Abstract
Posaconazole in oral suspension must be taken multiple times a day with food (preferably a high-fat meal) to ensure adequate exposure among patients. We evaluated the effect of food on the bioavailability of a new delayed-release tablet formulation of posaconazole at the proposed clinical dose of 300 mg once daily in a randomized, open-label, single-dose, two-period crossover study with 18 healthy volunteers. When a single 300-mg dose of posaconazole in tablet form (3 tablets × 100 mg) was administered with a high-fat meal, the posaconazole area under the concentration-time curve from 0 to 72 h (AUC0–72) and maximum concentration in plasma (Cmax) increased 51% and 16%, respectively, compared to those after administration in the fasted state. The median time to Cmax (Tmax) shifted from 5 h in the fasted state to 6 h under fed conditions. No serious adverse events were reported, and no subject discontinued the study due to an adverse event. Six of the 18 subjects reported at least one clinical adverse event; all of these events were mild and short lasting. The results of this study demonstrate that a high-fat meal only modestly increases the mean posaconazole exposure (AUC), ∼1.5-fold, after administration of posaconazole tablets, in contrast to the 4-fold increase in AUC observed previously for a posaconazole oral suspension given with a high-fat meal.
INTRODUCTION
Posaconazole (MK-5592) is a registered extended-spectrum triazole with demonstrated efficacy as antifungal prophylaxis for invasive fungal infections (IFIs) in high-risk patients (1, 2) and as treatment for refractory IFIs (3, 4). Posaconazole was initially developed and marketed as an oral suspension; the suspension must be administered multiple times a day and taken with food (preferably a high-fat meal) (5, 6) or a nutritional supplement or acidic beverage (6–8) to ensure adequate exposure. Reliable and adequate levels of posaconazole exposure are important to ensure continued antifungal prophylaxis. In fact, a clear exposure-efficacy relationship for posaconazole has been identified based on the results of prior pivotal studies conducted with the oral suspension (4, 9).
Antifungal prophylaxis recipients routinely include the following two key populations: (i) patients with acute myelogenous leukemia (AML), myelodysplastic syndrome (MDS), or other acute hematological malignancies, who may develop neutropenia and chemotherapy-induced side effects, namely, severe nausea or vomiting; and (ii) allogeneic hematopoietic stem cell transplant (HSCT) recipients, who routinely develop graft-versus-host disease (GVHD) and its associated complications, including severe mucositis or diarrhea. Adequate food intake to obtain optimal posaconazole exposure may be difficult for these patients (10, 11). In order to overcome the burden of taking multiple doses per day together with food intake for the target population, a new solid oral tablet formulation of posaconazole has been developed that can be taken once daily and without the need for food intake to support adequate absorption. The new posaconazole tablet is currently approved in the United States, Canada, Australia, Switzerland, Taiwan, and the European Union.
The posaconazole tablet formulation contains amorphous posaconazole dissolved in a pH-sensitive polymer matrix, hydroxypropylmethylcellulose acetate succinate (HPMCAS), by hot-melt extrusion technology to form a molecularly dispersed material. The pH-dependent solubility of HPMCAS limits posaconazole release from the solid dispersion in the low gastric pH of the stomach. At the elevated pH of the intestine, HPMCAS is highly soluble, and this property allows the molecularly dispersed posaconazole to be released. Furthermore, it is believed that the presence of HPMCAS in the intestinal fluid prevents the recrystallization of posaconazole, thus ensuring that a larger fraction of the dose is available for absorption. This mechanism of drug release and prevention of drug recrystallization is relatively independent of the fed/fasted state of the gastrointestinal tract.
A phase I study with a 100-mg prototype tablet formulation (12) showed that under fasted conditions, posaconazole solid tablets yielded substantially higher mean drug exposure than that obtained with the posaconazole oral suspension. In addition, peak and total exposures for the posaconazole tablet were not markedly affected by food, whereas a high-fat meal increased the area under the concentration-time curve (AUC) and the maximum concentration in plasma (Cmax) of posaconazole approximately 3-fold when the drug was given as 100 mg in oral suspension (12). At a higher dose of the posaconazole oral suspension (200 mg), a high-fat meal increased the AUC and Cmax approximately 4-fold compared to those for the fasted state (5). In the current study, we evaluated the effect of a high-fat meal on the bioavailability of the final market image of posaconazole tablets given to healthy volunteers as a single oral dose of 300 mg, which reflects the clinical dose of 300 mg once daily (13).
MATERIALS AND METHODS
Study design.
MK-5592 protocol 0112 was a randomized, open-label, single-dose, two-period crossover study conducted at a single center in the United States (QPS, Springfield, MO) from 25 June 2013 to 19 July 2013. The study was conducted in accordance with the principles of good clinical practice and was approved by the appropriate institutional review board and regulatory agencies. Written informed consent was obtained from each subject before enrollment. In this exploratory study, the sample size was based on empirical considerations, and no statistical hypothesis was tested.
Subjects.
Healthy, nonsmoking male and female subjects between 18 and 65 years of age and with a body mass index between 18 and 30 kg/m2 were eligible for the study. Enrolled subjects had clinical laboratory test results (hematology, blood chemistry, and urinalysis) within normal limits (or clinically acceptable to the investigator), vital signs without clinically relevant abnormalities, and 12-lead electrocardiogram (ECG) parameters within the gender-specific normal ranges. Female subjects were not allowed to be pregnant or breastfeeding. All subjects agreed to use a medically accepted, nonhormonal method of contraception during the study and up to 30 days after the last dose of study drug. Subjects with any surgical or medical condition that could significantly alter the absorption, distribution, metabolism, or excretion of any drug were excluded. Additional exclusion criteria were (i) cardiomyopathy or a history of any type of heart disease; (ii) any infectious disease within 4 weeks before drug administration; (iii) a positive result for hepatitis B virus surface antigen, hepatitis C virus antibodies, HIV, or alcohol or drugs with a high potential for abuse; (iv) blood donation, clinical study participation, or use of an investigational product in the past 30 days; (v) clinically significant allergic reactions; (vi) vegetarian diet; and (vii) excessive consumption of caffeinated beverages. Use of any concomitant drug (other than ibuprofen) was prohibited throughout the study period.
Study procedures.
Subjects were randomly assigned to receive treatment A or treatment B in period 1 and the alternate treatment in period 2. Treatment A consisted of a single oral dose of posaconazole (300 mg) given as 3 tablets of 100 mg each after fasting for at least 10 h; treatment B consisted of a single oral dose of posaconazole (300 mg) given as 3 tablets of 100 mg each after consumption of a high-fat meal. The washout between dosing days during periods 1 and 2 was a minimum of 7 days. Subjects remained in the clinic from the afternoon of day −1 until 24 h after dosing in each period, and they returned to the clinic for pharmacokinetic (PK) sample collection after this time point.
The total volume of water taken with treatments A and B was 240 ml per treatment. All subjects fasted for 4 h after dosing. Drinking water or other fluids was restricted from 1 h prior to dosing to 1 h after dosing, except for the fluids included in the high-fat meal. Subjects were to remain upright for 4 h postdose. Subjects received their dose of trial medication between 6 and 10 a.m., at the same clock-time in each period, as much as possible. The tablets were swallowed whole, not chewed or crushed, and a mouth check was performed after each dosing. Subjects received a standardized lunch at least 4 h after dosing, a standardized dinner 10 h after dosing, and a standardized snack 14 h after dosing. During housing, meal times were identical for all periods.
For treatment B, subjects began eating a high-fat meal approximately 30 min before study drug administration. The high-fat meal consisted of two eggs fried in butter, two strips of bacon, two slices of toast with butter, 4 oz of hash brown potatoes, and 8 oz of whole milk (or equivalent substitutions providing approximately 150 cal from protein, 250 cal from carbohydrates, and 500 to 600 cal from fat).
Pharmacokinetic evaluations.
Blood samples for pharmacokinetic evaluations were collected in each period, before dosing (0 h) and at 1, 2, 3, 4, 5, 6, 8, 12, 24, 48, and 72 h postdose. Plasma samples were analyzed for posaconazole concentrations by using a validated liquid chromatography method (14) with tandem mass spectrometric detection, with a calibration range of 5 to 5,000 ng/ml. The primary pharmacokinetic endpoints included the maximum plasma concentration (Cmax), the time to the maximum observed plasma concentration (Tmax), and the area under the plasma concentration-time curve from time zero to the last sampling time point (AUC0–72).
All available pharmacokinetic data were included in the analysis, and no imputations were made for missing data. Cmax and AUC0–72 were analyzed following log transformation in a linear mixed-effects model with fixed-effects terms for treatment and period. Ninety percent confidence intervals (CIs) were constructed for the differences in least-squares (LS) means on the log scale for the posaconazole Cmax and AUC. Exponentiating the log-scale 90% CI provided a 90% CI for the geometric mean ratio (GMR) for the fed versus fasted state. The pseudo within-subject percent coefficient of variation (% CV) was calculated as follows: , where σ2A and σ2B are the estimated variances on the log scale for the two treatment groups and σAB is the corresponding estimated covariance, each obtained from the linear mixed-effects model. Additionally, descriptive statistics were provided by treatment. The minimum, median, and maximum were provided for all pharmacokinetic parameters. Arithmetic means and standard deviations based on the raw scale were provided for the AUC0–72 and Cmax. In addition, the % CV of AUC0–72 and Cmax were also provided and calculated according to the following formula: , where s2 is the observed variance on the natural log scale.
Safety evaluations.
Safety evaluations included all reported adverse events as well as physical examination results, vital sign measurements (heart rate, blood pressure, and body temperature), a 12-lead ECG, and laboratory safety tests.
RESULTS
Subject demographics and disposition.
Eighteen healthy subjects (mean age, 45.1 years; standard deviation [SD], 16.4 years) were enrolled in the study. Six subjects (33.3%) were male, and 12 (66.7%) were female. Additional subject demographics and baseline characteristics are shown in Table 1. Sixteen subjects completed the study according to the protocol. Two subjects dropped out, each missing one period, resulting in a total of 17 subjects under fasting conditions and 17 subjects under fed conditions. Four subjects (three under fasting conditions and one under fed conditions) had detectable predose plasma concentrations in period 2 that were >5% of their Cmax values. Analyses conducted with and without these four subjects yielded generally similar results. Therefore, only the primary analysis (which excluded these four subjects) is presented here.
TABLE 1.
Subject demographics and baseline characteristics
| Characteristic | Value for all patients (n = 18) |
|---|---|
| Gender (no. [%]) | |
| Male | 6 (33.3) |
| Female | 12 (66.7) |
| Age (yr) | |
| Mean (SD) | 45.1 (16.4) |
| Median (minimum, maximum) | 52.5 (19, 65) |
| Race (no. [%]) | |
| White or Caucasian | 15 (83.3) |
| Black or African American | 2 (11.1) |
| Asian | 1 (5.6) |
| Ethnicity (no. [%]) | |
| Non-Hispanic | 18 (100) |
| Tobacco use (no. [%]) | |
| Non-tobacco user | 16 (88.9) |
| Former tobacco user | 2 (11.1) |
| Height (cm) | |
| Mean (SD) | 167 (8.7) |
| Median (minimum, maximum) | 168 (155, 187) |
| Weight (kg) | |
| Mean (SD) | 72.9 (10.6) |
| Median (minimum, maximum) | 72.0 (54, 92) |
| Body mass index | |
| Mean (SD) | 26.0 (2.4) |
| Median (minimum, maximum) | 26.2 (20.7, 29.4) |
Pharmacokinetics.
When a single dose of 300 mg posaconazole (as 3 tablets of 100 mg each) was administered with a high-fat meal, posaconazole exposure in terms of AUC0–72 and Cmax increased 51% and 16%, respectively, compared to administration in the fasted state (Table 2). The median Tmax shifted slightly, from 5 h under fasting conditions to 6 h under fed conditions. The plasma concentration-time profiles were affected by the coadministration of food (Fig. 1). The effect of food on the plasma concentration of posaconazole at 24 h postdose was consistent with the change in AUC0–72, with a C24 value (arithmetic mean) of 443 ng/ml under fasting conditions versus 686 ng/ml under fed conditions (geometric means, 425 and 677 ng/ml, respectively). The individual GMRs for Cmax ranged from 0.51 to 1.89 among the 14 subjects for whom individual fed and fasted pharmacokinetics of posaconazole were available. For most subjects, Cmax was higher under fed conditions, but four subjects showed the opposite effect (Fig. 2). The individual GMRs for AUC0–72 ranged from 0.93 to 2.23 among these 14 subjects. The two subjects with the highest GMRs (2.23 and 2.14) had the lowest AUC value (14,600 ng · h/ml for both) in the fasted state.
TABLE 2.
Plasma pharmacokinetics of posaconazole following single doses of 300 mg of posaconazole in tablet form (3 tablets × 100 mg) administered to healthy subjects under fasting and fed conditionsa
| Parameter | GM (95% CI) for fasting conditions (n = 14) | GM (95% CI) for fed conditions (n = 16) | GMR (90% CI) for fed/fasting ratio | Pseudo within-subject % CV |
|---|---|---|---|---|
| Cmax (ng/ml) | 893 (731, 1,090) | 1,040 (915, 1,190) | 1.16 (0.96, 1.41) | 29.4 |
| AUC0–72 (h · ng/ml) | 25,600 (21,500, 30,400) | 38,700 (35,000, 42,700) | 1.51 (1.33, 1.72) | 17.2 |
| Tmax (h) | 5.00 (3.00, 8.00) | 6.00 (5.00, 24.00) |
GM, geometric least-squares mean; CI, confidence interval; GMR, geometric least-squares mean ratio; % CV, pseudo within-subject % coefficient of variance. Exceptions to the column headings are the data for Tmax, which are medians (minimums, maximums).
FIG 1.
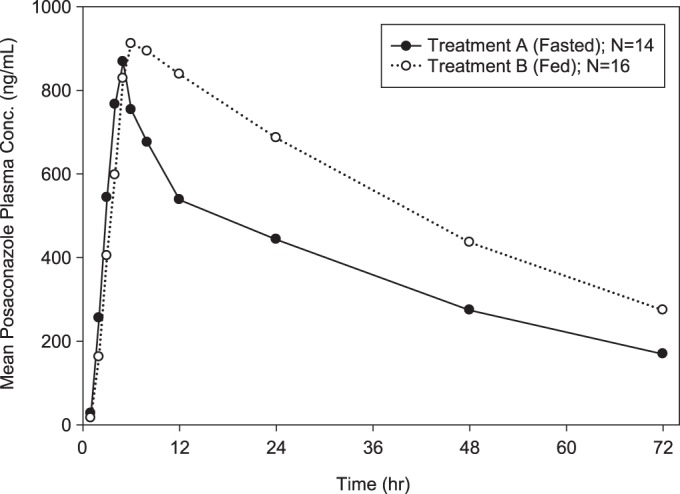
Arithmetic mean posaconazole plasma concentration-time profiles following a single oral dose of 300 mg of posaconazole in tablet form (3 tablets × 100 mg) administered to healthy subjects under fasting and fed conditions.
FIG 2.
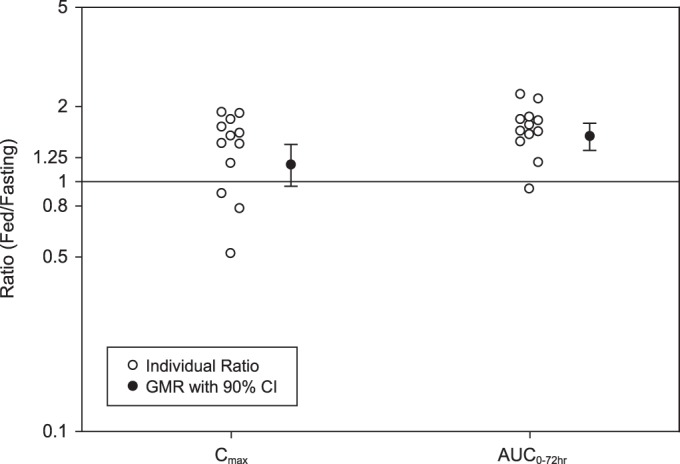
Individual ratios and GMRs (90% CI) for plasma posaconazole Cmax and AUC0–72 (fed/fasting) following a single oral dose of 300 mg of posaconazole in tablet form (3 tablets × 100 mg) administered to healthy subjects under fasting (n = 14) and fed (n = 16) conditions.
Safety and tolerability.
All 18 subjects were included in the assessment of safety and tolerability. No serious adverse events were reported, and no subject discontinued the study due to an adverse event. Six subjects (33%) reported at least one adverse event. The most frequently reported adverse event was fatigue, which was reported by five subjects; all other adverse events (headache, chills, and decreased appetite) were reported by one subject each. All clinical adverse events were of mild intensity and short duration and were considered possibly related to the study drug. No adverse events required concomitant medication, nonmedical therapies, or any action to be taken with the study drug. No clinically significant findings were reported for laboratory values, vital signs, or ECG parameters.
DISCUSSION
The results of this food-effect study demonstrate that a high-fat meal (∼70 g fat) only modestly increases the mean posaconazole exposure (AUC), ∼1.5-fold, after administration of the new posaconazole solid tablet formulation. For comparison, the AUC of posaconazole was 4 times greater (than in the fasted state) when the oral suspension (200 mg) was administered with a high-fat meal (∼50 g fat) and about 2.6 times greater when it was administered with a nonfat meal or nutritional supplement (14 g fat) (5). In the new tablet formulation, posaconazole is dispersed in a polymer matrix (HPMCAS) that is highly soluble at the elevated pH of the intestine but not at the low pH of the stomach and that prevents recrystallization of posaconazole after it is released. A previous study showed that peak and total exposures for a prototype posaconazole tablet, which also used the HPMCAS technology, were not markedly affected by food (12). The current study confirms that dissolving posaconazole in the pH-sensitive HPMCAS matrix significantly reduces the food effect observed with the posaconazole oral suspension.
The fed/fasted GMRs for AUC and Cmax were somewhat higher in the current study than in the earlier study of the prototype tablet, which showed a maximal GMR of 1.08 (90% CI, 0.98 to 1.19) for AUC and 0.94 (90% CI, 0.83 to 1.07) for Cmax (P. Dogterom, J. Xu, H. Waskin, W. Kersemaekers, and M. van Iersel, presented at the American Society for Clinical Pharmacology and Therapeutics 115th Annual Meeting, Atlanta, GA, 20 to 22 March 2014). These two studies used slightly different tablet formulations in terms of the excipients and color, but the ratio of pH-sensitive polymer (HPMCAS) to active ingredient (posaconazole) was not changed throughout development of the formulation. Only changes in excipient quantities to account for tablet tensile strength, friability, and aesthetics were made; these changes would not be expected to influence the effect of food on posaconazole exposure. Another difference between the studies was the dose of posaconazole that was evaluated. The current study used the final approved clinical dose of 300 mg (as a single dose of three 100-mg tablets), while the earlier study used a 100-mg dose (one tablet). The food effect observed with the oral suspension is greater at higher doses of posaconazole: a 4-fold increase in exposure was observed at 200 mg (5), versus a 2.5- to 3-fold increase at 100 mg (12). Thus, the higher dose of 300 mg used in the current study is the most likely explanation for the somewhat larger food effect.
Since the food effect estimated by this study is based on a high-fat meal, it likely represents a worst-case extreme of the potential food effect in patients for whom posaconazole is indicated, since lighter meals are more typical in this population. With the posaconazole oral suspension (200 mg), a smaller food effect was observed after a low-fat meal (2.6- to 3-fold increase in AUC) than after a high-fat meal (4-fold increase in AUC) (5). Thus, the effect of lighter (i.e., lower fat) meals on posaconazole exposure after tablet administration may be less than the ∼50% effect observed in the current study. In a study of posaconazole tablets given irrespective of food intake for prophylaxis of IFI, an ∼30% higher plasma exposure of posaconazole was observed in HSCT patients than in neutropenic AML/MDS patients (Dogterom et al., presented at the American Society for Clinical Pharmacology and Therapeutics 115th Annual Meeting). This difference may be related to differences in the ability of patients to take their doses with food (i.e., AML/MDS patients with neutropenia from cytotoxic chemotherapy also routinely suffer from severe nausea and anorexia, thereby complicating their food intake), as well as to underlying characteristics related to the disease. Most importantly, despite the likely limited food intake in the neutropenic patients, adequate exposure of posaconazole was obtained.
In summary, the results of this study show that posaconazole exposure is only modestly affected by a high-fat meal after administration of the solid tablet formulation at the approved dose of 300 mg. In addition, a single dose of posaconazole at 300 mg was safe and well tolerated in healthy subjects when given in either the fed or fasted state. These results suggest that posaconazole tablets can be taken without regard to food, which represents a significant improvement over the oral suspension, particularly for patients who may have difficulty ensuring adequate food intake.
ACKNOWLEDGMENTS
All funding for this study was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
Medical writing and editorial assistance were provided by Kim Strohmaier and Karyn Davis of Merck Sharp & Dohme Corp.
All authors are responsible for the work described in this paper. All authors were involved in (i) conception, design, acquisition, analysis, statistical analysis, and/or interpretation of the data; and (ii) drafting the manuscript and/or revising the manuscript for important intellectual content. All authors provided final approval of the version to be published.
All authors are current or former employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., and may own stock and/or stock options in the Company.
REFERENCES
- 1.Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott D, Holowiecki J, Stockelberg D, Goh Y-T, Petrini M, Hardalo C, Suresh R, Angulo-Gonzalez D. 2007. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 356:348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 2.Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, Greinix H, Morais de Azevedo W, Reddy V, Boparai N, Pedicone L, Patino H, Durrant S. 2007. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med 356:335–347. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- 3.Raad II, Hachem RY, Herbrecht R, Graybill JR, Hare R, Corcoran G, Kontoyiannis DP. 2006. Posaconazole as salvage treatment of invasive fusariosis in patients with underlying hematologic malignancy and other conditions. Clin Infect Dis 42:1398–1403. doi: 10.1086/503425. [DOI] [PubMed] [Google Scholar]
- 4.Walsh TJ, Raad I, Patterson TF, Chandrasekar P, Donowitz GR, Graybill R, Greene RE, Hachem R, Hadley S, Herbrecht R, Langston A, Louie A, Ribaud P, Segal BH, Stevens DA, van Burik J-AH, White CS, Corcoran G, Gogate J, Krishna G, Pedicone L, Hardalo C, Perfect JR. 2007. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis 44:2–12. doi: 10.1086/508774. [DOI] [PubMed] [Google Scholar]
- 5.Courtney R, Wexler D, Radwanski E, Lim J, Laughlin M. 2004. Effect of food on the relative bioavailability of two oral formulations of posaconazole in healthy adults. Br J Clin Pharmacol 57:218–222. doi: 10.1046/j.1365-2125.2003.01977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishna G, Moton A, Ma L, Medlock MM, McLeod J. 2009. The pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob Agents Chemother 53:958–966. doi: 10.1128/AAC.01034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishna G, Ma L, Vickery D, Yu X, Wu I, Power E, Beresford E, Komjathy S. 2009. Effect of varying amounts of a liquid nutritional supplement on the pharmacokinetics of posaconazole in healthy volunteers. Antimicrob Agents Chemother 53:4749–4752. doi: 10.1128/AAC.00889-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sansone-Parsons A, Krishna G, Calzetta A, Wexler D, Kantesaria B, Rosenberg MA, Saltzman MA. 2006. Effect of a nutritional supplement on posaconazole pharmacokinetics following oral administration to healthy volunteers. Antimicrob Agents Chemother 50:1881–1883. doi: 10.1128/AAC.50.5.1881-1883.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jang SH, Colangelo PM, Gobburu JVS. 2010. Exposure-response of posaconazole used for prophylaxis against invasive fungal infections: evaluating the need to adjust doses based on drug concentrations in plasma. Clin Pharmacol Ther 88:115–119. doi: 10.1038/clpt.2010.64. [DOI] [PubMed] [Google Scholar]
- 10.Gorschlüter M, Mey U, Strehl J, Ziske C, Schepke M, Schmidt-Wolf IG, Sauerbruch T, Glasmacher A. 2005. Neutropenic enterocolitis in adults: systematic analysis of evidence quality. Eur J Haematol 75:1–13. doi: 10.1111/j.1600-0609.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- 11.Pille S, Bohmer D. 1998. Options for artificial nutrition of cancer patients. Strahlenther Onkol 174(Suppl 3):S52–S55. [PubMed] [Google Scholar]
- 12.Krishna G, Ma L, Martinho M, O'Mara E. 2012. Single-dose phase I study to evaluate the pharmacokinetics of posaconazole in new tablet and capsule formulations relative to oral suspension. Antimicrob Agents Chemother 56:4196–4201. doi: 10.1128/AAC.00222-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merck. 2014. Prescribing information for Noxafil (posaconazole) injection/delayed-release tablets/oral suspension. Merck & Co., Inc., Whitehouse Station, NJ. [Google Scholar]
- 14.Shen JX, Krishna G, Hayes RN. 2007. A sensitive liquid chromatography and mass spectrometry method for the determination of posaconazole in human plasma. J Pharm Biomed Anal 43:228–236. doi: 10.1016/j.jpba.2006.06.011. [DOI] [PubMed] [Google Scholar]


