Abstract
A population pharmacokinetic (PK) analysis was conducted to characterize the voriconazole pharmacokinetic profiles in immunocompromised Japanese pediatric subjects and to compare them to those in immunocompromised non-Japanese pediatric subjects. A previously developed two-compartment pharmacokinetic model with first-order absorption and mixed linear and nonlinear elimination adequately described the voriconazole intravenous and oral data from Japanese pediatric subjects with few modifications. Bayesian priors were applied to this analysis by using the NONMEM routine NWPRI, which allowed priors for the fixed-effect parameter vector and variance matrix of the random-effect parameters to be a normal distribution and an inverse Wishart distribution, respectively. Large intersubject variabilities in oral bioavailability and voriconazole exposure were observed in these pediatric subjects. The mean oral bioavailability estimated in Japanese pediatric subjects was 73% (range, 17% to 99%), which is consistent with the reported estimates of 64% in the previous model and less than what was originally estimated for healthy adults—96%. Voriconazole exposures in Japanese pediatric subjects were generally comparable to those in non-Japanese pediatric subjects receiving the same dosing regimens, given the large intersubject variability. Consistent with the previous findings, the CYP2C19 genotyping status did not have a clinically relevant effect on voriconazole exposure in Japanese pediatric subjects, although it was identified as a covariate in the model to help explain the intersubject variability in voriconazole exposure. The CYP2C19 genotyping status alone does not warrant dose adjustment of voriconazole. No other factors besides age and weight were identified to explain the PK variability of voriconazole.
INTRODUCTION
Voriconazole is a broad-spectrum triazole antifungal agent for the treatment of patients with opportunistic fungal infections, such as invasive aspergillosis (1). It exhibits potent and wide-ranging in vitro activity, including fungicidal activity against Aspergillus species and other fungi. Immunocompromised children, particularly those with hematological malignancies, are at higher risk for systemic fungal infections, which significantly affect the prognosis of such patients. Voriconazole is suggested as a treatment option for fungal infections in children in Japanese and overseas guidelines.
Voriconazole is extensively metabolized by and is also an inhibitor of the cytochrome P450 (CYP) isozymes CYP2C19 (major), CYP2C9, and CYP3A4 (2), which results in extensive drug interactions with concomitant medications. Voriconazole exhibits nonlinear pharmacokinetics (PK), and intersubject variability in voriconazole exposure is high. It has been demonstrated that in healthy adults CYP2C19 genotype, gender, and age are key factors that help explain this variability (3). CYP2C19 exhibits genetic polymorphism, and approximately 3% to 5% of Caucasians and African Americans and up to 20% of Asians are poor metabolizers (PMs) (4, 5).
Previously, a PK model was developed based on pooled data from non-Japanese immunocompromised children (2 to <12 years old), immunocompromised adolescents (12 to <17 years old), and healthy adults (6). According to the deterministic simulations based on individual parameter estimates from this PK model, the predicted total exposure (area under the concentration-time curve over a 12-hour dosing interval [AUC12]) in children following a 9-mg/kg of body weight intravenous (i.v.) loading dose was comparable to that in adults following a 6-mg/kg i.v. loading dose, the predicted AUC12 in children following 8 mg/kg i.v. every 12 h (q12h) was comparable to that in adults following 4 mg/kg i.v. q12h, and the predicted AUC12 in children following 9 mg/kg (maximum, 350 mg) q12h orally (p.o.) was comparable to that in adults following 200 mg p.o. q12h. The dosing in young adolescents (12 to 14 years old) was dependent on their weight; adolescents weighing <50 kg should be dosed like children and adolescents weighing ≥50 kg should be dosed like adults in order to achieve a voriconazole AUC12 comparable to those in adults.
Due to limited experience with and safety information on the proposed dosing regimens of voriconazole in Japanese children, an additional PK study was conducted to evaluate optimal doses of voriconazole in Japanese pediatric subjects (7). An exploratory analysis of the effect of CYP2C19 genetic polymorphisms and predicted metabolic enzyme activity on voriconazole plasma concentrations was performed in this study. The voriconazole exposure results based on the noncompartmental analysis method and the safety results from the study have been published elsewhere (7).
Here, we further analyzed these voriconazole concentration data using a population PK modeling approach to assess if the previously developed PK model could fit the concentration data from these immunocompromised Japanese pediatric subjects, to predict individual exposure parameters (e.g., AUC12 and trough concentration [Cmin]) based on the final PK parameter estimates, to identify and characterize patient factors that influence the variability in the PK of voriconazole, and to compare the voriconazole exposure between Japanese and non-Japanese pediatric populations at the same dosing regimens using the same modeling approach.
MATERIALS AND METHODS
Study design.
This was an open-label, multicenter, phase 2 study to evaluate the PK, safety, and tolerability of voriconazole following an i.v.-to-oral switch regimen in immunocompromised Japanese children aged 2 to <15 years who were at high risk for systemic fungal infection. Voriconazole was administered according to the dosing regimens listed in Table 1. Voriconazole i.v. doses were administered at an infusion rate of about 3 mg/kg/h, and voriconazole oral doses (powder for oral suspension) were administered at least 1 h before or 1 h after a meal. Another detailed description of this study was published separately (7). This study was conducted in six centers in Japan, in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and in compliance with the Independent Ethics Committee, informed consent regulations, and the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Good Clinical Practices (GCP) Guidelines. In addition, all local regulatory requirements were followed, in particular, those affording greater protection to the safety of trial participants. Written informed consent was obtained prior to the subject entering the study.
TABLE 1.
Study design
| Population | Dosing regimen |
Planned PK sampling |
|||
|---|---|---|---|---|---|
| Day(s) | Dose, route, dosing interval | Day(s) | No. of samples/subject | Sampling plan | |
| Age 2 to <12 yr or 12 to <15 yr and wt <50 kg | 1 | 9 mg/kg i.v. q12h | |||
| 2–7 | 8 mg/kg i.v. q12h | 2 | 1 | Predose | |
| 7 | 7 | Predose; 1 h after start of infusion; 10–20 min after end of infusion; and 4, 6, 8, and 12 h after start of infusion | |||
| 8–14 | 9 mg/kg p.o. q12h (maximum, 350 mg p.o. q12h) | 14 | 7 | Predose and 1, 2, 4, 6, 8, and 12 h postdose | |
| Age 12 to <15 yr and wt ≥50 kg | 1 | 6 mg/kg i.v. q12h | |||
| 2–7 | 4 mg/kg i.v. q12h | 2 | 1 | Predose | |
| 7 | 7 | Predose; 1 h after start of infusion; 10–20 min after end of infusion; and 4, 6, 8, and 12 h after start of infusion | |||
| 8–14 | 200 mg p.o. q12h | 14 | 7 | Predose and 1, 2, 4, 6, 8, and 12 h postdose | |
Population pharmacokinetic modeling method.
Concentration-time data were analyzed using the nonlinear mixed-effect modeling software package NONMEM (version 7 level 2; ICON Development Solutions, Hanover, MD, USA). The software R 2.12.2 was used for exploratory analysis and to assist the model-building process. Perl-speaks-NONMEM (PsN) version 3.5.4 was used for model evaluation. The R package Xpose version 4.3.2 (http://xpose.sourceforge.net) was used to support the analysis. The first-order conditional estimation (FOCE) method was employed to estimate all model parameters.
A previously developed PK model based on data from 5 studies, including children, adolescents, and adults, was adapted to the current data (6). This was a two-compartment model with first-order absorption and mixed linear and time-dependent nonlinear (Michaelis-Menten) elimination. All clearance terms (maximum elimination rate [Vmax] at 1 h after the start of dosing [Vmax,1], linear clearance [CL], and intercompartmental clearance [Q]) were scaled allometrically, using weight to a power of 0.75, and all volume terms (volumes of distribution for the central and peripheral compartments, V2 and V3) were scaled allometrically, using weight to a power of 1. In the previous model, the differences in PK model structure between children, adolescents, and adults were the covariate effect of CYP2C19 genotype on the maximum fraction of Vmax inhibition (Vmax,inh) and the correlation between Km and Vmax (6). The covariate effect of the CYP2C19 genotype (ultrarapid metabolizer [UM]/extensive metabolizer [EM] versus heterozygous extensive metabolizer [HEM]/PM) on Vmax,inh was included for adults only, where HEM/PM were predicted to have full inhibition (Vmax,inh = 100%; Vmax = 0) of the nonlinear pathway in adults at maintenance dosing. In addition, for both adolescents and adults, Km and Vmax were predicted to be 100% correlated within an individual.
The final model used the FOCE method on log-transformed concentrations. Intersubject variability was estimated using exponential random effects, additive random effects on a logit scale, or Manly-transformed random effects, as appropriate. Within-subject variability (residual error) was modeled as additive errors on the log-transformed concentrations (analogous to the proportional-error model on the untransformed concentrations).
Since the previous model was complex and used various parameters and the number of subjects (n = 21) in this study was limited compared to the previous 173 subjects, prior information from the previous final model was included in the current PK model to stabilize the model (8).
To leverage prior information from the previous studies, Bayesian priors were applied to this analysis by using the NONMEM routine NWPRI, which allowed priors for the fixed-effect parameter vector θ and the variance matrix of the random-effect parameter Ω to be a normal distribution and an inverse Wishart distribution, respectively (9). This analysis used both normal and inverse Wishart priors. The normal prior was specified with mean, θ̂, and variance, Γ̂. The θ̂ value was derived from parameter estimates of the fixed-effect parameters in the previous analysis. The Γ̂ value was obtained from the variance of the parameter estimates based on nonparametric bootstrap of the previous analysis, where the off-diagonal elements of Γ̂ were assumed to be zero. The inverse Wishart prior was specified with mode Ω̂ and degree of freedom (df). The Ω̂ value was obtained from the variance matrix estimate in the previous analysis, whereas the df was elicited from the number of subjects in the previous studies.
Assessment of model predictive performance (validation).
The PK models were assessed with goodness-of-fit plots, including prediction-corrected visual predictive checks (pcVPCs) (10). For pcVPC evaluations, 1,000 replicates of the study design were simulated.
Evaluation of estimated exposure parameters.
In order to predict voriconazole exposures, a simulation data set for subjects who received the recommended doses defined in the study (Table 1) was created using dense sampling time points, with individual estimated PK parameters incorporated. Then, the concentrations were estimated, and AUC12 values were computed using numerical integration in NONMEM; the Cmax was estimated as the maximum concentration; the Cmin was estimated at 12 h postdose at steady state.
RESULTS
Data for analysis.
The voriconazole plasma concentrations were available for 21 subjects (276 observations): 152 concentration records were obtained from 21 subjects during the i.v. period, and 124 concentration records were obtained from 18 subjects during the oral period.
The demographics tested as potential covariates (predictors) for these data are presented in Table 2.
TABLE 2.
Demographic characteristics
| Characteristic | Median (range) or counts |
|---|---|
| Body wt (kg) | 31.5 (11.5–55.2) |
| Body mass index (kg/m2) | 16.4 (11.9–22.9) |
| Age (yr) | 10 (3–14) |
| Gender | 9 males, 12 females |
| CYP2C19 genotyping status | 9 EM, 2 PM, 10 HEM |
Final model.
The normal-inverse-Wishart prior approach could not be fully implemented because the previously developed model did not converge successfully and the bootstrap results from the previous model could not support a full block covariance matrix. Therefore, the assumption was made that there was no covariance between the fixed-effect parameters. Moreover, the degrees of freedom for random-effects parameter uncertainty reflected the number of subjects in the previous data set (n = 173).
When the previously developed voriconazole PK model for children was fitted to the current data as a base model, the condition number was over 1,000 (i.e., 4,163), and ETA (η, empirical Bayes prediction of the intersubject random effect) distributions did not show normal distribution.
When the covariate effect of the CYP2C19 genotype on Vmax,inh and Km-Vmax correlation were included in the base model (similar to what was implemented for adults in the previous PK model), the condition number and diagnostic plots (including ETA distributions) significantly improved. The condition number is 21.3, suggesting no notable colinearity. The parameter estimates for the final voriconazole model are presented in Table 3.
TABLE 3.
Voriconazole parameter estimates from the population PK model and the prior distributions used in the analysis
| Parameter | Mean (%RSEa) of normal prior for θ | Estimate for θ (%RSE) | Interindividual variability | Mode of inverse Wishart prior for Ωh | Estimate for Ω SDb (%RSE) |
|---|---|---|---|---|---|
| Km (μg/ml) | 1.15 (28) | 0.922 (30) | Km,i = Km × exp(ηKm-Vmax,1) | ||
| ωKm-Vmax,1 | 1.85 | 1.36 (11) | |||
| Vmax,1 (mg/h/70 kgc) | 114 (16) | 118 (14) | Vmax,1,i = Vmax,1 × exp(ηKm-Vmax,1 × θVmax,scale) | ||
| ωKm-Vmax,1 | 1.85 | 1.36 (11) | |||
| θVmax,scale | 1.25 (12) | ||||
| Vmax,inhd | NSe | 2.61 (19) | NSe | NS | |
| T50 (h) | 2.41(6.6) | 2.45 (6.3) | NS | NS | |
| CL (liters/h/70 kgc) | 6.16 (13) | 6.02 (11) | CLi = CL × exp(ηCL) | ||
| ωCL | 0.509 | 0.696 (10) | |||
| V2 (liters/70 kg) | 79.0 (3.1) | 75.0 (3.2) | V2,i = V2 × exp(ηV2) | ||
| ωV2 | 0.0186 | 0.142 (11) | |||
| V3 (liters/70 kg) | 103 (6.0) | 101 (6.1) | V3,i = V3 × exp(ηV3) | ||
| ωV3 | 0.591 | 0.784 (11) | |||
| Q (liters/h/70 kgc) | 25.4 (4.2) | 24.6 (4.4) | Qi = Q × exp(ηQ) | ||
| ωQ | 0.180 | 0.434 (11) | |||
| F1d | 0.585 (13) | 0.597 (13) | logit(F1,i) = logit(F1) + ETATRf | ||
| ωF1 | 2.79 | 1.69 (11) | |||
| θBC-F | 0.367 (42) | 0.330 (23) | |||
| ka (1/h) | 1.19 (20) | 1.38 (14) | ka,i = ka × exp(ηka) | ||
| ωka | 0.806 | 0.894 (11) | |||
| Alag (h)j | 0.120 (2.8) | 0.121 (2.8) | NS | NS | |
| Residual errorg | NAi | 0.239 (5.8) | |||
| Corr(ηKm,ηV3) | −0.51 | −0.52 (17) | |||
| Corr(ηKm,ηCL) | 0.48 | 0.26 (30) | |||
| Corr(ηV3,ηCL) | 0.27 | 0.15 (52) | |||
| Corr(ηKm,ηQ) | −0.60 | −0.61 (15) | |||
| Corr(ηV3,ηQ) | 0.87 | 0.88 (12) | |||
| Corr(ηCL,ηQ) | 0.18 | 0.097 (77) |
%RSE (percent relative standard error of the estimate) = SE/parameter estimate × 100 (for variability and correlation terms, this is the %RSE of the variance and covariance estimate, respectively). SE in the original model was computed based on a limited nonparametric bootstrap (n = 10), and SE in this study was obtained from $COVARIANCE reported by NONMEM.
Standard deviation of random effects (ω).
Note that a power function of 0.75 was applied for clearance terms, i.e., the relationship to weight is not linear.
logit(Vmax,inh), Vmax,inh = exp(θVmax,inh)/[1 + exp(θVmax,inh)]. Vmax,inh is equal to 100% if CYP2C19 is equal to HEM or PM. logit(F1), F1 = exp(θF1)/[1+exp(θF1)].
NS, not supported in the model.
ETATR = {[exp(ηF1)]θBC-F1 − 1}/θBC-F1.
Residual error was estimated independently of the original model.
Four Ω priors were specified: prior for Km, CL, V3, and Q (df = 169); prior for V2 (df = 172); prior for F1 (df = 172); and prior for ka (df = 172).
NA, not applicable.
Alag, absorption lag time.
Overall, the parameter estimates are in agreement with those reported previously, with the exception of Vmax,inh in association with the inclusion of the CYP2C19 genotype effect (Table 3). For example, in a typical 20-kg Japanese child with CYP2C19 EM status or HEM/PM status, the maximum fraction of the inhibition in Vmax (Vmax,inh) compared to the Vmax value at 1 h was estimated to be 93% or 100% in the current model (Table 4), while it was 75% in the original model for a 20-kg child regardless of the CYP2C19 genotyping status (6).
TABLE 4.
Voriconazole PK parameter estimates for typical pediatric subjects
| Parameter | Wt or status of subject | Typical value | IIVa (CV%) |
|---|---|---|---|
| Km (μg/ml) | 0.922 | 136 | |
| Vmax,1(mg/h)b | 50 kg | 91.7 | 170 |
| 20 kg | 46.1 | ||
| Vmax,inh (%)c | UM/EM | 93.2 | NSe |
| PM/HEM | 100 | ||
| T50 (h) | 2.45 | NS | |
| CL (liters/h)b | 50 kg | 4.68 | 70 |
| 20 kg | 2.35 | ||
| V2 (liters)b | 50 kg | 53.6 | 14 |
| 20 kg | 21.4 | ||
| V3 (liters)b | 50 kg | 72.1 | 78 |
| 20 kg | 28.9 | ||
| Q (liters /h)b | 50 kg | 19.1 | 43 |
| 20 kg | 9.61 | ||
| F1 (%)c | 64.5 | −d | |
| Alag (h) | 0.121 | NS | |
| ka (h−1) | 1.38 | 89 | |
| Residual error (CV%) | 23.9 |
IIV (CV%), interindividual variability (expressed as percent coefficient of variation). The model for IIV estimation also included covariance between Km, Vmax,1, CL, V3 and Q. The IIV for Km and the IIV for Vmax,1 were 100% correlated.
The values reflect the effect of allometric scaling.
Vmax,inh and F1 were modeled on the logit scale {exp(q)/[1 + exp(q)]}, where q estimates were 2.61 (Vmax,inh) and 0.597 (F1), respectively.
The variability in F1 was Manly transformed (11). ETATR = {[exp(ηF1)]0.330 − 1}/0.330; logit(F1,i) = logit(F1) + ETATR. The SD of ηF1 was estimated to be 1.69.
NS, not supported in the model.
Parameters were estimated with generally good precision. It was noted that the estimated value of η shrinkage in the oral absorption rate constant (ka) was slightly high (i.e., >40%), as would be expected for a complex model with a small sample size to inform the parameter, especially for absorption. Assessment of the model performance metrics suggests acceptable model performance.
Attempts to identify new covariates (e.g., the CYP2C19 genotype, age, gender, and liver function parameters) on other voriconazole PK parameters were not successful, and no trend was observed based on graphical evaluations.
Predictive performance.
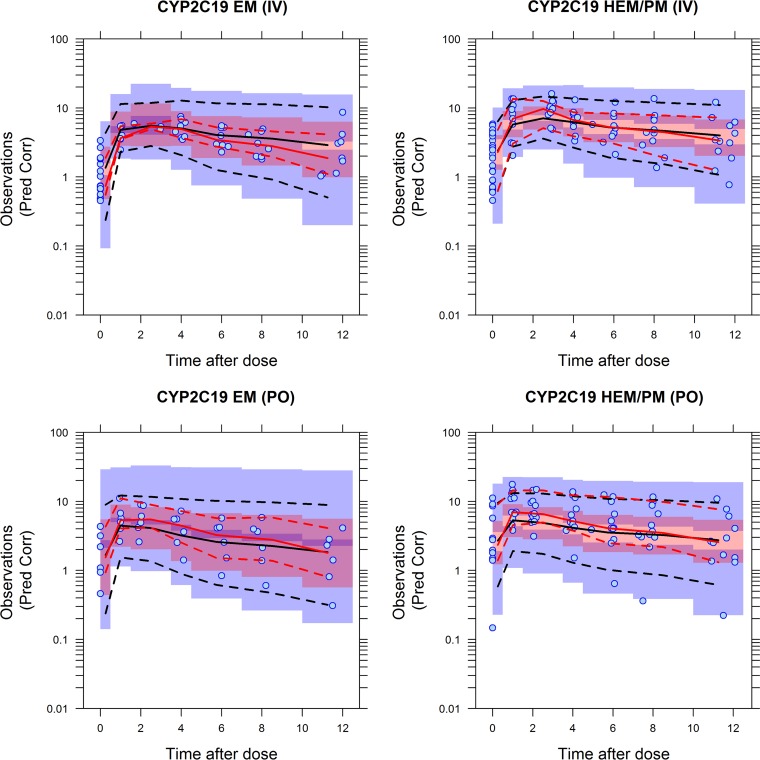
The pcVPC plots of i.v. data and p.o. data versus time after last dose stratified by CYP2C19 genotype status are presented in Fig. 1. The percentiles of the prediction-corrected observed voriconazole concentrations were compared with the same metrics derived from 1,000 simulations of individuals from the original data set. An appropriate structural and statistical model would have the observed percentiles within the 95% confidence intervals of the corresponding percentiles of the simulated data. As shown in Fig. 1, the model describes the data adequately for the different routes of administration and CYP2C19 genotype status.
FIG 1.
pcVPC with observed and simulated voriconazole median and 10th and 90th percentile prediction-corrected (Pred Corr) concentrations and 80% prediction interval stratified by CYP2C19 genotype status. The symbols are observed data. The black solid and dashed lines and red solid and dashed lines represent the medians and 10th and 90th percentiles of the simulated and observed voriconazole concentrations, respectively. The bands around the simulated percentiles represent the 95% confidence intervals of the simulated concentrations.
Summary of voriconazole exposure by CYP2C19 genotype.
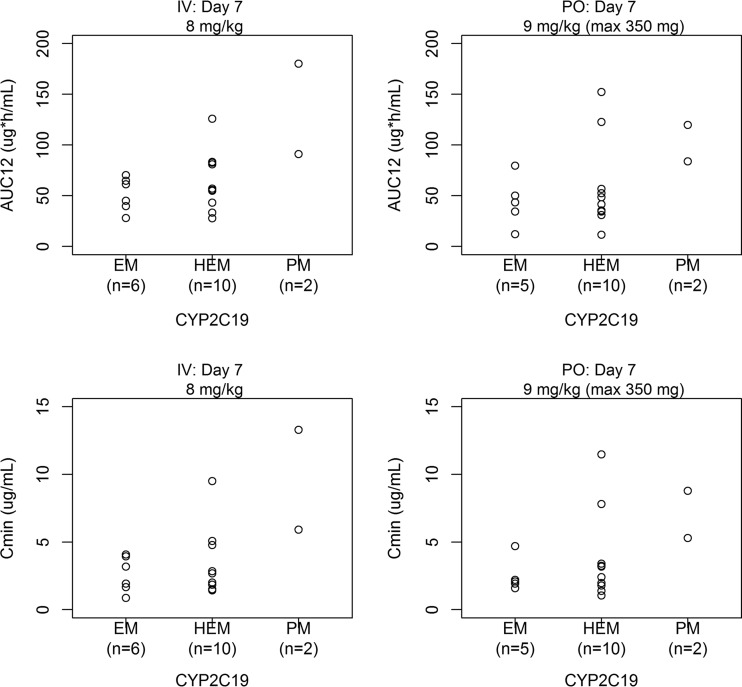
Comparisons of estimated voriconazole AUC12 and Cmin by CYP2C19 genotype status in these Japanese pediatric subjects at the 8-mg/kg i.v. dose and 9-mg/kg p.o. dose are presented in Fig. 2. Although there were some visual trends with CYP2C19 genotype status, i.e., higher average exposure in subjects who were HEMs/PMs, the voriconazole exposures varied widely within each genotype and overlapped considerably across CYP2C19 genotypes.
FIG 2.
Comparison of estimated AUC12 (top) and Cmin (bottom) by CYP2C19 genotype status.
Correlation between voriconazole AUC12 and Cmin.
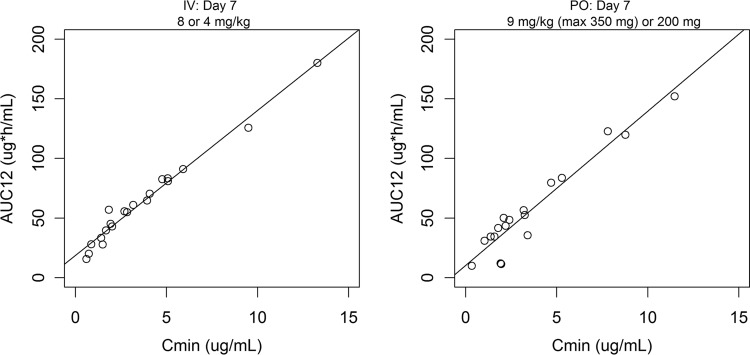
There was good correlation between the estimated voriconazole AUC12 and Cmin in this analysis (Fig. 3), which is consistent with the noncompartmental analysis results (7).
FIG 3.
Correlation between estimated voriconazole AUC12 versus Cmin at steady state by administration route. The solid lines are from a linear-regression model (the R2 values, where R2 is the coefficient of determination, are 0.976 and 0.920 for the i.v. and p.o. routes, respectively).
Exposure comparison between Japanese and non-Japanese pediatric subjects.
As shown in Table 5, the estimated average voriconazole exposures in Japanese pediatric subjects at 8 mg/kg i.v. q12h and 9 mg/kg (maximum, 350 mg) p.o. q12h in this study were higher than the simulated average exposures in non-Japanese pediatric subjects using the original model (6). Nonetheless, the individual AUC12 values estimated in these Japanese pediatric subjects were within the range of simulated exposures in non-Japanese pediatric subjects, except for one Japanese adolescent subject with higher exposure, who was a 12-year-old 44.7-kg male with CYP2C19 PM status (Fig. 4). This indicated that voriconazole exposures in Japanese pediatric subjects were generally comparable to those in non-Japanese pediatric subjects receiving the same dosing regimens, given the large intersubject variability.
TABLE 5.
Overall summary of estimated voriconazole exposure parameters in Japanese pediatric subjects (current model) compared to non-Japanese pediatric subjects (original model)
| Parameter | Value [geometric mean (CV%)]a |
|||
|---|---|---|---|---|
| 8 mg/kg i.v. q12h |
9 mg/kg p.o. q12h (maximum, 350 mg) |
|||
| Non-Japaneseb | Japanesec | Non-Japaneseb | Japanesec | |
| AUC12 (μg · h/ml) | 29.2 (99) | 60.3 (55) | 15.7 (113) | 47.8 (67) |
| Cmax (μg/ml) | 4.90 (64) | 7.83 (37) | 2.67 (72) | 6.21 (51) |
| Cmin (μg/ml) | 1.04 (140) | 3.12 (79) | 0.480 (175) | 2.98 (78) |
| F1 (%) | 64d | 73 (17–99)e | ||
The summary statistics were based on the PK parameters calculated from the simulated concentrations using individual PK parameters and an infusion rate of 3 mg/kg/h for i.v. administration.
Based on pooled data from 40 non-Japanese children, 8 young adolescents weighing <50 kg, using the original integrated population PK model (6).
Based on 18 Japanese children and young adolescents weighing <50 kg using the current final model. For the p.o. regimen, n = 17.
Parameter estimates in children and adolescents from the original integrated population PK model (6). The variability in F was Manly transformed (11). ETATR = {[exp(ηF1)]0.367 − 1}/0.367; logit(F1,i) = logit(F1) + ETATR. The SD of ηF1 was estimated to be 1.67.
Arithmetic mean (range).
FIG 4.
Comparisons of steady-state simulated exposures (AUC12) at recommended i.v. and oral dosing regimens from the original model and those from the current model. Each box represents the interquintile distance, with the median indicated by a solid line in the center of the box; the whiskers represent approximately 99% of the data (≤1.5 times the interquartile range), and outliers outside the whiskers are represented by points. The data for these box plots are from the original integrated PPK model (6). Estimated individual AUC12 values from the current model were overlaid on the box plots. Peds, children 2 to 11 years old; Adol 1, adolescents 12 to 14 years old and weighing <50 kg; Adol 2, adolescents 12 to 14 years old and weighing ≥50 kg. For i.v. steady state, Peds and Adol 1 received 8 mg/kg q12h and Adol 2 received 4 mg/kg q12h; for p.o. steady state, Peds and Adol 1 received 9 mg/kg (maximum, 350 mg) q12h and Adol 2 received 200 mg q12h.
DISCUSSION
The current analysis demonstrated that the previously developed PK model based on non-Japanese data (6) was able to fit the Japanese pediatric data adequately with few modifications. Specifically, the covariate effect of the CYP2C19 genotype on Vmax,inh (originally included for adults only) was included, and Km-Vmax correlation (100%) was included for the estimation of intersubject variability of parameters (originally included for adolescents and adults only). Other differences between children (2 to <12 years old) and adolescents (12 to <17 years old) in the original model were the different estimates for two PK parameters: the first-order absorption rate constant (ka) and Vmax,inh. Since the sample size of the current analysis was small (n = 21), especially for adolescents (only 6 subjects aged 12 to <15 years), these two parameters (ka and Vmax,inh) were not estimated separately for children and young adolescents in this analysis, and the corresponding variability was reflected in individual parameter estimates. All the parameter estimates are in agreement with those previously reported, except for Vmax,inh in association with inclusion of the CYP2C19 genotype effect (shown in Table 3). Overall, this indicated that the PK model developed for voriconazole is acceptable, although it is relatively complex.
The population PK modeling approach has an advantage over the noncompartmental analysis approach, which allows the evaluation of different potential covariates (e.g., CYP2C19 genotype, age, gender, and liver function parameters) in the model development process. Nonetheless, besides those already identified in the previous PK model, no new covariates on other voriconazole PK parameters were identified in this analysis, probably due to the small sample size and confounding by other factors also contributing to the intersubject variability in voriconazole exposures in these patients (e.g., many concomitant medications and complicated background therapy).
It is recognized that the number of subjects (n = 2) with CYP2C19 PM status was limited in this analysis, although extensive effort was made to enroll this type of patient. The CYP2C19 PM subjects were combined with the CYP2C19 HEM subjects during the covariate evaluation process. The CYP2C19 genotyping status (HEM/PM) was identified as a covariate (predictor) for Vmax,inh in this analysis, which is similar to what was identified in healthy adults in the previous model (6). As shown in Fig. 4, although the estimated average voriconazole exposures in CYP2C19 HEMs and PMs were higher than that in CYP2C19 EMs, there were substantial overlaps in voriconazole exposure distributions across CYP2C19 genotypes in these Japanese pediatric subjects. This indicated that the genotyping status helps explain the variability in voriconazole exposure to only a certain extent and the CYP2C19 genotyping status alone does not warrant dose adjustment in Japanese pediatric subjects. This is consistent with what has been observed in adults. For instance, in healthy adult subjects, on average, CYP2C19 PMs have 4-fold and 2-fold higher voriconazole concentrations than their EM and HEM counterparts, respectively (3). Nonetheless, voriconazole exposures vary widely within each genotype and overlap considerably across CYP2C19 genotypes. These differences are not of a clinically relevant magnitude, and no dose adjustment based on CYP2C19 genotyping status is warranted for adults, which is reflected in the product label (3). In addition, the analysis of non-Japanese pediatric data did not identify CYP2C19 genotype as a covariate in the original PK model (6). Based on the analyses conducted with the CYP2C19 genotyping data collected thus far, even if more CYP2C19 PM subjects were included in the current analysis, it is unlikely to produce different findings.
The mean oral bioavailability (F) estimated in Japanese pediatric subjects was 73% (range, 17% to 99%), which is consistent with the reported estimates of 64% in the previous model and less than what was originally estimated for healthy adults—96% (3, 6). The large intersubject variabilities in oral bioavailability and voriconazole exposure in these pediatric subjects were not unexpected, since this patient population would be on many concomitant medications and would have various serious underlying conditions, which could affect the oral absorption and disposition processes.
As noted above, voriconazole exposures in Japanese pediatric subjects were generally comparable to those in non-Japanese pediatric subjects receiving the same dosing regimens, given the large intersubject variability; however, the average exposure values in Japanese pediatric subjects were higher (Table 5 and Fig. 4). As for the slight trend of increased exposure in Japanese pediatric subjects, it may be partially explained by a lower prevalence of CYP2C19 EMs in Japanese pediatric subjects (e.g., 33% versus 44%). Also, note that the sample size of Japanese pediatric subjects used for comparison with non-Japanese pediatric subjects is relatively small (i.e., 18 versus 48).
Taken together, the dosing regimens evaluated in this study are deemed appropriate as the starting doses for Japanese pediatric patients. As with adults, dose adjustment can be made subsequently based on the pediatric patient's clinical response and the tolerability and/or voriconazole trough concentrations (if available). Detailed justification of the appropriateness of these dosing regimens as the initial recommendation for pediatric patients and discussion on how to use therapeutic drug monitoring as an additional tool to facilitate voriconazole management have been published elsewhere (7).
In summary, a two-compartment PK model with first-order absorption and mixed linear and nonlinear (Michaelis-Menten and time-dependent Vmax) elimination adequately described the voriconazole i.v. and oral data from Japanese pediatric subjects. Individual AUC12 estimates across different age groups in Japanese pediatric subjects were generally in agreement with the simulated AUC12 in non-Japanese pediatric subjects on the same dosing regimens using the same modeling approach. Consistent with the previous findings, the CYP2C19 genotyping status did not have a clinically relevant effect on voriconazole exposure in Japanese pediatric subjects, although it was identified as a covariate for Vmax,inh to help explain the intersubject variability in voriconazole exposure. Thus, the CYP2C19 genotyping status alone does not warrant dose adjustment of voriconazole. No other factors besides age and weight were identified to explain the PK variability of voriconazole.
ACKNOWLEDGMENTS
The study used in the analyses was sponsored by Pfizer Inc.
We are all employees of Pfizer.
APPENDIX
| Vmax,1 = θVmax,1 × (wt/70)0.75 |
| T50 = θT50 |
| CL = θCL × (wt/70)0.75 |
| V2 = θV2 × wt/70 |
| V3 = θV3 × wt/70 |
| Q = θQ × (wt/70)0.75 |
| logit(F1) = θF1 |
| ka = θka |
| Alag = θAlag |
Km is the Michaelis-Menten constant, Vmax,1 is the maximum elimination rate at 1 h after the start of dosing, Vmax,inh is the maximum fraction of Vmax inhibition, T is the time after the first dose, T50 is the time in hours where half of the maximum inhibition occurred, CL is the linear clearance, V2 is the volume of distribution for the central compartment, V3 is the volume of distribution for the peripheral compartment, Q is the intercompartmental clearance, F1 is the oral bioavailability, ka is the first-order absorption rate constant, Alag is the absorption lag time, θ is the fixed-effect parameter, and wt is body weight.
REFERENCES
- 1.Johnson LB, Kauffman CA. 2003. Voriconazole: a new triazole antifungal agent. Clin Infect Dis 36:630–637. doi: 10.1086/367933. [DOI] [PubMed] [Google Scholar]
- 2.Hyland R, Jones BC, Smith DA. 2003. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab Dispos 31:540–547. doi: 10.1124/dmd.31.5.540. [DOI] [PubMed] [Google Scholar]
- 3.Theuretzbacher U, Ihle F, Derendorf H. 2006. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin Pharmacokinet 45:649–663. doi: 10.2165/00003088-200645070-00002. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein JA, Ishizaki T, Chiba K, de Morais SM, Bell D, Krahn PM, Evans DA. 1997. Frequencies of the defective CYP2C19 alleles responsible for the mephenytoin poor metabolizer phenotype in various Oriental, Caucasian, Saudi Arabian and American black populations. Pharmacogenetics 7:59–64. doi: 10.1097/00008571-199702000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Kubota T, Chiba K, Ishizaki T. 1996. Genotyping of S-mephenytoin 4′-hydroxylation in an extended Japanese population. Clin Pharmacol Ther 60:661–666. doi: 10.1016/S0009-9236(96)90214-3. [DOI] [PubMed] [Google Scholar]
- 6.Friberg LE, Ravva P, Karlsson MO, Liu P. 2012. Integrated population pharmacokinetic analysis of voriconazole in children, adolescents, and adults. Antimicrob Agents Chemother 56:3032–3042. doi: 10.1128/AAC.05761-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mori M, Kobayashi R, Kato K, Maeda N, Fukushima K, Goto H, Inoue M, Muto C, Okayama A, Watanabe K, Liu P. 2015. Pharmacokinetics and safety of voriconazole intravenous-to-oral switch regimens in immunocompromised Japanese pediatric patients. Antimicrob Agents Chemother 59:1004–1013. doi: 10.1128/AAC.04093-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gisleskog P, Karlsson M, Beal S. 2002. Use of prior information to stabilize a population data analysis. J Pharmacokinet Pharmacodyn 29:473–505. doi: 10.1023/A:1022972420004. [DOI] [PubMed] [Google Scholar]
- 9.Boeckmann AJ, Sheiner LB, Beal SL. 2009. NONMEM users guides. ICON Development Solutions, Ellicott City, MD. [Google Scholar]
- 10.Bergstrand M, Hooker A, Wallin J, Karlsson M. 2011. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13:143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersson KF, Hanze E, Savic R, Karlsson M. 2009. Semiparametric distributions with estimated shape parameters. Pharm Res 26:2174–2185. doi: 10.1007/s11095-009-9931-1. [DOI] [PubMed] [Google Scholar]