Abstract
We describe the pharmacokinetics of dolutegravir (DTG) in a premature neonate after maternal intensification of an antiretroviral (ARV) regimen by adding DTG. During the last 2 weeks of pregnancy, the ARV was tenofovir-emtricitabine, atazanavir-ritonavir, and DTG (50 mg once daily). From the interaction between atazanavir and DTG via CYP3A4 and UGT1A1 and placental efflux transporter inhibition and considering the infant's probable enzymatic immaturity, the DTG elimination half-life was estimated to be 4-fold longer in neonates than in adults.
TEXT
Dolutegravir (DTG) is an HIV-1 integrase strand transfer inhibitor; data for pregnant women are limited. From animal reproduction studies failing to demonstrate any risk to the fetus and with a lack of adequate and well-controlled studies with pregnant women, DTG was classified as a category B drug by the U.S. Food and Drug Administration (1). Consequently, a DTG-containing regimen could be used during pregnancy to sharply decrease the plasma HIV-1 RNA level (plasma viral load [pVL]) near delivery in late HIV infection presenters. It could also be used for infection with viruses harboring resistance substitutions in the integrase coding region with limited alternative antiretroviral treatment (ARV) to prevent mother-to-child transmission.
Here we report on DTG intensification in a 35-year-old African woman with HIV-1 infection since 2002 that was well controlled until pregnancy who was admitted in an emergency to a hospital for premature membrane rupture at 35 weeks of gestation. In accordance with the national guidelines of France (2), the HIV infection was successfully treated with tenofovir disoproxil fumarate and emtricitabine (FTC) at 300 and 200 mg once daily and atazanavir (ATV) and ritonavir (RTV) at 300 and 100 mg once daily with an undetectable pVL and approximately 500 CD4 cells/mm3 until pregnancy. At 22 and 32 weeks of gestation, for no apparent reason, the pVL rebounded to 279 and 453 copies/ml, respectively.
To document the safety of and adherence to ARV, plasma drug concentrations were determined by ultraperformance liquid chromatography with tandem mass spectrometry (Acquity UPLC-TQD; Waters, Milford, MA) after sample pretreatment as previously described (3). At 26 weeks of gestation, the maternal ARV concentrations at 24 h postdose (C24h) were as follows: ATV, 1,319 ng/ml; RTV, 48 ng/ml; FTC, 91 ng/ml; TFV, 50 ng/ml. The FTC and TFV C24h were considered adequate, and the ATV C24h was considered active but elevated, according to the 200- to 850-ng/ml efficacy-tolerance interval (2). At the time of virological failure, the genotypic resistance test of the HIV-1 subtype CRF06_cpx revealed four thymidine-associated substitutions (M41L, K70R, L210W, and T215Y) and an M184V substitution conferring resistance to FTC and possible resistance to TFV. With these results, with the absence of a genotypic resistance test for the integrase coding region, and to retain a once-daily regimen, DTG at 50 mg once daily was added to the therapy regimen at 33 weeks of gestation.
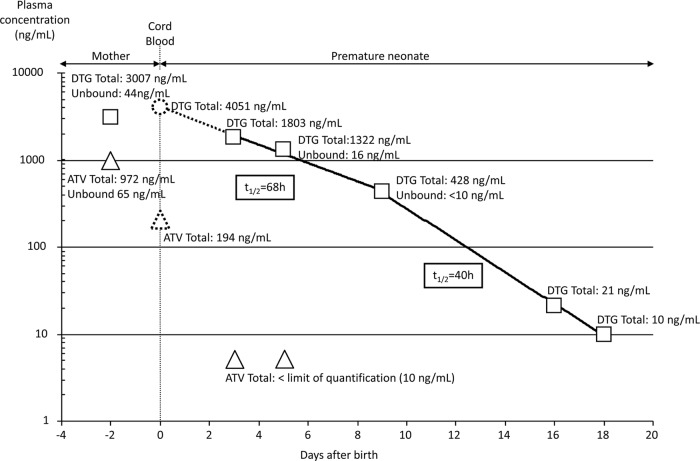
At 35 weeks of gestation, 2 days before delivery and 2 weeks after DTG intensification, the maternal steady-state total and unbound ATV C24h were 972 and 65 ng/ml, respectively, and the DTG C24h were 3,007 and 44 ng/ml, respectively. The calculated maternal free fractions of ATV and DTG were 6.6 and 1.5%, respectively. The total DTG C24h was interpreted according to the supposed 1,000-ng/ml effective threshold predictive of a virological response in the SAILING study (4). The RTV C24h of 86 ng/ml, the FTC C24h of 221 ng/ml, and the TFV C24h of 85 ng/ml were considered adequate.
Delivery was by caesarian section 48 h after hospitalization. The newborn boy appeared hypotrophic, with a weight of 1,600 g, a length of 43 cm, and an Apgar score of 10/10. He received 5 days of phototherapy from day 4 to day 9; the maximum total bilirubinemia was 175 μmol/liter on day 5. At delivery, the maternal pVL was undetectable and the cord blood was negative for proviral DNA as assessed by real-time PCR for polymerase and long terminal repeat sequences (limit of detection, <10 copies/million peripheral blood mononuclear cells).
In the neonate, on day 1, postexposure prophylaxis was started with intravenous zidovudine at 2 mg/kg twice a day. At 3 and 5 days of age, the plasma DTG concentrations resulting from maternal placental transfer were 1,803 and 1,322 ng/ml, respectively. In both samples, the plasma ATV concentration was <10 ng/ml. At day 5, the unbound plasma DTG concentration was 16 ng/ml, corresponding to a free fraction of 1.2%. At day 9, the total and unbound plasma DTG concentrations were 428 and <10 ng/ml, respectively. Both results of free fractions for DTG in the neonate were consistent with values in adults (1). At day 18 after delivery, the total plasma DTG concentration was <10 ng/ml, demonstrating complete DTG elimination by the neonate.
In this premature neonate, the calculated terminal half-life of DTG in plasma, based on a one-phase pharmacokinetic elimination model, was approximately 46 h. With a two-phase elimination model, the calculated half-lives were 68 h (first) and 40 h (terminal). With the latter pharmacokinetic model, the plasma DTG concentration in cord blood might be extrapolated to approximately 4,051 ng/ml at delivery. As an illustration, 2 days before delivery, the total and unbound maternal DTG C24h were 3,007 and 44 ng/ml, respectively. The materno-fetal ratio of plasma DTG concentrations was estimated to be 1.3 (using the total cord blood plasma DTG concentration at delivery and the last measured total maternal DTG C24h, 2 days before delivery), for probable accumulation in the fetal compartment. Knowing that 20% of the total ATV crosses the placenta (5), we estimated the plasma ATV concentration in cord blood to be approximately 194 ng/ml.
To our knowledge, this is the first report of a case demonstrating the unexpected placental transfer of DTG with fetal accumulation and then slow neonatal clearance. Several mechanistic hypotheses might explain this DTG placental transfer as the drug interaction between ATV and DTG in both the mother and the neonate (1, 6). ATV acts as a perpetuator of DTG pharmacokinetics, leading to increased half-lives in both the mother and the neonate via inhibition of CYP3A4 and UGT1A1, corresponding to the first slope of the DTG elimination curve in the neonate with the longer half-life (Fig. 1). In healthy adults, the trough plasma DTG concentration is increased by 121% and the half-life is 24 h, for a 2-fold increased value (7). ATV is also a placental ABC transporter (P-glycoprotein) inhibitor, which possibly limits the fetal efflux of DTG (8, 9). Moreover, the premature infant's glucuronyl-transferase immaturity might increase the neonatal DTG accumulation more than extrapolation of the maternal free fraction would predict, which might explain the terminal half-life of DTG.
FIG 1.
Two-phase elimination model of total and unbound plasma ATV and DTG concentrations in a mother with HIV infection and her infant. Shown are maternal plasma ATV (triangles) and DTG (squares) concentrations 2 days before delivery, neonate plasma ATV (triangles) and DTG (squares) concentrations, and extrapolated plasma ATV (dashed triangle) and DTG (dashed circle) concentrations at delivery in cord blood. Elimination half-lives of DTG were determined by linear regression. The limit of quantification (LOQ) of ATV and DTG is 10 ng/ml. By convention, values below the LOQ were plotted as LOQ/2.
Finally, and despite the unexpected plasma DTG overexposure and the 4-fold longer half-life of DTG in the premature neonate than in adults, it was well tolerated.
REFERENCES
- 1.Anonymous. 2014. Tivicay (dolutegravir), summary of product characteristics. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204790s002lbl.pdf Last accessed, January 2015.
- 2.Anonymous. 2013. Prise en charge médicale des personnes infectées par le VIH. Recommandations du groupe d'experts rapport 2013 sous la direction du Philippe Morlat et sous l'égide du CNS et de l'ANRS. http://www.sante.gouv.fr/IMG/pdf/Rapport_Morlat_2013_Mise_en_ligne.pdf Last accessed, January 2015 (In French.)
- 3.Jung BH, Rezk NL, Bridges AS, Corbett AH, Kashuba AD. 2007. Simultaneous determination of 17 antiretroviral drugs in human plasma for quantitative analysis with liquid chromatography-tandem mass spectrometry. Biomed Chromatogr 21:1095–1104. doi: 10.1002/bmc.865. [DOI] [PubMed] [Google Scholar]
- 4.Song I, Chen S, Piscitelli S, Min S. 2013. Pharmacokinetics (PK) and PK-pharmacodynamic (PD) relationship of dolutegravir (DTG) in integrase inhibitor (INI)-naïve subjects, abstr A-1573 53rd ICAAC, 10 to 13 September 2013, Denver, CO. [Google Scholar]
- 5.Ivanovic J, Nicastri E, Anceschi MM, Ascenzi P, Signore F, Pisani G, Vallone C, Mattia E, Notari S, Tempestilli M, Pucillo LP, Narciso P, Pregnancy and Newborn Clinical Outcome Group in HIV Infection (PANCOH). 2009. Transplacental transfer of antiretroviral drugs and newborn birth weight in HIV-infected pregnant women. Curr HIV Res 7:620–625. doi: 10.2174/157016209789973628. [DOI] [PubMed] [Google Scholar]
- 6.Anonymous. 2014. Reyataz (atazanavir), summary of product characteristics. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206352s000,021567s035lbl.pdf Last accessed, January 2015.
- 7.Song I, Borland J, Chen S, Lou Y, Peppercorn A, Wajima T, Min S, Piscitelli SC. 2011. Effect of atazanavir and atazanavir/ritonavir on the pharmacokinetics of the next-generation HIV integrase inhibitor, S/GSK1349572. Br J Clin Pharmacol 72:103–108. doi: 10.1111/j.1365-2125.2011.03947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gulati A, Gerk PM. 2009. Role of placental ATP-binding cassette (ABC) transporters in antiretroviral therapy during pregnancy. J Pharm Sci 98:2317–2335. Rev. doi: 10.1002/jps.21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyagi SJ, Collier AC. 2011. The development of UDP-glucuronosyltransferases 1A1 and 1A6 in the pediatric liver. Drug Metab Dispos 39:912–919. doi: 10.1124/dmd.110.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]