Abstract
Despite modern prevention and treatment strategies, human cytomegalovirus (HCMV) remains a common opportunistic pathogen associated with serious morbidity and mortality in immunocompromised individuals, such as transplant recipients and AIDS patients. All drugs currently licensed for the treatment of HCMV infection target the viral DNA polymerase and are associated with severe toxicity issues and the emergence of drug resistance. Letermovir (AIC246, MK-8228) is a new anti-HCMV agent in clinical development that acts via a novel mode of action and has demonstrated anti-HCMV activity in vitro and in vivo. For the future, drug combination therapies, including letermovir, might be indicated under special medical conditions, such as the emergence of multidrug-resistant virus strains in transplant recipients or in HCMV-HIV-coinfected patients. Accordingly, knowledge of the compatibility of letermovir with other HCMV or HIV antivirals is of medical importance. Here, we evaluated the inhibition of HCMV replication by letermovir in combination with all currently approved HCMV antivirals using cell culture checkerboard assays. In addition, the effects of letermovir on the antiviral activities of selected HIV drugs, and vice versa, were analyzed. Using two different mathematical techniques to analyze the experimental data, (i) additive effects were observed for the combination of letermovir with anti-HCMV drugs and (ii) no interaction was found between letermovir and anti-HIV drugs. Since none of the tested drug combinations significantly antagonized letermovir efficacy (or vice versa), our findings suggest that letermovir may offer the potential for combination therapy with the tested HCMV and HIV drugs.
INTRODUCTION
Human cytomegalovirus (HCMV) is a widespread opportunistic pathogen that rarely causes clinical manifestations in the healthy population; however, HCMV infection of immunocompromised individuals, such as transplant recipients or AIDS patients, is associated with serious and life-threatening diseases (1–3).
In human stem cell transplant (HSCT) recipients, a primary infection with HCMV or reactivation of HCMV from persistently infected cells can lead to multiorgan disease, including hepatitis, pneumonia, gastroenteritis, and encephalitis, accounting for considerable mortality, particularly in HCMV-seropositive (R+) patients (4). After solid-organ transplantation (SOT), clinical disease is most common during primary HCMV infection of a seronegative individual receiving an organ from an HCMV-seropositive donor (D+ R−). The most common clinical presentation is HCMV syndrome (fever, arthralgias, myalgias, and myelosuppression), though clinical disease may also present with evidence of end organ involvement, such as gastrointestinal disease or pneumonia, which is associated with increased morbidity and mortality and poor long-term outcomes after SOT (5, 6).
HCMV is also the most common and most severe viral opportunistic infection in patients with HIV infection. Over 90% of all patients with HIV infection are seropositive for HCMV, and the virus can reactivate when the body's immune defenses are low, as can be seen in patients with AIDS (2). Before the availability of highly active antiretroviral therapy (HAART), up to 40% of HIV-infected patients developed HCMV disease, most commonly manifested as retinitis leading to blindness (7, 8). Following initiation of HAART, the incidence of HCMV disease has decreased dramatically due to the reconstitution of humoral immunity against HCMV. However, for patients experiencing HAART failure (limited access to HAART, intolerable HAART regimens, poor compliance, or HIV resistance), the occurrence of HCMV-associated diseases remains a therapeutic challenge (2, 9, 10). In addition, evidence is emerging that accelerated aging and immunosenescence observed in HIV-HCMV-coinfected individuals during HAART is associated with asymptomatic HCMV replication (11, 12).
Current therapeutic options for the prevention and treatment of HCMV infections are limited to nucleoside analogues, e.g., oral or intravenous ganciclovir (GCV) or its oral prodrug, valganciclovir, together with the second-line treatments, foscarnet (FOS), cidofovir (CDV), and acyclovir (ACV) (the last drug is approved only for the prophylaxis of HCMV infections in SOT patients in a limited number of countries). Although these products are effective antivirals, all current treatments for HCMV are limited by dose-related toxicities, such as myelosuppression, neutropenia, and nephrotoxicity, which may eventually lead to treatment failure (3, 13, 14). Moreover, since all the drugs act by inhibiting the viral DNA polymerase pUL54, another treatment concern is the emergence of drug-resistant virus due to prolonged and repeated therapy. This is of particular relevance, since cross-resistance to all approved anti-HCMV drugs is increasingly observed (15–17).
Letermovir (also known as AIC246 or MK-8228) is a new, efficacious, first-in-class anti-HCMV drug currently being developed for the prevention of HCMV disease in immunocompromised transplant patients (18–20). In contrast to all the anti-HCMV drugs marketed, letermovir's novel mechanism of action targets the pUL56 subunit of the viral terminase complex and thus interferes with viral DNA maturation and packaging (21). Owing to the absence of a human counterpart for the viral terminase enzyme, the drug appears to have the advantage of reduced toxicity, due to the unlikely prospect of mechanism-based side effects. In fact, its novel mode of action not only appears to avoid the toxicities seen with the nucleoside analogues (19, 20), but also offers new treatment options for patients infected with HCMV strains resistant to approved antivirals. The latter drug property is strongly supported by in vitro data, as well as initial clinical data relating to letermovir use in a lung transplant patient infected with a multiresistant HCMV strain (18, 22–24).
Combining antiviral agents with different mechanisms of action is a proven strategy to increase antiviral potency, provided that the antiviral effects of the combined drugs are at least additive and without a concomitant increase in cytotoxicity. Although letermovir retains full efficacy against infections with HCMV variants resistant to approved anti-HCMV drugs, combination therapies including letermovir might be indicated in the future for special medical conditions, such as resistant infections in hard-to-treat compartments like the central nervous system (CNS) (25, 26), a clinical manifestation predominantly seen in HIV-HCMV-coinfected patients (2, 12, 27). Given this, we sought to determine the potential for combining letermovir with approved anti-HCMV and anti-HIV drugs and to explore whether letermovir represents a therapeutic option for HIV-HCMV-coinfected patients.
In the present study, we used two-drug combination experiments in order to analyze the in vitro efficacy of letermovir in combination with (i) approved HCMV polymerase inhibitors and (ii) a selection of anti-HIV agents representing the majority of currently marketed drug classes. Our results show that none of the tested drug combinations antagonized letermovir efficacy or vice versa and thus suggest that letermovir offers the potential for combination therapy with both approved anti-HCMV and anti-HIV drugs.
MATERIALS AND METHODS
Cells, cell culture, and viruses.
Normal human dermal fibroblasts (NHDFs) were purchased from Clonetics (no. CC-2511) and were cultured as described previously (18). The human T cell line MT-4 was obtained from the NIH AIDS Research and Reference Reagent Program (no. 120) and was cultured in RPMI 1640 medium as described previously (28). The HCMV AD169-derived recombinant virus RV-HG was reconstituted from HCMV-BAC pHG, kindly provided by E. Borst and M. Messerle (29). Inserted in the unique short region, HCMV-BAC pHG contains an enhanced green fluorescent protein (EGFP) reporter gene expressed under the control of the major immediate-early promoter (30). HCMV stocks were propagated using NHDFs and titrated by means of IE1p72 fluorescence, as described previously (31, 32). The HIV-1 strain LAI, obtained from the NIH AIDS Research and Reference Reagent Program (no. 2522), was propagated using MT-4 cells and titrated in an endpoint dilution assay using MT-4 cells and the alamarBlue cell viability assay (Invitrogen, Germany).
Antiviral compounds.
Letermovir was synthesized at the medical chemistry department of Bayer Pharma AG, Wuppertal, Germany, and stored as a 50 mM stock solution in dimethyl sulfoxide (DMSO) for in vitro use. The intravenous formulations of ganciclovir (Cymevene; Roche), foscarnet (Foscavir; AstraZeneca), cidofovir (Vistide; Gilead), and acyclovir (Zovirax; GlaxoSmithKline) were used as 50 mM solutions in 0.9% saline. Efavirenz (Sustiva; BMS), etravirine (Intelence; Tibotec), nevirapine (Viramune; BI), atazanavir (Reyataz; BMS), ritonavir (Norvir; Abbott), darunavir (Prezista; Tibotec), and lopinavir (Kaletra; AbbVie) were extracted from commercial formulations. Emtricitabine was purchased from AK Scientific Inc. (USA). Tenofovir and rilpivirine were purchased from Beta Pharma Co. Ltd. (China) and Medicilon Inc. (China), respectively, and raltegravir and elvitegravir were purchased from Selleck Chemicals LLC (USA). All anti-HIV drugs were stored as DMSO stock solutions for in vitro use. The clinically relevant therapeutic drug concentration in human serum for letermovir was deduced from pharmacokinetic studies in patients (unpublished data), and the respective maximum therapeutic concentrations for anti-HIV drugs were calculated from maximum concentrations of drug in serum (Cmax values) taken from “PK fact sheets” (http://www.hiv-druginteractions.org) (see Table 3).
TABLE 3.
In vitro activities and therapeutic concentrations of HCMV and HIV-1 drugs
| Antiviral agent | Cell culture EC50 (μM)a | Therapeutic concn (μM)b |
|---|---|---|
| HCMV terminase inhibitor | ||
| Letermovir | 0.0040 ± 0.0008 | 3.0 |
| HCMV polymerase inhibitors | ||
| Ganciclovir (GCV) | 2.0 ± 0.9 | NN |
| Cidofovir (CDV) | 0.2 ± 0.02 | NN |
| Foscarnet (FOS) | 98 ± 19 | NN |
| Aciclovir (ACV) | 20 ± 6 | NN |
| HIV NRTIs | ||
| Emtricitabine (FTC) | 0.0641 ± 0.0399 | 7.3 |
| Tenofovir (TDF) | 3.2770 ± 0.9829 | 1.1 |
| HIV NNRTIs | ||
| Efavirenz (EFV) | 0.0003 ± 0.0001 | 12.9 |
| Etravirine (ETR) | 0.0004 ± 0.0002 | 1.8 |
| Nevirapine (NVP) | 0.2128 ± 0.0161 | 18.8 |
| Rilpivirine (RPV) | 0.0002 ± 0.0001 | 0.7 |
| HIV protease inhibitors | ||
| Atazanavir (ATV) | 0.0016 ± 0.0012 | 6.3 |
| Darunavir (DRV) | 0.0036 ± 0.0005 | 11.9 |
| Lopinavir (LPV) | 0.0029 ± 0.0006 | 10.0 |
| Ritonavir (RTV) | 0.0094 ± 0.0002 | 15.5 |
| HIV integrase inhibitors | ||
| Raltegravir (RAL) | 0.0069 ± 0.0008 | 4.5 |
| Elvitegravir (ELV) | 0.0006 ± 0.0001 | 3.8 |
EC50 values were determined by a fluorescence reduction assay (anti-HCMV activities of HCMV drugs) or using an alamarBlue viability assay (anti-HIV-1 activities of HIV drugs). The data are means of results from at least two independent experiments ± standard deviations.
Assumed clinically therapeutic drug concentrations in human serum derived from pharmacokinetic studies in patients (letermovir) or taken from “PK fact sheets” (Cmax) (http://www.hiv-druginteractions.org). NN, not needed.
Preparation of drug combination matrices and antiviral and cytotoxicity assays.
Combinations of letermovir with approved anti-HCMV compounds were prepared in a checkerboard fashion, as described previously (33). For each drug combination, the two compounds were prepared separately by serial 3-fold dilution and were mixed in 96-well plates (without using the edges of the plates) to create a two-dimensional matrix of single and combined diluted drugs. Five concentrations of letermovir, starting at a maximum concentration of 0.036 μM (9 times the 50% effective concentration [EC50]), were tested in all possible combinations with seven concentrations of the other anti-HCMV drugs, starting at a maximum drug concentration 9 to 27 times the EC50. For the single-drug dilutions, the concentrations in the middle of the series were defined as the EC50s of the corresponding drugs. In order to test for putative drug interactions of letermovir with selected anti-HIV drugs, a fixed dose of the drug at a clinically relevant concentration (Cmax) (see Table 3) was combined with serial 3-fold letermovir dilutions at concentrations spanning and including the EC50 and vice versa. In parallel, an identical dilution series was prepared for each active drug alone.
The antiviral effects of the drug combinations on HCMV replication were determined by a sensitive and reproducible fluorescence reduction assay closely correlated with plaque reduction assays (18, 22). Briefly, NHDFs cultured in black 96-well plates (Greiner Bio-One, Germany) were infected with the green fluorescent protein (GFP)-expressing virus RV-HG at a multiplicity of infection (MOI) of 0.2. After adsorption, the virus inoculum was replaced with medium containing the test compound combinations described above. The plates were incubated at 37°C for 7 days before GFP units (GFPU) were determined for each well by a charge-coupled-device camera-based fluorescence detector (FluoBox; Bayer Technology Services GmbH, Leverkusen, Germany). The effect on HIV-1 replication was measured by determining the HIV-1 cytopathic effect using an alamarBlue cell viability assay (28). Briefly, MT-4 cells were infected with HIV-1 LAI (MOI = 0.01) and incubated in 96-well plates with medium containing the test compound combinations described above for 5 days at 37°C and 5% CO2 before alamarBlue reagent was added. The fluorescence signal in each well was measured after 3 h at 550 and 595 nm using a SpectraFluor Plus fluorescence reader (Tecan, Germany). Each two-drug combination was tested 5 times in at least three independent experiments, and for each combination, a concurrent cytotoxicity study was performed with uninfected cells and matched drug exposure. Potential synergistic cytotoxicity was assessed by determination of the viability of uninfected cells using the alamarBlue cell viability assay as described by the manufacturer.
Statistical evaluation of drug combination effects.
To assess the antiviral effects of different HCMV drug combinations, (i) volumes of synergy or antagonism were assessed according to the Bliss independence method described by Prichard and Shipman (33) and (ii) combination indices (CIs) were calculated according to the Loewe additivity method described by Chou and Talalay (34). For the Bliss independence model, the raw data were analyzed at the 95% confidence level using the MacSynergy II software developed by Prichard and Shipman (33). The program uses the independent-effects definition of additive interactions, meaning that theoretical additive interactions are calculated from the dose-response curves for each drug alone. This calculated additive surface, which represents predicted or additive interactions, is then subtracted from the experimentally determined dose-response surface to reveal regions of nonadditive activity. The resulting surface appears as a horizontal plane at 0% inhibition above calculated if the interactions are merely additive (no interaction). Any peaks above this plane of additivity indicate synergism, and any depressions below the plane indicate antagonism. The volumes of the peaks/depressions were calculated to quantify the effect of the drug combination on antiviral activity (synergy/antagonism volumes [μM2%]). For these studies, the synergy/antagonism volumes (μM2%) of drug combinations were defined as follows: values of less than −100 reflect strong antagonism, values in the range of greater than or equal to −100 to less than or equal to −50 indicate slight antagonism, and values between greater than −50 and less than +50 were considered additive. Volumes of greater than or equal to +50 to less than or equal to +100 and of greater than +100 were considered slight and strong synergism, respectively (35). For the Loewe additivity evaluation, experimental data were analyzed with software (CalcuSyn; Biosoft, Cambridge, United Kingdom) based on the method of Chou and Talalay (34, 36). In the first step, the program converts the dose-effect curves for each drug or drug combination to median effect plots (36). A CI for each experimental combination is then calculated by the following formula: [(D)1/(Dx)1] + [(D)2/(Dx)2] + [(D)1(D)2/(Dx)1(Dx)2], where (Dx)1 and (Dx)2 are the doses of drug 1 and drug 2 that have x effect when each drug is used alone and (D)1 and (D)2 are the doses of drug 1 and drug 2 that have the same x effect when they are used in combination. The software calculates the CIs at 50% (CI50), 75% (CI75), and 90% (CI90) antiviral effects of combinations. A weighted average CI (CIwt) was calculated by the use of the following formula: CIwt = (CI50 + 2CI75 + 3CI90)/6. CI values of <0.8, >1.2 and ≥0.8, and ≤1.2 indicate synergy, antagonism, or additivity between drugs, respectively (37). Drug combinations were analyzed at three different fixed drug ratios spanning and including the approximate ratio of their EC50s (see Tables 2 and 3).
TABLE 2.
Analysis of two-drug combinations at fixed concentration ratios by use of the Loewe additivity model
| Drug combination | Molar ratio | CI valuea at level of HCMV inhibition of: |
CIwtb | Overall result | ||
|---|---|---|---|---|---|---|
| 50% | 75% | 90% | ||||
| Letermovir + GCV | 1:166 | 0.90 ± 0.22 | 0.83 ± 0.13 | 0.90 ± 0.25 | 0.88 | Additive |
| 1:500 | 1.03 ± 0.08 | 1.00 ± 0.11 | 1.09 ± 0.27 | 1.05 | ||
| 1:1,500 | 1.00 ± 0.05 | 1.06 ± 0.06 | 1.15 ± 0.09 | 1.09 | ||
| Letermovir + CDV | 1:16.6 | 1.21 ± 0.18 | 1.18 ± 0.05 | 1.18 ± 0.07 | 1.18 | Additive |
| 1:50 | 1.19 ± 0.25 | 1.19 ± 0.18 | 1.22 ± 0.14 | 1.20 | ||
| 1:150 | 1.01 ± 0.30 | 1.08 ± 0.14 | 1.19 ± 0.04 | 1.12 | ||
| Letermovir + FOS | 1:8,333 | 1.15 ± 0.36 | 1.18 ± 0.18 | 1.30 ± 0.01 | 1.23 | Additive/minor antagonism |
| 1:25,000 | 1.25 ± 0.14 | 1.35 ± 0.03 | 1.49 ± 0.20 | 1.40 | ||
| 1:75,000 | 1.15 ± 0.11 | 1.20 ± 0.09 | 1.28 ± 0.17 | 1.23 | ||
| Letermovir + ACV | 1:1,666 | 1.09 ± 0.13 | 0.86 ± 0.16 | 0.83 ± 0.20 | 0.89 | Additive |
| 1:5,000 | 1.18 ± 0.24 | 1.04 ± 0.11 | 0.97 ± 0.05 | 1.03 | ||
| 1:15,000 | 1.19 ± 0.16 | 1.21 ± 0.11 | 1.26 ± 0.12 | 1.23 | ||
CI values were determined for the indicated two-drug combinations at 50%, 75%, and 90% inhibition of HCMV replication according to the method described by Chou and Talalay (34) using the CalcuSyn program (Biosoft, Ferguson, MO, USA). CIs of <0.8, 0.8 to 1.2, or >1.2 indicate synergism, additive effect, and antagonism respectively.
The CIwt value was assigned as follows: (CI50 + 2CI75 + 3CI90)/6. Datasets from at least 3 independent experiments were combined, and arithmetic means ± standard deviations were calculated for each drug-drug combination.
To assess the potential impact of anti-HIV drugs on the anti-HCMV activity of letermovir and vice versa, EC50s were calculated from fixed-dose drug combinations and single-drug dilutions (see above) using nonlinear regression curve fitting with a variable slope (GraphPad Prism 4.0; GraphPad Software Inc., La Jolla, CA, USA). EC50 ratios were calculated as follows: EC50 of letermovir alone divided by EC50 of letermovir plus anti-HIV drug (anti-HCMV activity) or EC50 of anti-HIV drug alone divided by EC50 of anti-HIV drug plus letermovir (anti-HIV activity). Shifts in the EC50 of the active drug of >2.5 in the presence or absence of the inactive drug were considered potential drug-drug interactions.
RESULTS
Two-drug combinations of letermovir with approved anti-HCMV compounds.
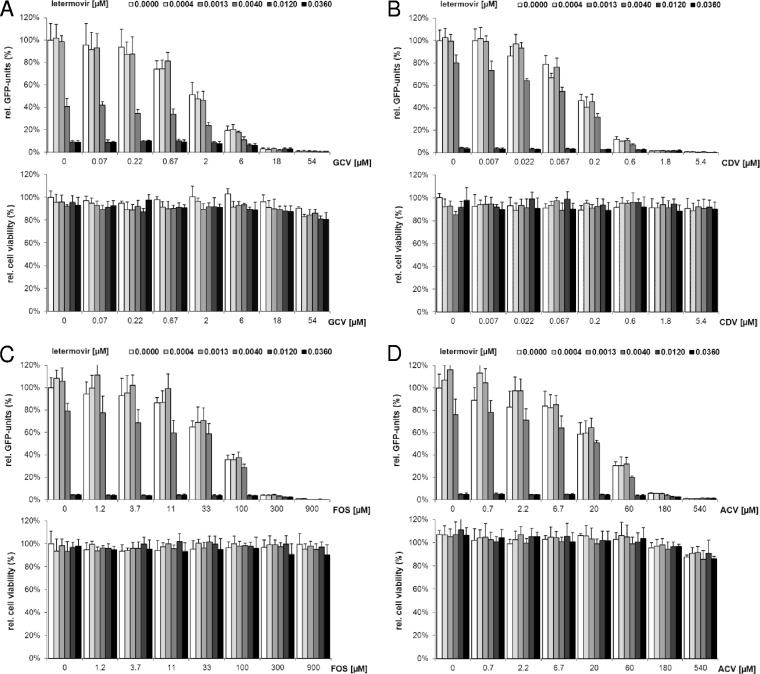
In vitro two-drug combination experiments are widely used to identify drug combinations that give enhanced antiviral effects or to exclude combinations with antagonism in either or both directions (36). Accordingly, we sought to evaluate the antiviral effects of combining letermovir singly with all currently approved drugs for treatment or prevention of HCMV infections: GCV, CDV, FOS, and ACV. For this, human fibroblasts infected with a GFP-expressing HCMV strain (RV-HG) were treated with various concentrations of the cleavage/packaging inhibitor letermovir, one of the currently approved polymerase inhibitors, or the two drugs in combination. The drugs were titrated in a checkerboard fashion so that synergy/antagonism volumes and CIs could be determined from the same data set (see below). HCMV inhibition was determined 7 days postinfection (p.i.) by quantification of GFP units in the infected cells. As shown in Fig. 1A to D, top (see Table 3), letermovir and the tested polymerase inhibitors were effective inhibitors of HCMV replication when used as single agents, and combinations of the drugs were even more effective. In addition, in concurrent viability assays, none of the tested two-drug combinations showed synergistic cytotoxicity within the range of drug concentrations examined (Fig. 1A to D, bottom).
FIG 1.
Anti-HCMV activities and cytotoxicities for letermovir in combination with GCV (A), CDV (B), FOS (C), and ACV (D). Each bar represents the average of five cell culture replicates with standard deviations. (Top) Anti-HCMV activities of the indicated two-drug combinations were determined in a checkerboard fashion using a fluorescence reduction assay (18). The concentrations of approved polymerase inhibitors are given on the x axis, and the concentrations of letermovir are indicated by shading, as shown above the graphs. Drug effects were calculated as percent reduction of GFP units in the presence of each drug combination compared to GFP units determined in the absence of drug. (Bottom) The viability of NHDFs following compound treatment for 7 days was determined by the alamarBlue viability assay and is presented as a percentage of the viability of cells that were left untreated.
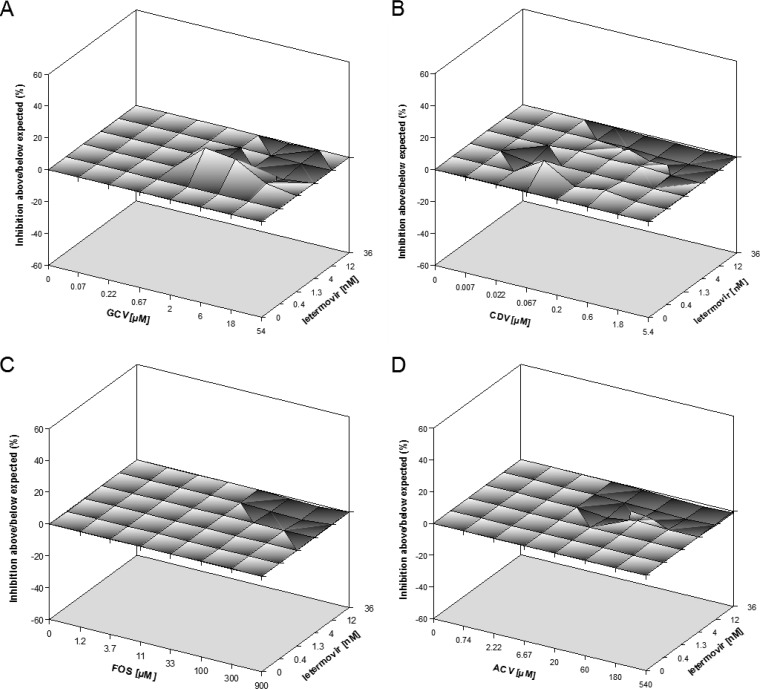
In order to assess whether the observed antiviral activities of the drug combinations were synergistic, antagonistic, or simply additive, the experimental data shown in Fig. 1 were analyzed with the two predominant models for defining drug interaction: Bliss independence and Loewe additivity (38). For the Bliss independence method, data were evaluated with the MacSynergy II program, which displays synergy and antagonism as peaks above or below a predicted additive plane in a 3-dimensional graph (33). Figure 2 shows a representative experiment for each of the drug combinations analyzed. The corresponding mean synergy and antagonism volumes at the 95% confidence level from at least three independent combination experiments are summarized in Table 1. The calculated synergy and antagonism volumes for all tested two-drug combinations were well within the range of −50 to +50 μM2%. Thus, by definition, all combinations were scored as additive and yielded no antagonism.
FIG 2.
Efficacy analysis of two-drug combinations by use of the Bliss independence model. Three-dimensional surface plots of the anti-HCMV activities of the indicated two-drug combinations are shown. Indicated are representative single-experiment plots of the combinations of letermovir and GCV (A), CDV (B), FOS (C), and ACV (D). The plots were generated using MacSynergy II software (33). The x and y axes show drug concentrations. The zero plane across the z axis represents the theoretical additive effect; a positive value, displayed as a peak above the plane, indicates synergy, and a negative value, shown as a valley below the plane, indicates antagonism. Each experimental data point represents the average of five cell culture replicates, and 95% confidence intervals were used to evaluate the data.
TABLE 1.
Analysis of the combination of letermovir with approved anti-HCMV drugs by use of the Bliss independence model
| Drug combination | Mean vol (μM2%)a |
Antiviral effect | |
|---|---|---|---|
| Synergy | Antagonism | ||
| Letermovir + GCV | 15.6 ± 13.8 | −5.1 ± 6.5 | Additive |
| Letermovir + CDV | 3.3 ± 4.7 | −12.6 ± 4.1 | Additive |
| Letermovir + FOS | 5.1 ± 5.8 | −15.2 ± 14.3 | Additive |
| Letermovir + ACV | 0.5 ± 0.6 | −12.4 ± 7.7 | Additive |
Antiviral activities of drug combinations were determined in a checkerboard fashion using a GFP-based fluorescence reduction assay. MacSynergy II software was used to calculate the synergy and antagonism volumes of the antiviral effects of two-drug combinations at the 95% confidence level. The positive and negative values from five cell culture replicates were individually summed to give the mean synergy and antagonism volumes. Datasets from at least 3 independent experiments were combined, and arithmetic means were calculated for each drug-drug combination. Volumes of synergy or antagonism greater than ±50 μM2% can be considered significant and may be important in vivo.
Comparable results were obtained when data were analyzed by the Loewe additivity model (Table 2). In this model, the antiviral effects produced by the combinations of letermovir with the different polymerase inhibitors at various fixed ratios of concentrations were compared to those produced by letermovir or the respective polymerase inhibitor alone (34, 36). CIs were computed at the EC50, EC75, and EC90 levels using CalcuSyn software (Biosoft). Since large effects are thought to be more therapeutically relevant than small effects, the CIwt (see Materials and Methods) was calculated, in addition (36, 39).
As summarized in Table 2, combinations of letermovir with GCV or CDV at three different ratios yielded consistently additive responses, with CIwts of 0.88 to 1.2. Overall additive CIwts were also obtained for the combination of letermovir with ACV, although at one of the three tested drug ratios (1:15,000), weakly antagonistic values (>1.2) were seen at the higher levels of HCMV inhibition. However, none of the respective CI values (1.21 ± 0.11 and 1.26 ± 0.12 [Table 2]) was significantly outside the 0.8 to 1.2 range, which is considered to indicate additivity (37). The CIwts for the letermovir-FOS combination were consistently just above 1.2 at all three drug ratios, with individual CIs ranging from additive to weak antagonism at the higher effective doses. Taken alone, this suggests a minor antagonistic response, which stands, however, in contrast to the clear additive drug interaction observed with the McSynergy evaluation (compare Table 1). In summary, taking into account both the Loewe and Bliss models, the antiviral interactions between letermovir and GCV, CDV, or ACV were generally additive, and the same appears to be the case for FOS, though there was a slight discrepancy between the two models.
Two-drug combinations of letermovir with approved HIV compounds.
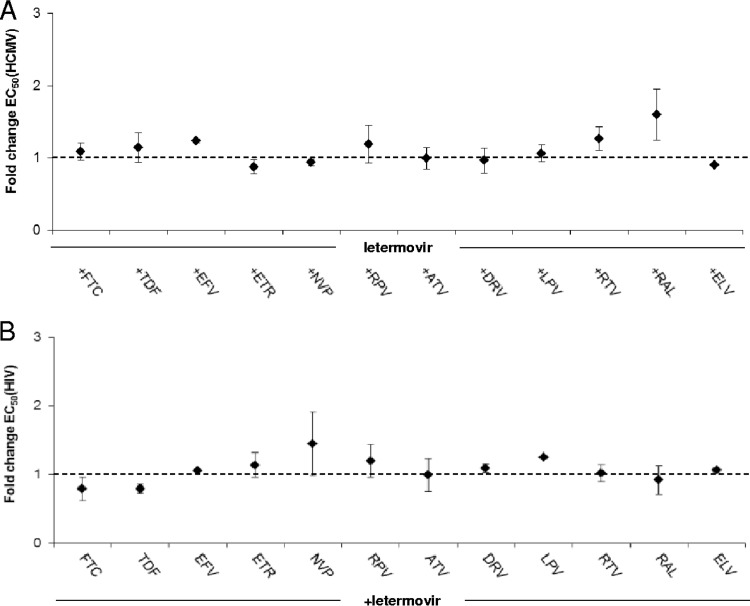
As outlined in the introduction, almost all individuals with HIV infection are coinfected with HCMV, and reactivation of the virus is still a major cause of morbidity and mortality in patients with AIDS (2). Therefore, we sought to gain initial insights into the efficacy and toxicity of letermovir when coadministered with currently approved anti-HIV drugs and vice versa. Following current guidelines, we used at least two representatives of each class of antivirals (40). Accordingly, combinations of letermovir with selected nucleoside reverse transcriptase inhibitors (NRTIs) (emtricitabine and tenofovir), nonnucleoside reverse transcriptase inhibitors (NNRTIs) (efavirenz, etravirine, nevirapine, and rilpivirine), proteinase inhibitors (atazanavir, darunavir, lopinavir, and ritonavir), and integrase inhibitors (raltegravir and elvitegravir) were assessed using in vitro activity studies against HCMV and HIV-1. Given that none of the tested HIV drugs has antiviral activity against HCMV (data not shown) and letermovir is not active against HIV-1 (22), HCMV-HIV two-drug combinations could not be assessed by the Loewe or the Bliss model (36, 38). Alternatively, we created composite dose-response curves and determined whether the EC50 of the active inhibitor letermovir alone was affected by adding a clinically relevant fixed dose of a second, noninhibitory HIV compound and vice versa. The concentration of the “inactive” drug in the combination was chosen on the basis of the maximum clinically therapeutic levels of the respective drug in human serum (Cmax). Table 3 summarizes the individual EC50s for the active drug alone and depicts the therapeutic drug concentration that was applied if the compound was given as the “inactive” combination partner. Figure 3 graphically depicts the EC50 fold changes of the indicated drug combinations compared with the EC50 determined for the active drug alone. We found that the in vitro anti-HCMV activity of letermovir was not significantly modified in the presence of any of the tested HIV drugs administered at near-physiological plasma drug concentrations (Fig. 3A), suggesting no interaction of the compounds (inertism). On the other hand, we also determined the effect of a therapeutic dose of letermovir (3 μM) on the anti-HIV efficacies of the tested antiretroviral drugs. Figure 3B shows that the maximal anti-HIV EC50 change observed in the presence of letermovir was 1.4-fold for the nevirapine-letermovir combination. Thus, these results indicate drug inertism. Of note, all combination studies included concurrent evaluations of cell viability (data not shown) to exclude nonspecific effects.
FIG 3.
(A) Effects of therapeutic drug concentrations of selected anti-HIV drugs (Table 3 lists the drug abbreviations) on the letermovir EC50 value for inhibition of HCMV replication. (B) Effects of a clinically relevant letermovir dose on the EC50 values of the indicated anti-HIV drugs for inhibition of HIV-1 replication. The EC50 values were determined for the indicated fixed-dose drug combinations and for single-drug dilutions. EC50 fold changes of the indicated drug combinations were calculated as outlined in Materials and Methods (dashed line: no effect/inertism; for consensus terminology for two-agent combined action concepts, see reference 38). Each data point represents the average of the results of three independent experiments and is shown with the standard deviation.
Taken together, all the analyzed two-drug combinations described in this report showed additive effects (HCMV drug combinations) or demonstrated inertism (HIV drugs). Importantly, none of the tested combinations yielded significant antagonism.
DISCUSSION
Combination therapy has emerged as a powerful approach to HIV treatment, particularly if the drugs in the combination have different modes of action. Accordingly, a similar approach is taken in the therapy of other chronic infections with highly mutagenic RNA viruses, like hepatitis C virus (HCV) (41, 42). In contrast, a comparable strategy is not routinely applied to manage infections with large DNA viruses, like HCMV (3, 4). The reasons for this are, among others, the relatively high toxicity of currently approved HCMV antivirals and the comparatively low mutation rate of DNA viruses, reducing the need for drug combinations. Thus, monotherapy will most likely remain the standard of care for HCMV prophylaxis in transplant recipients. Nevertheless, combination therapy might prove useful in the future, either in controlling HCMV replication in hard-to-treat conditions (e.g., infection with multiresistant viruses or treatment of established HCMV disease) or in hard-to-treat compartments, like the CNS or eye (27, 43, 44).
The novel anti-HCMV drug letermovir may combine properties of an efficacious standalone drug and those of a drug that may be suitable for use in combination therapy alongside currently approved antivirals, given its (i) novel mode of action, (ii) favorable toxicity and tolerability profile, and (iii) proven clinical efficacy in treatment and prevention of HCMV infections (18–23). However, drug-drug interactions may be complex and can result in synergistic, additive, or even antagonistic effects, Thus, as a first step and a prerequisite for clinical use, we sought to evaluate the potential benefits or risks of combining letermovir with currently approved drugs for HCMV therapy using two-drug in vitro combinations while being aware that cell culture experiments by nature are not able to detect more complex interactions, such as metabolic effects, that would be expected in vivo.
In this study, a single checkerboard dataset was analyzed by the two main mathematical models of interaction: Loewe additivity and Bliss independence. Despite an ongoing academic debate, so far there is no generally accepted agreement on which of the two models is more appropriate, and moreover, discordant results produced by the different methods are not uncommon (38, 45). Consequently, we decided to analyze our data with both algorithms in order to obtain more comprehensive evaluations of drug combinations and to avoid potential biasing of the results of our studies by choosing only one specific interaction model. When letermovir was tested in combination with GCV, CDV, or ACV, it displayed clear-cut additive antiviral activity, demonstrated by both models, indicating validity of the results and excluding potential bias. The reason why discordant results were obtained for the letermovir-FOS combination is unclear. However, taking into account that the drug combination (i) clearly gave additive antiviral effects in the Bliss evaluation and (ii) did not deviate significantly from additivity at the EC50 and/or EC75 level when data were analyzed by the Loewe algorithm, an overall additive drug interaction, as was seen with the other polymerase inhibitors, is most likely. Nevertheless, the possibility of a low level of antagonism at some drug ratios or inhibition levels cannot be entirely excluded and warrants clinical evaluation.
Only a few data are available in the literature on combinations of viral terminase inhibitors with polymerase inhibitors. Evers et al. studied interactions of the discontinued cleavage/packaging inhibitors BAY38-4766 and BDCRB with GCV using the Bliss independence model (46). It is noteworthy that, although they target the HCMV terminase complex, letermovir, BAY38-4766, and BDCRB are all chemically unrelated and are characterized by entirely distinct resistance profiles, rendering it unlikely that the molecules share the same mode of terminase interaction (18, 21, 24, 46). Accordingly, significant antagonism was found between BAY38-4766 and GCV (−350 μM2%), while BDCRB in combination with GCV displayed minimal synergy (+53 μM2%). However, since this minor synergy was coupled with increased cytotoxicity, the overall effect of the BDCRB-GCV combination was considered additive and is then consistent with our results obtained for the letermovir-GCV combination (46).
Besides transplant recipients, HCMV infections are of particular concern among individuals with HIV (2). This prompted us to further explore the therapeutic potential of letermovir by evaluating its in vitro drug-drug interactions in combination with anti-HIV drugs used in HAART regimens. Since neither the Loewe nor the Bliss model can be applied if one of the two drugs in a combination does not by itself act as an inhibitor, data analysis of these experiments to determine whether the EC50 of the active drug in the combination was affected by the second, noninhibitor compound was reduced. For these studies, an attempt was made to mimic drug levels anticipated in patients. Our studies indicate that (i) the anti-HCMV activity of letermovir will not be changed when administered concurrently with maximum therapeutic doses (Cmax) of one of the tested anti-HIV drugs and (ii) the efficacy of the currently most relevant anti-HIV drugs will be unaffected when administered concomitantly with a physiological concentration of letermovir. Although these in vitro results likewise warrant clinical confirmation, the absence of antagonism across a broad panel of commonly used anti-HIV agents is encouraging, since it suggests that letermovir treatment of an active HCMV infection in HCMV-HIV-coinfected patients would not be compromised by concurrent HAART and vice versa.
In summary, none of the analyzed two-drug combinations described in this report yielded clear-cut significant antagonism, and none of the combinations exhibited synergistic cytotoxicity in cell culture. Thus, within the limits of the combinations assessed, our in vitro results suggest that letermovir may indeed offer the potential for combination therapy with approved anti-HCMV drugs and support the use of letermovir in HCMV-HIV-coinfected individuals undergoing HAART.
ACKNOWLEDGMENTS
The excellent technical assistance of Marion Heidtmann, Ilva Leckebusch, and Lars von Gehr is gratefully acknowledged. We thank Rob Saunders, biomed context, and the Merck (MSD) medical writing team for critical reading of the manuscript.
We were all affiliated with AiCuris GmbH Co. KG at the time the work was performed.
REFERENCES
- 1.Sampathkumar P, Paya C. 2007. Pathogenesis in transplant recipients. In Emery VC. (ed), Human cytomegalovirus. International Medical Press, London, United Kingdom. [Google Scholar]
- 2.Steininger C, Puchhammer-Stockl E, Popow-Kraupp T. 2006. Cytomegalovirus disease in the era of highly active antiretroviral therapy (HAART). J Clin Virol 37:1–9. doi: 10.1016/j.jcv.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Kotton CN. 2013. CMV: prevention, diagnosis and therapy. Am J Transplant 13(Suppl 3):S24–S40. doi: 10.1111/ajt.12006. [DOI] [PubMed] [Google Scholar]
- 4.Ljungman P, Hakki M, Boeckh M. 2011. Cytomegalovirus in hematopoietic stem cell transplant recipients. Hematol Oncol Clin North Am 25:151–169. doi: 10.1016/j.hoc.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beam E, Razonable RR. 2012. Cytomegalovirus in solid organ transplantation: epidemiology, prevention, and treatment. Curr Infect Dis Rep 14:633–641. doi: 10.1007/s11908-012-0292-2. [DOI] [PubMed] [Google Scholar]
- 6.Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Danziger-Isakov L, Humar A. 2013. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 96:333–360. doi: 10.1097/TP.0b013e31829df29d. [DOI] [PubMed] [Google Scholar]
- 7.Pertel P, Hirschtick R, Phair J, Chmiel J, Poggensee L, Murphy R. 1992. Risk of developing cytomegalovirus retinitis in persons infected with the human immunodeficiency virus. J Acquir Immune Defic Syndr 5:1069–1074. [PubMed] [Google Scholar]
- 8.Gallant JE, Moore RD, Richman DD, Keruly J, Chaisson RE. 1992. Incidence and natural history of cytomegalovirus disease in patients with advanced human immunodeficiency virus disease treated with zidovudine. Zidovudine Epidemiology Study Group. J Infect Dis 166:1223–1227. [DOI] [PubMed] [Google Scholar]
- 9.Kedhar SR, Jabs DA. 2007. Cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Herpes 14:66–71. [PubMed] [Google Scholar]
- 10.Losina E, Schackman BR, Sadownik SN, Gebo KA, Walensky RP, Chiosi JJ, Weinstein MC, Hicks PL, Aaronson WH, Moore RD, Paltiel AD, Freedberg KA. 2009. Racial and sex disparities in life expectancy losses among HIV-infected persons in the United States: impact of risk behavior, late initiation, and early discontinuation of antiretroviral therapy. Clin Infect Dis 49:1570–1578. doi: 10.1086/644772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Appay V, Fastenackels S, Katlama C, Ait-Mohand H, Schneider L, Guihot A, Keller M, Grubeck-Loebenstein B, Simon A, Lambotte O, Hunt PW, Deeks SG, Costagliola D, Autran B, Sauce D. 2011. Old age and anti-cytomegalovirus immunity are associated with altered T-cell reconstitution in HIV-1-infected patients. AIDS 25:1813–1822. doi: 10.1097/QAD.0b013e32834640e6. [DOI] [PubMed] [Google Scholar]
- 12.Hunt PW, Martin JN, Sinclair E, Epling L, Teague J, Jacobson MA, Tracy RP, Corey L, Deeks SG. 2011. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis 203:1474–1483. doi: 10.1093/infdis/jir060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biron KK. 2006. Antiviral drugs for cytomegalovirus diseases. Antiviral Res 71:154–163. doi: 10.1016/j.antiviral.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Lischka P, Zimmermann H. 2008. Antiviral strategies to combat cytomegalovirus infections in transplant recipients. Curr Opin Pharmacol 8:541–548. doi: 10.1016/j.coph.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Harter G, Michel D. 2012. Antiviral treatment of cytomegalovirus infection: an update. Expert Opin Pharmacother 13:623–627. doi: 10.1517/14656566.2012.658775. [DOI] [PubMed] [Google Scholar]
- 16.Lurain NS, Chou S. 2010. Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev 23:689–712. doi: 10.1128/CMR.00009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Page AK, Jager MM, Iwasenko JM, Scott GM, Alain S, Rawlinson WD. 2013. Clinical aspects of cytomegalovirus antiviral resistance in solid organ transplant recipients. Clin Infect Dis 56:1018–1029. doi: 10.1093/cid/cis1035. [DOI] [PubMed] [Google Scholar]
- 18.Lischka P, Hewlett G, Wunberg T, Baumeister J, Paulsen D, Goldner T, Ruebsamen-Schaeff H, Zimmermann H. 2010. In vitro and in vivo activities of the novel anticytomegalovirus compound AIC246. Antimicrob Agents Chemother 54:1290–1297. doi: 10.1128/AAC.01596-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoelben S, Arns W, Renders L, Hummel J, Muhlfeld A, Stangl M, Fischereder M, Gwinner W, Suwelack B, Witzke O, Durr M, Beelen DW, Michel D, Lischka P, Zimmermann H, Rubsamen-Schaeff H, Budde K. 2014. Preemptive treatment of cytomegalovirus infection in kidney transplant recipients with letermovir: results of a phase 2a study. Transpl Int 27:77–86. doi: 10.1111/tri.12225. [DOI] [PubMed] [Google Scholar]
- 20.Chemaly RF, Ullmann AJ, Stoelben S, Richard MP, Bornhauser M, Groth C, Einsele H, Silverman M, Mullane KM, Brown J, Nowak H, Kolling K, Stobernack HP, Lischka P, Zimmermann H, Rubsamen-Schaeff H, Champlin RE, Ehninger G. 2014. Letermovir for cytomegalovirus prophylaxis in hematopoietic-cell transplantation. N Engl J Med 370:1781–1789. doi: 10.1056/NEJMoa1309533. [DOI] [PubMed] [Google Scholar]
- 21.Goldner T, Hewlett G, Ettischer N, Ruebsamen-Schaeff H, Zimmermann H, Lischka P. 2011. The novel anticytomegalovirus compound AIC246 (letermovir) inhibits human cytomegalovirus replication through a specific antiviral mechanism that involves the viral terminase. J Virol 85:10884–10893. doi: 10.1128/JVI.05265-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marschall M, Stamminger T, Urban A, Wildum S, Ruebsamen-Schaeff H, Zimmermann H, Lischka P. 2012. In vitro evaluation of the activities of the novel anticytomegalovirus compound AIC246 (letermovir) against herpesviruses and other human pathogenic viruses. Antimicrob Agents Chemother 56:1135–1137. doi: 10.1128/AAC.05908-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaul DR, Stoelben S, Cober E, Ojo T, Sandusky E, Lischka P, Zimmermann H, Rubsamen-Schaeff H. 2011. First report of successful treatment of multidrug-resistant cytomegalovirus disease with the novel anti-CMV compound AIC246. Am J Transplant 11:1079–1084. doi: 10.1111/j.1600-6143.2011.03530.x. [DOI] [PubMed] [Google Scholar]
- 24.Goldner T, Hempel C, Ruebsamen-Schaeff H, Zimmermann H, Lischka P. 2014. Geno- and phenotypic characterization of human cytomegalovirus mutants selected in vitro after letermovir (AIC246) exposure. Antimicrob Agents Chemother 58:610–613. doi: 10.1128/AAC.01794-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy SM, Winston DJ, Territo MC, Schiller GJ. 2010. CMV central nervous system disease in stem-cell transplant recipients: an increasing complication of drug-resistant CMV infection and protracted immunodeficiency. Bone Marrow Transplant 45:979–984. doi: 10.1038/bmt.2010.35. [DOI] [PubMed] [Google Scholar]
- 26.Maschke M, Kastrup O, Diener HC. 2002. CNS manifestations of cytomegalovirus infections: diagnosis and treatment. CNS Drugs 16:303–315. doi: 10.2165/00023210-200216050-00003. [DOI] [PubMed] [Google Scholar]
- 27.Griffiths P. 2004. Cytomegalovirus infection of the central nervous system. Herpes 11(Suppl 2):95A–104A. [PubMed] [Google Scholar]
- 28.Wildum S, Paulsen D, Thede K, Ruebsamen-Schaeff H, Zimmermann H. 2013. In vitro and in vivo activities of AIC292, a novel HIV-1 nonnucleoside reverse transcriptase inhibitor. Antimicrob Agents Chemother 57:5320–5329. doi: 10.1128/AAC.01377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borst EM, Wagner K, Binz A, Sodeik B, Messerle M. 2008. The essential human cytomegalovirus gene UL52 is required for cleavage-packaging of the viral genome. J Virol 82:2065–2078. doi: 10.1128/JVI.01967-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borst EM, Messerle M. 2005. Analysis of human cytomegalovirus oriLyt sequence requirements in the context of the viral genome. J Virol 79:3615–3626. doi: 10.1128/JVI.79.6.3615-3626.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andreoni M, Faircloth M, Vugler L, Britt WJ. 1989. A rapid microneutralization assay for the measurement of neutralizing antibody reactive with human cytomegalovirus. J Virol Methods 23:157–167. doi: 10.1016/0166-0934(89)90129-8. [DOI] [PubMed] [Google Scholar]
- 32.Lorz K, Hofmann H, Berndt A, Tavalai N, Mueller R, Schlotzer-Schrehardt U, Stamminger T. 2006. Deletion of open reading frame UL26 from the human cytomegalovirus genome results in reduced viral growth, which involves impaired stability of viral particles. J Virol 80:5423–5434. doi: 10.1128/JVI.02585-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prichard MN, Shipman C Jr. 1990. A three-dimensional model to analyze drug-drug interactions. Antiviral Res 14:181–205. doi: 10.1016/0166-3542(90)90001-N. [DOI] [PubMed] [Google Scholar]
- 34.Chou TC, Talalay P. 1984. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 35.Lai MT, Munshi V, Touch S, Tynebor RM, Tucker TJ, McKenna PM, Williams TM, Distefano DJ, Hazuda DJ, Miller MD. 2009. Antiviral activity of MK-4965, a novel nonnucleoside reverse transcriptase inhibitor. Antimicrob Agents Chemother 53:2424–2431. doi: 10.1128/AAC.01559-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chou TC. 2006. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 37.Azijn H, Tirry I, Vingerhoets J, de Bethune MP, Kraus G, Boven K, Jochmans D, Van CE, Picchio G, Rimsky LT. 2010. TMC278, a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV-1. Antimicrob Agents Chemother 54:718–727. doi: 10.1128/AAC.00986-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greco WR, Bravo G, Parsons JC. 1995. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev 47:331–385. [PubMed] [Google Scholar]
- 39.Bassit L, Grier J, Bennett M, Schinazi RF. 2008. Combinations of 2′-C-methylcytidine analogues with interferon-alpha2b and triple combination with ribavirin in the hepatitis C virus replicon system. Antivir Chem Chemother 19:25–31. doi: 10.1177/095632020801900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Food and Drug Administration Center for Drug Evaluation and Research. 2006. Guidance for industry: antiviral product development—conducting and submitting virology studies to the agency. FDA, Washington, DC. [Google Scholar]
- 41.Volberding PA, Deeks SG. 2010. Antiretroviral therapy and management of HIV infection. Lancet 376:49–62. doi: 10.1016/S0140-6736(10)60676-9. [DOI] [PubMed] [Google Scholar]
- 42.Ilyas JA, Vierling JM. 2014. An overview of emerging therapies for the treatment of chronic hepatitis C. Med Clin North Am 98:17–38. doi: 10.1016/j.mcna.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 43.Butler NJ, Thorne JE. 2012. Current status of HIV infection and ocular disease. Curr Opin Ophthalmol 23:517–522. doi: 10.1097/ICU.0b013e328358ba85. [DOI] [PubMed] [Google Scholar]
- 44.Griffiths PD, Emery VC. 2014. Taming the transplantation troll by targeting terminase. N Engl J Med 370:1844–1846. doi: 10.1056/NEJMe1401567. [DOI] [PubMed] [Google Scholar]
- 45.Delaney WE, Yang H, Miller MD, Gibbs CS, Xiong S. 2004. Combinations of adefovir with nucleoside analogs produce additive antiviral effects against hepatitis B virus in vitro. Antimicrob Agents Chemother 48:3702–3710. doi: 10.1128/AAC.48.10.3702-3710.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evers DL, Komazin G, Shin D, Hwang DD, Townsend LB, Drach JC. 2002. Interactions among antiviral drugs acting late in the replication cycle of human cytomegalovirus. Antiviral Res 56:61–72. doi: 10.1016/S0166-3542(02)00094-3. [DOI] [PubMed] [Google Scholar]