83 [95% CI, 0.76 to 0.90]), 92.0% concordance between AmpliVue and illumigene (κ = 0.80 [95% CI, 0.73 to 0.88]), and 93.0% concordance between illumigene and BD Max (κ = 0.83 [95% CI, 0.75 to 0.90]).
TABLE 2.
Discordant NAAT results
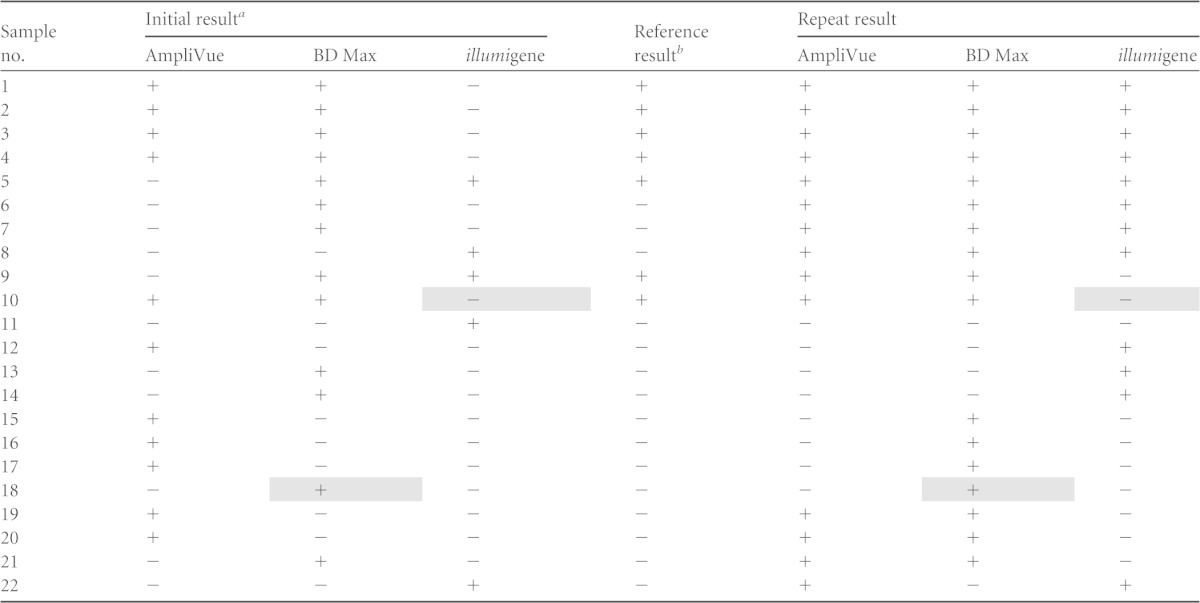
Shading indicates results that were consistently discordant with the other 2 NAAT methods.
The reference results are concordant results from 2 of 3 NAATs in initial testing.
TABLE 3.
Performance of resolved test results for three NAATs in detecting GBS in antenatal specimens
| Assay | Sensitivity (95% CI) (%) | Specificity (95% CI) (%) |
|---|---|---|
| AmpliVue | 96.4 (86.6–99.3) | 95.8 (90.8–98.3) |
| BD Max | 100 (91.9–100) | 95.8 (90.8–98.3) |
| illumigene | 90.9 (79.3–96.6) | 97.9 (93.6–99.5) |
Repeat testing with all three NAATs in parallel was performed for the 22 specimens with discordant NAAT results (Table 2). Of note, this repeat testing yielded a different result than that obtained in the first round of testing for several specimens (Table 2). For example, specimen 9, which was considered a true-positive specimen because 2 of the 3 initial NAAT results were positive, had an initial negative result by AmpliVue but was positive in repeat testing. In contrast, this specimen had an initial positive result by illumigene but was negative in repeat testing (Table 2). Ten (45.4%) of the 22 specimens had both a positive result and a negative result by two of the three NAATs, all of which had positive amplification curves that came up after cycle 35 on the BD Max system (data not shown). Such results suggest GBS loads near the limit of detection (LOD) for these assays (AmpliVue, 1.39 × 106 CFU/ml; BD Max, 2 × 104 CFU/ml; illumigene, 2.56 × 104 CFU/ml). It should be noted that discordant AmpliVue results could be due to the extended storage of enriched Lim broths. The study protocol provided at the time of testing did not specify storage requirements; however, the current package insert for the FDA-cleared AmpliVue assay specifies up to 25 h at 2 to 8°C. When testing could not be conducted immediately, enriched Lim broths were stored according to the specifications for the BD Max and illumigene assays (up to 7 days at 2 to 8°C). While sensitivity and specificity varied among the assays, it should be noted that only one specimen was repeatedly negative by the illumigene assay but positive by the other two NAATs (specimen 10) and one specimen was repeatedly positive by the BD Max GBS assay and negative by the other two NAATs (specimen 18) (Table 2), suggesting that platform performances were not appreciably different. However, the small sample size (n = 200) precluded detailed assessment of differences between the assays and any statistical significance. As only the specimens with discordant results were retested, significant bias would be introduced into the data set if an attempt was made to calculate performance characteristics using the data from the second round of testing, and such an analysis was not performed. Lim-broth-enriched culture was the least sensitive method for the detection of GBS, with a sensitivity of 53.6%. While by definition culture showed high specificity (100%) and a high positive predictive value (PPV) (100%), the negative predictive value (NPV) was low (17.5%), suggesting that this method is not ideal for a screening test. Some reports indicate that culture recovery of beta-hemolytic GBS can be improved through the use of a pigmented broth, but identification rates are still significantly lower than with NAAT-based methods (13). In addition, while our study attempted to evaluate all cultures for the presence of nonhemolytic GBS, interpretation can be subjective and recovery of these organisms can be difficult, especially when Lim broth subcultures yield mixed cultures.
GBS colonization rates for women are reported to range between 10 and 30%, based on culture results (14, 15). Studies evaluating NAAT performance for GBS detection have demonstrated increased GBS positivity rates, which vary by the type of NAAT, the targeted gene, and the study population (7–9, 16).
To our knowledge, this is the first study that provides a direct comparison of the performances of AmpliVue, BD Max, and illumigene molecular platforms with culture results. In the present study, the positivity rate increased from 15% with culture to 30% with AmpliVue, 31% with BD Max, and 27% with illumigene. Our findings of increases in GBS positivity rates are consistent with other studies (9, 16). Similar to our results, Davies et al. reported the sensitivity of antenatal cultures to be 54%, improving to 94% with the use of a molecular assay (IDI-Strep B) (16). The performance of the BD Max assay in this study was comparable to observations by other investigators, who showed 95 to 100% sensitivity (6, 8, 17). The sensitivity of the illumigene assay in our study (90.9%) was lower than that reported by Couturier et al. (6) (98.5%); however, the differences between the NAATs did not achieve statistical significance (P > 0.5).
One limitation in this study is the lack of an alternative, and perhaps more sensitive, testing method for the 22 specimens with discrepant results. For example, a NAAT method with an initial extraction step, such as the Roche analyte-specific reagent (ASR) LightCycler assay used by Goodrich and Miller (7), has a lower LOD and might have helped resolve some or all of the discrepant results in this study. For specimens for which both a positive result and a negative result were obtained with a platform during initial and repeat testing, it is suspected that GBS DNA from viable or nonviable organisms is at the limit of detection for those assays. Because the concentrations of the targets were so low in those samples, as implied by the late amplification curves in the BD Max assay, attempts to use an alternative PCR followed by bidirectional sequencing from the Lim broth were not made. A recent study, similar to the one presented here, evaluated the performance of three GBS molecular tests (BD Max GBS, illumigene, and BD GeneOhm StrepB assays). While the BD Max assay performed comparably to our study, Couturier et al. also found that most specimens with discordant results had higher threshold cycle (CT) values (CT of >35) when positive and some specimens persistently exhibited discordant results with repeat testing (6). Investigators in that study also concluded that this was likely due to low levels of the target organisms. The clinical significance of false-negative NAAT results for these low-burden specimens is currently unknown, but laboratories that perform verification studies for GBS PCR assays should be cognizant that such specimens may be encountered and should have a predetermined strategy for how to resolve discrepant results. Importantly, all three NAATs performed better than culture.
The onset of labor or premature rupture of membranes prior to 35 to 37 weeks of gestation is a risk factor for early-onset GBS disease, particularly because GBS status is often unknown (1). The reduced turnaround time and increased sensitivity of PCR in comparison with culture may be beneficial in these scenarios, in which rapid results can aid decision-making regarding antibiotic prophylaxis. However, one disadvantage of GBS NAATs currently on the market is that most require 18 to 24 h of enrichment prior to testing. While some groups tested enriched Lim broths with shorter incubation times, optimal sensitivity was achieved with the recommended 18 to 24 h, following manufacturer and CDC recommendations (6, 18). In-house verification studies will be necessary for most assays, to prove that shorter enrichment times can provide accurate results.
Overall, the three assays compared in the study presented here were easy to perform, involved minimal hands-on time, and had rapid turnarounds. The AmpliVue assay allows samples to be run individually or up to 24 at a time with the heat blocks provided and individual packaging of the detection cartridges. The BD Max system also can test specimens in batches of 1 to 24 and requires no user manipulations after a run is initiated. An additional benefit is the semi-random-access ability; a second batch of 24 samples can be loaded into the system once the first batch has been extracted and is at the PCR stage. The major disadvantage of the BD Max GBS assay is the requirement for the BD Max system. The illumigene assay, like the AmpliVue assay, is perhaps best suited for medium-volume laboratories, because up to 10 specimens can be tested with one instrument. Additionally, the illumigene assay is compatible with samples in enriched Trans-Vag broth or carrot broth. All three platforms can be used for other assays manufactured by the companies, and the BD Max system can accommodate laboratory-developed tests in runs separate from those for in vitro diagnostic assays. The use of molecular methods, coupled with appropriate intrapartum antibiotic prophylaxis, should further reduce the rates of GBS-associated early-onset infections by improving antenatal GBS detection rates. Further molecular studies to evaluate GBS colonization rates among women during antenatal screening and at the time of birth, combined with clinical outcome studies, could facilitate assessment of whether prophylaxis for culture-negative, PCR-positive women is beneficial in preventing neonatal GBS infections.
ACKNOWLEDGMENTS
We are grateful to Quidel Corp., BD Diagnostics, and Meridian Bioscience for providing reagents for this study. We thank Diane Kawa for critical review of the manuscript.
Funding for this study was provided by Quidel Corp.
REFERENCES
- 1.Verani JR, McGee L, Schrag SJ. 2010. Prevention of perinatal group B streptococcal disease: revised guidelines from CDC, 2010. MMWR Recomm Rep 59(RR-10):1–36. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 1996. Prevention of perinatal group B streptococcal disease: a public health perspective. MMWR Recomm Rep 45(RR-7):1–24. [PubMed] [Google Scholar]
- 3.Schrag SJ, Zell ER, Lynfield R, Roome A, Arnold KE, Craig AS, Harrison LH, Reingold A, Stefonek K, Smith G, Gamble M, Schuchat A. 2002. A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N Engl J Med 347:233–239. doi: 10.1056/NEJMoa020205. [DOI] [PubMed] [Google Scholar]
- 4.Schrag SJ, Zywicki S, Farley MM, Reingold AL, Harrison LH, Lefkowitz LB, Hadler JL, Danila R, Cieslak PR, Schuchat A. 2000. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med 342:15–20. doi: 10.1056/NEJM200001063420103. [DOI] [PubMed] [Google Scholar]
- 5.Stoll BJ, Hansen NI, Sanchez PJ, Faix RG, Poindexter BB, Van Meurs KP, Bizzarro MJ, Goldberg RN, Frantz ID III, Hale EC, Shankaran S, Kennedy K, Carlo WA, Watterberg KL, Bell EF, Walsh MC, Schibler K, Laptook AR, Shane AL, Schrag SJ, Das A, Higgins RD. 2011. Early onset neonatal sepsis: the burden of group B streptococcal and E. coli disease continues. Pediatrics 127:817–826. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couturier BA, Weight T, Elmer H, Schlaberg R. 2014. Antepartum screening for group B Streptococcus by three FDA-cleared molecular tests and effect of shortened enrichment culture on molecular detection rates. J Clin Microbiol 52:3429–3432. doi: 10.1128/JCM.01081-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodrich JS, Miller MB. 2007. Comparison of culture and 2 real-time polymerase chain reaction assays to detect group B Streptococcus during antepartum screening. Diagn Microbiol Infect Dis 59:17–22. doi: 10.1016/j.diagmicrobio.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 8.Riedlinger J, Beqaj SH, Milish MA, Young S, Smith R, Dodd M, Hankerd RE, Lebar WD, Newton DW. 2010. Multicenter evaluation of the BD Max GBS assay for detection of group B streptococci in prenatal vaginal and rectal screening swab specimens from pregnant women. J Clin Microbiol 48:4239–4241. doi: 10.1128/JCM.00947-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rallu F, Barriga P, Scrivo C, Martel-Laferriere V, Laferriere C. 2006. Sensitivities of antigen detection and PCR assays greatly increased compared to that of the standard culture method for screening for group B streptococcus carriage in pregnant women. J Clin Microbiol 44:725–728. doi: 10.1128/JCM.44.3.725-728.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quidel Corp. 2014. AmpliVue Group B Streptococcus assay package insert. Quidel Corp., San Diego, CA. [Google Scholar]
- 11.BD Diagnostics. 2013. BD MAX GBS assay package insert. BD Diagnostics, Franklin Lakes, NJ. [Google Scholar]
- 12.Meridian Bioscience. 2012. Illumigene Group B Streptococcus assay package insert. Meridian Bioscience, Cincinnati, OH. [Google Scholar]
- 13.Block T, Munson E, Culver A, Vaughan K, Hryciuk JE. 2008. Comparison of carrot broth- and selective Todd-Hewitt broth-enhanced PCR protocols for real-time detection of Streptococcus agalactiae in prenatal vaginal/anorectal specimens. J Clin Microbiol 46:3615–3620. doi: 10.1128/JCM.01262-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Regan JA, Klebanoff MA, Nugent RP. 1991. The epidemiology of group B streptococcal colonization in pregnancy. Obstet Gynecol 77:604–610. [PubMed] [Google Scholar]
- 15.Yancey MK, Schuchat A, Brown LK, Ventura VL, Markenson GR. 1996. The accuracy of late antenatal screening cultures in predicting genital group B streptococcal colonization at delivery. Obstet Gynecol 88:811–815. doi: 10.1016/0029-7844(96)00320-1. [DOI] [PubMed] [Google Scholar]
- 16.Davies HD, Miller MA, Faro S, Gregson D, Kehl SC, Jordan JA. 2004. Multicenter study of a rapid molecular-based assay for the diagnosis of group B Streptococcus colonization in pregnant women. Clin Infect Dis 39:1129–1135. doi: 10.1086/424518. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz J, Robinson-Dunn B, Makin J, Boyanton BL Jr. 2012. Evaluation of the BD MAX GBS assay to detect Streptococcus group B in LIM broth-enriched antepartum vaginal-rectal specimens. Diagn Microbiol Infect Dis 73:97–108. doi: 10.1016/j.diagmicrobio.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Munson E, Napierala M, Munson KL, Culver A, Hryciuk JE. 2010. Temporal characterization of carrot broth-enhanced real-time PCR as an alternative means for rapid detection of Streptococcus agalactiae from prenatal anorectal and vaginal screenings. J Clin Microbiol 48:4495–4500. doi: 10.1128/JCM.01734-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


