Abstract
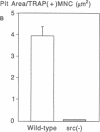
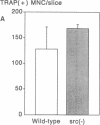
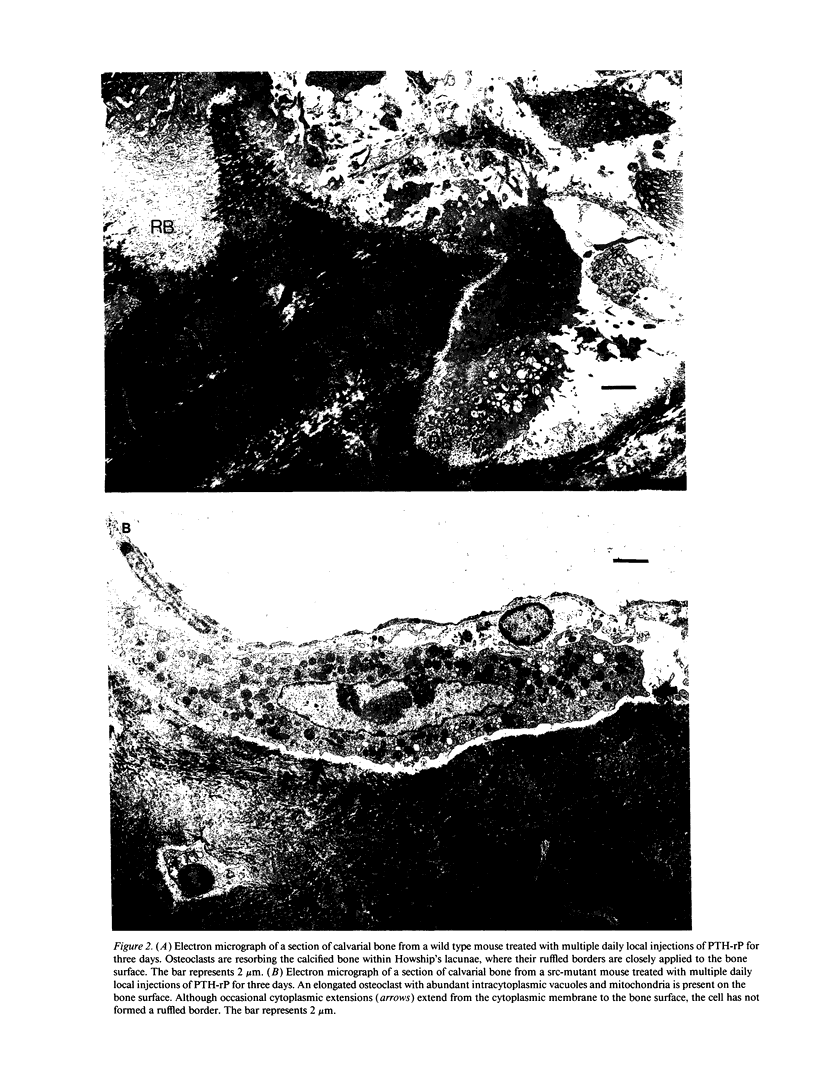
Targeted disruption of the c-src proto-oncogene in mice has shown that src expression is required for normal bone resorption, since the src-deficient mutants develop osteopetrosis. To evaluate the mechanisms by which src-deficiency affects osteoclast function, we treated src-deficient mice with the stimulants of bone resorption, IL-1, parathyroid hormone, and parathyroid hormone-related protein, and analyzed the effects by quantitative bone histomorphometry and electron microscopy. Increased numbers of multinucleated cells with the morphological characteristics of osteoclasts appeared on bone surfaces, but these cells did not form ruffled borders or normal resorption lacunae. To confirm these in vivo findings, we cultured src-mutant bone marrow cells on dentine slices in the presence of 1,25 dihydroxyvitamin D3. Increased numbers of multinucleated cells were formed, but unlike normal murine bone marrow cells, they did not form resorption pits. These results indicate that osteoclasts appear in the absence of pp60c-src, but that pp60c-src expression is required for mature osteoclasts to form ruffled borders and resorb bone.
Full text
PDF
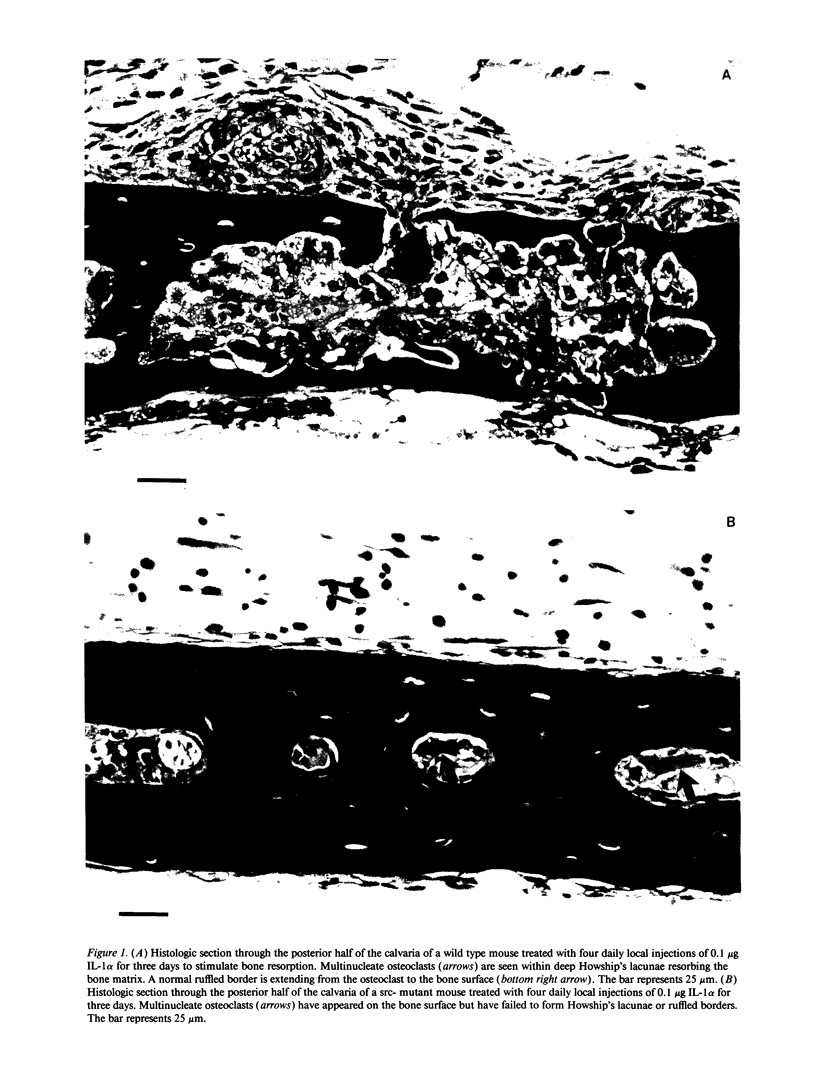

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyce B. F., Aufdemorte T. B., Garrett I. R., Yates A. J., Mundy G. R. Effects of interleukin-1 on bone turnover in normal mice. Endocrinology. 1989 Sep;125(3):1142–1150. doi: 10.1210/endo-125-3-1142. [DOI] [PubMed] [Google Scholar]
- Boyce B. F., Yates A. J., Mundy G. R. Bolus injections of recombinant human interleukin-1 cause transient hypocalcemia in normal mice. Endocrinology. 1989 Nov;125(5):2780–2783. doi: 10.1210/endo-125-5-2780. [DOI] [PubMed] [Google Scholar]
- Cheng N., Sahyoun N. The growth cone cytoskeleton. Glycoprotein association, calmodulin binding, and tyrosine/serine phosphorylation of tubulin. J Biol Chem. 1988 Mar 15;263(8):3935–3942. [PubMed] [Google Scholar]
- Felix R., Cecchini M. G., Fleisch H. Macrophage colony stimulating factor restores in vivo bone resorption in the op/op osteopetrotic mouse. Endocrinology. 1990 Nov;127(5):2592–2594. doi: 10.1210/endo-127-5-2592. [DOI] [PubMed] [Google Scholar]
- Garrett I. R., Boyce B. F., Oreffo R. O., Bonewald L., Poser J., Mundy G. R. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest. 1990 Mar;85(3):632–639. doi: 10.1172/JCI114485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellie S., Horvath A. R., Elmore M. A. Cytoskeletal targets for oncogenic tyrosine kinases. J Cell Sci. 1991 Jun;99(Pt 2):207–211. doi: 10.1242/jcs.99.2.207. [DOI] [PubMed] [Google Scholar]
- Kodama H., Yamasaki A., Nose M., Niida S., Ohgame Y., Abe M., Kumegawa M., Suda T. Congenital osteoclast deficiency in osteopetrotic (op/op) mice is cured by injections of macrophage colony-stimulating factor. J Exp Med. 1991 Jan 1;173(1):269–272. doi: 10.1084/jem.173.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchisio P. C., Cirillo D., Teti A., Zambonin-Zallone A., Tarone G. Rous sarcoma virus-transformed fibroblasts and cells of monocytic origin display a peculiar dot-like organization of cytoskeletal proteins involved in microfilament-membrane interactions. Exp Cell Res. 1987 Mar;169(1):202–214. doi: 10.1016/0014-4827(87)90238-2. [DOI] [PubMed] [Google Scholar]
- Marks S. C., Jr Osteopetrosis--multiple pathways for the interception of osteoclast function. Appl Pathol. 1987;5(3):172–183. [PubMed] [Google Scholar]
- Matten W. T., Aubry M., West J., Maness P. F. Tubulin is phosphorylated at tyrosine by pp60c-src in nerve growth cone membranes. J Cell Biol. 1990 Nov;111(5 Pt 1):1959–1970. doi: 10.1083/jcb.111.5.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan G. A., Martin T. J. Role of osteoblasts in hormonal control of bone resorption--a hypothesis. Calcif Tissue Int. 1981;33(4):349–351. doi: 10.1007/BF02409454. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991 Feb 22;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Udagawa N., Akatsu T., Tanaka H., Isogai Y., Suda T. Deficiency of osteoclasts in osteopetrotic mice is due to a defect in the local microenvironment provided by osteoblastic cells. Endocrinology. 1991 Apr;128(4):1792–1796. doi: 10.1210/endo-128-4-1792. [DOI] [PubMed] [Google Scholar]
- Yates A. J., Gutierrez G. E., Smolens P., Travis P. S., Katz M. S., Aufdemorte T. B., Boyce B. F., Hymer T. K., Poser J. W., Mundy G. R. Effects of a synthetic peptide of a parathyroid hormone-related protein on calcium homeostasis, renal tubular calcium reabsorption, and bone metabolism in vivo and in vitro in rodents. J Clin Invest. 1988 Mar;81(3):932–938. doi: 10.1172/JCI113406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Hayashi S., Kunisada T., Ogawa M., Nishikawa S., Okamura H., Sudo T., Shultz L. D., Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990 May 31;345(6274):442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]