Abstract
We investigated the activity of meropenem-clavulanic acid (MEM-CLA) against 68 Mycobacterium tuberculosis isolates. We included predominantly multi- and extensively drug-resistant tuberculosis (MDR/XDR-TB) isolates, since the activity of MEM-CLA for resistant isolates has previously not been studied extensively. Using Middlebrook 7H10 medium, all but four isolates showed an MIC distribution of 0.125 to 2 mg/liter for MEM-CLA, below the non-species-related breakpoint for MEM of 2 mg/liter defined by EUCAST. MEM-CLA is a potential treatment option for MDR/XDR-TB.
TEXT
Multidrug-resistant and extensively drug-resistant tuberculosis (MDR/XDR-TB) is unrelentingly increasing worldwide. As MDR/XDR-TB is notoriously difficult to treat, already approved drugs, such as trimethoprim-sulfamethoxazole, are being investigated as treatment options (1). The activity of penicillin against Mycobacterium tuberculosis was investigated in the 1940s (2), but β-lactams were deemed ineffective. However, it was later shown that the β-lactamase BlaC causes the hydrolysis of β-lactam antibiotics (3–6). This hydrolysis can be inhibited by the β-lactamase inhibitor clavulanic acid (CLA), which irreversibly inactivates BlaC (6, 7). Meropenem (MEM) is a β-lactam antibiotic of the carbapenem group. Even though MEM is a relatively poor substrate for BlaC (8), MEM on its own shows conflicting evidence regarding antituberculous activity (9–12). Hence, the combination meropenem-clavulanic acid (MEM-CLA) is an interesting treatment alternative for drug-resistant TB, but there is a lack of in vitro and in vivo studies for this combination. The aim of this study was to investigate the in vitro effect of MEM-CLA against M. tuberculosis, predominantly MDR/XDR-TB isolates.
Using Middlebrook 7H10, 94 M. tuberculosis isolates were studied. The isolates consisted of clinical isolates and isolates submitted to the Public Health Agency of Sweden for proficiency drug susceptibility testing, with all isolates being globally sourced. A total of 68 isolates showed sufficient growth to be studied further, and they were categorized into three resistance groups consisting of 36 MDR-TB, 13 XDR-TB, and 19 with mixed resistance patterns (non-MDR/XDR-TB). Strain H37Rv (ATCC 27294) was used as the control. Middlebrook 7H10 agar (Becton Dickinson AB, Stockholm, Sweden) enriched with 10% oleic acid–albumin–dextrose–catalase (OADC) and 5% glycerol was prepared in 14-cm petri dishes, each dish containing 60 ml of agar. A stock solution was prepared by diluting MEM with water, and then applied in serial two-step dilutions, reaching a final antibiotic concentration range of 0.002 to 512 mg/liter of MEM. CLA was added to all dishes at a concentration of 64 mg/liter in order to ensure a sufficient concentration of the β-lactamase inhibitor throughout the whole experiment. Due to the short half-life of CLA in solid media (1.4 days) (13), the concentration of CLA after the first week of our experiment was expected to be around 2 mg/liter, similar to the concentration of CLA seen in serum after a dose of 500 mg/125 mg of amoxicillin-clavulanic acid (AMX-CLA) (14).
The MICs of the 68 M. tuberculosis isolates were determined by inoculating bacterial suspensions onto the Middlebrook 7H10 medium using a 96-stick replicator, as previously described (15). The mycobacterial cultures were incubated at 37°C and growth was evaluated after 3 weeks. The MIC was defined as the lowest concentration with less growth than the 1:100 diluted control. Sterile water was used as a negative control (15).
The MIC distribution of MEM-CLA (expressed as the concentration of MEM) was 0.125 to 32 mg/liter (Fig. 1). All but four isolates had MICs of ≤2 mg/liter of MEM-CLA, which is the non-species-related susceptibility breakpoint of MEM defined by EUCAST (16). The MIC90 and the MIC of the control strain H37Rv were each 1 mg/liter.
FIG 1.
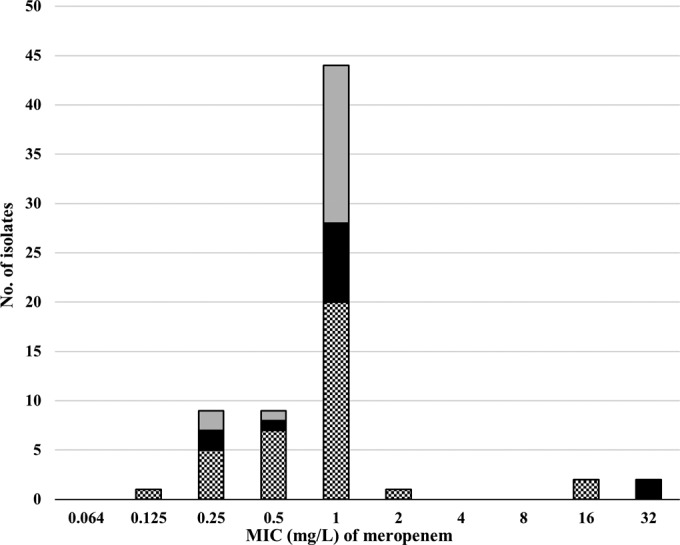
The MIC distribution of meropenem-clavulanic acid for the M. tuberculosis isolates (n = 68) tested using Middlebrook 7H10 medium, shown by bars shaded as follows: non-MDR/XDR-TB (gray); XDR-TB (black), and MDR-TB (hatched). The MIC distribution shows the concentration of meropenem, as the concentration of clavulanic acid was fixed uniformly at 64 mg/liter.
Overall, we observed low MICs for MEM-CLA against M. tuberculosis in vitro, even for highly drug-resistant isolates. The majority of the isolates followed a Gaussian wild-type distribution, with MICs below or equal to the EUCAST non-species-related susceptibility breakpoint for MEM of 2 mg/liter (16). Four isolates (two MDR-TB and two XDR-TB isolates) had very high MIC levels of 16 and 32 mg/liter, drug concentrations unlikely to be achieved in serum even with high-dose regimens (17). The four M. tuberculosis isolates with very high MICs (16 and 32 mg/liter) for MEM-CLA were already highly resistant to other pharmacologically unrelated anti-TB drugs, which could be due to a generally lower permeability, as previously suggested for highly resistant M. tuberculosis isolates (13).
β-Lactam antibiotics have little intrinsic activity against M. tuberculosis (18–21), but are effective in vitro when combined with a β-lactamase inhibitor (18, 20–23). The combination of β-lactam antibiotics and β-lactamase inhibitors has shown anti-TB effect in humans (24, 25), rodents (26), within macrophages (10), and against nonreplicating M. tuberculosis in vitro (8). MEM has poor intrinsic activity (12) but is bactericidal in vitro when combined with CLA (8, 27). The in vitro effect of MEM-CLA has been investigated previously for H37Rv (10, 26) and 13 isolates of M. tuberculosis, all XDR-TB (8). All isolates were susceptible to ≤1 mg/liter of MEM-CLA, which is in line with our tentative breakpoint of 2 mg/liter. However, the concentration of the β-lactamase inhibitor seems important. When Gonzalo et al. used 2.5 mg/liter of CLA, they found a higher MIC of ≤3 mg/liter for MEM-CLA for 28 mainly drug-resistant M. tuberculosis isolates. On the other hand, a synergistic effect was seen when AMX-CLA and MEM were combined, resulting in 25 of 28 M. tuberculosis isolates with MICs of ≤1.28 mg/liter (11). CLA is presently only available in combination with AMX (i.e., Augmentin), necessitating the use of MEM in combination with AMX-CLA for drug-resistant TB.
Clinically, MEM in combination with AMX-CLA has been used in 44 drug-resistant cases (32 MDR-TB, 12 XDR) (27–29). However, the successful drug regimens also contained moxifloxacin and linezolid, thus making the attributable effect of combined MEM and AMX-CLA difficult to assess.
A dose of 1 g MEM thrice daily ensures a coverage of 40% of the dosing interval with free serum drug concentrations exceeding the MIC of 2 mg/liter (fT > MIC ≥ 40%), which is regarded as an appropriate pharmacodynamic target for Gram-positive and Gram-negative bacteria. The need of frequent dosing of β-lactam antibiotics has not been evaluated systematically for M. tuberculosis. Nevertheless, when AMX-CLA was divided into three daily doses, the early bactericidal activity (EBA) was similar to the EBA of fluoroquinolones (1,000 mg/250 mg AMX-CLA three times daily) (24), but the drug combination showed no effect on the EBA when given as a single high dose (3,000 mg/750 mg AMX-CLA once daily) (30). The drawback of MEM-CLA is that it requires intravenous access. The advantages are tolerable side effects (31), low plasma protein binding (32), and good lung penetration (33).
Our study was limited by the absence of wild-type M. tuberculosis isolates, the use of only one method of MIC determination (Middlebrook 7H10), and a high exclusion rate of isolates due to poor growth (27%). Perhaps the use of Middlebrook 7H11 media, considered better for the growth of drug-resistant strains (34), would have resulted in improved growth and a lower exclusion rate. However, most of the excluded isolates displayed a higher degree of susceptibility to CLA alone in a concurrent experiment (data not shown). This raises the question of whether the high concentration of CLA contributed to the poor growth of the excluded isolates. Previous studies have shown no (19, 21) or very little (13) intrinsic activity of CLA, although the concentrations tested (16 mg/liter and 19.9 mg/liter, respectively) were lower than the concentration used in our study and liquid media was used. Nevertheless, the excluded isolates were distributed among all three of our resistance groups, and the exclusion rate is unlikely to have compromised the validity of our results.
In conclusion, our study is unique because of the high number of MDR/XDR-TB isolates investigated showing clinically achievable MICs of MEM-CLA. MEM-CLA is a potential treatment option for selected cases of multidrug-resistant tuberculosis.
ACKNOWLEDGMENTS
This work was supported by the Swedish Society of Medicine (grant SLS 169241 presented to K.Ä.), and through support to T.S. by the Marianne and Marcus Wallenberg Foundation, the Swedish Heart and Lung Foundation (Oscar II Jubilée Foundation), the Swedish Society of Antimicrobial Chemotherapy, and the Research Council of Southeast Sweden (FORSS).
We thank Brian Davies for language correction.
REFERENCES
- 1.Davies Forsman L, Schon T, Simonsson US, Bruchfeld J, Larsson M, Jureen P, Sturegard E, Giske CG, Angeby K. 2014. Intra- and extracellular activities of trimethoprim-sulfamethoxazole against susceptible and multidrug-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother 58:7557–7559. doi: 10.1128/AAC.02995-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham EP, Chain E, Fletcher CM, Florey HW, Gardner AD, Heatley NG, Jennings MA. 1992. Further observations on penicillin. 1941. Eur J Clin Pharmacol 42:3–9. [PubMed] [Google Scholar]
- 3.Zhang Y, Steingrube VA, Wallace RJ Jr. 1992. Beta-l actamase inhibitors and the inducibility of the beta-lactamase of Mycobacterium tuberculosis. Am Rev Respir Dis 145:657–660. doi: 10.1164/ajrccm/145.3.657. [DOI] [PubMed] [Google Scholar]
- 4.Voladri RK, Lakey DL, Hennigan SH, Menzies BE, Edwards KM, Kernodle DS. 1998. Recombinant expression and characterization of the major beta-lactamase of Mycobacterium tuberculosis. Antimicrob Agents Chemother 42:1375–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flores AR, Parsons LM, Pavelka MS Jr. 2005. Genetic analysis of the beta-lactamases of Mycobacterium tuberculosis and Mycobacterium smegmatis and susceptibility to beta-lactam antibiotics. Microbiology 151:521–532. doi: 10.1099/mic.0.27629-0. [DOI] [PubMed] [Google Scholar]
- 6.Hugonnet JE, Blanchard JS. 2007. Irreversible inhibition of the Mycobacterium tuberculosis beta-lactamase by clavulanate. Biochemistry 46:11998–12004. doi: 10.1021/bi701506h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tremblay LW, Hugonnet JE, Blanchard JS. 2008. Structure of the covalent adduct formed between Mycobacterium tuberculosis beta-lactamase and clavulanate. Biochemistry 47:5312–5316. doi: 10.1021/bi8001055. [DOI] [PubMed] [Google Scholar]
- 8.Hugonnet JE, Tremblay LW, Boshoff HI, Barry CE III, Blanchard JS. 2009. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science 323:1215–1218. doi: 10.1126/science.1167498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watt B, Edwards JR, Rayner A, Grindey AJ, Harris G. 1992. In vitro activity of meropenem and imipenem against mycobacteria: development of a daily antibiotic dosing schedule. Tuber Lung Dis 73:134–136. doi: 10.1016/0962-8479(92)90145-A. [DOI] [PubMed] [Google Scholar]
- 10.England K, Boshoff HI, Arora K, Weiner D, Dayao E, Schimel D, Via LE, Barry CE III. 2012. Meropenem-clavulanic acid shows activity against Mycobacterium tuberculosis in vivo. Antimicrob Agents Chemother 56:3384–3387. doi: 10.1128/AAC.05690-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalo X, Drobniewski F. 2013. Is there a place for beta-lactams in the treatment of multidrug-resistant/extensively drug-resistant tuberculosis? Synergy between meropenem and amoxicillin/clavulanate. J Antimicrob Chemother 68:366–369. doi: 10.1093/jac/dks395. [DOI] [PubMed] [Google Scholar]
- 12.Coban AY, Bilgin K, Tasdelen Fisgin N, Uzun M, Durupinar B. 2008. Effect of meropenem against multidrug-resistant Mycobacterium tuberculosis. J Chemother 20:395–396. doi: 10.1179/joc.2008.20.3.395. [DOI] [PubMed] [Google Scholar]
- 13.Chambers HF, Moreau D, Yajko D, Miick C, Wagner C, Hackbarth C, Kocagoz S, Rosenberg E, Hadley WK, Nikaido H. 1995. Can penicillins and other beta-lactam antibiotics be used to treat tuberculosis? Antimicrob Agents Chemother 39:2620–2624. doi: 10.1128/AAC.39.12.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burke A, Cunha MD. 2008. Antibiotic essentials, 7th ed. Jones and Bartlett Learning, Burlington, MA. [Google Scholar]
- 15.Schon T, Jureen P, Giske CG, Chryssanthou E, Sturegard E, Werngren J, Kahlmeter G, Hoffner SE, Angeby KA. 2009. Evaluation of wild-type MIC distributions as a tool for determination of clinical breakpoints for Mycobacterium tuberculosis. J Antimicrob Chemother 64:786–793. doi: 10.1093/jac/dkp262. [DOI] [PubMed] [Google Scholar]
- 16.EUCAST 2015. Clinical breakpoints. http://www.eucast.org/clinical_breakpoints/.
- 17.Dulhunty JM, Roberts JA, Davis JS, Webb SA, Bellomo R, Gomersall C, Shirwadkar C, Eastwood GM, Myburgh J, Paterson DL, Lipman J. 2013. Continuous infusion of beta-lactam antibiotics in severe sepsis: a multicenter double-blind, randomized controlled trial. Clin Infect Dis 56:236–244. doi: 10.1093/cid/cis856. [DOI] [PubMed] [Google Scholar]
- 18.Cynamon MH, Palmer GS. 1983. In vitro activity of amoxicillin in combination with clavulanic acid against Mycobacterium tuberculosis. Antimicrob Agents Chemother 24:429–431. doi: 10.1128/AAC.24.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abate G, Miorner H. 1998. Susceptibility of multidrug-resistant strains of Mycobacterium tuberculosis to amoxycillin in combination with clavulanic acid and ethambutol. J Antimicrob Chemother 42:735–740. doi: 10.1093/jac/42.6.735. [DOI] [PubMed] [Google Scholar]
- 20.Segura C, Salvado M, Collado I, Chaves J, Coira A. 1998. Contribution of beta-lactamases to beta-lactam susceptibilities of susceptible and multidrug-resistant Mycobacterium tuberculosis clinical isolates. Antimicrob Agents Chemother 42:1524–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorg TB, Cynamon MH. 1987. Comparison of four beta-lactamase inhibitors in combination with ampicillin against Mycobacterium tuberculosis. J Antimicrob Chemother 19:59–64. doi: 10.1093/jac/19.1.59. [DOI] [PubMed] [Google Scholar]
- 22.Dincer I, Ergin A, Kocagoz T. 2004. The vitro efficacy of beta-lactam and beta-lactamase inhibitors against multidrug resistant clinical strains of Mycobacterium tuberculosis. Int J Antimicrob Agents 23:408–411. doi: 10.1016/j.ijantimicag.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Casal M, Rodriguez F, Benavente M, Luna M. 1986. In vitro susceptibility of Mycobacterium tuberculosis, Mycobacterium fortuitum and Mycobacterium chelonei to augmentin. Eur J Clin Microbiol 5:453–454. doi: 10.1007/BF02075706. [DOI] [PubMed] [Google Scholar]
- 24.Chambers HF, Kocagoz T, Sipit T, Turner J, Hopewell PC. 1998. Activity of amoxicillin/clavulanate in patients with tuberculosis. Clin Infect Dis 26:874–877. doi: 10.1086/513945. [DOI] [PubMed] [Google Scholar]
- 25.Nadler JP, Berger J, Nord JA, Cofsky R, Saxena M. 1991. Amoxicillin-clavulanic acid for treating drug-resistant Mycobacterium tuberculosis. Chest 99:1025–1026. doi: 10.1378/chest.99.4.1025. [DOI] [PubMed] [Google Scholar]
- 26.Veziris N, Truffot C, Mainardi JL, Jarlier V. 2011. Activity of carbapenems combined with clavulanate against murine tuberculosis. Antimicrob Agents Chemother 55:2597–2600. doi: 10.1128/AAC.01824-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Payen MC, De Wit S, Martin C, Sergysels R, Muylle I, Van Laethem Y, Clumeck N. 2012. Clinical use of the meropenem-clavulanate combination for extensively drug-resistant tuberculosis. Int J Tuberc Lung Dis 16:558–560. doi: 10.5588/ijtld.11.0414. [DOI] [PubMed] [Google Scholar]
- 28.Dauby N, Muylle I, Mouchet F, Sergysels R, Payen MC. 2011. Meropenem/clavulanate and linezolid treatment for extensively drug-resistant tuberculosis. Pediatr Infect Dis J 30:812–813. doi: 10.1097/INF.0b013e3182154b05. [DOI] [PubMed] [Google Scholar]
- 29.De Lorenzo S, Alffenaar JW, Sotgiu G, Centis R, D'Ambrosio L, Tiberi S, Bolhuis MS, van Altena R, Viggiani P, Piana A, Spanevello A, Migliori GB. 2013. Efficacy and safety of meropenem-clavulanate added to linezolid-containing regimens in the treatment of MDR-/XDR-TB. Eur Respir J 41:1386–1392. doi: 10.1183/09031936.00124312. [DOI] [PubMed] [Google Scholar]
- 30.Donald PR, Sirgel FA, Venter A, Parkin DP, Van de Wal BW, Barendse A, Smit E, Carman D, Talent J, Maritz J. 2001. Early bactericidal activity of amoxicillin in combination with clavulanic acid in patients with sputum smear-positive pulmonary tuberculosis. Scand J Infect Dis 33:466–469. doi: 10.1080/00365540152029954. [DOI] [PubMed] [Google Scholar]
- 31.Norrby SR, Newell PA, Faulkner KL, Lesky W. 1995. Safety profile of meropenem: international clinical experience based on the first 3125 patients treated with meropenem. J Antimicrob Chemother 36(Suppl):207–223. [DOI] [PubMed] [Google Scholar]
- 32.Harrison MP, Moss SR, Featherstone A, Fowkes AG, Sanders AM, Case DE. 1989. The disposition and metabolism of meropenem in laboratory animals and man. J Antimicrob Chemother 24(Suppl):265–277. [DOI] [PubMed] [Google Scholar]
- 33.Tomaselli F, Maier A, Matzi V, Smolle-Juttner FM, Dittrich P. 2004. Penetration of meropenem into pneumonic human lung tissue as measured by in vivo microdialysis. Antimicrob Agents Chemother 48:2228–2232. doi: 10.1128/AAC.48.6.2228-2232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohn ML, Waggoner RF, McClatchy JK. 1968. The 7H11 medium for the cultivation of mycobacteria. Am Rev Respir Dis 98:295–296. [DOI] [PubMed] [Google Scholar]


