Abstract
There is an urgent need for new antimalarial agents and strategies to treat and control malaria. This study shows an antiplasmodium effect of tulathromycin in mice infected with Plasmodium yoelii. The administration of tulathromycin around the time of infection prevented the progression of disease in 100% of the animals. In addition, highly parasitized mice treated with tulathromycin showed a decreased parasite burden and cleared the parasite faster than did untreated infected mice.
TEXT
Malaria is one of the most significant global medical and scientific burdens, affecting nearly 40% to 50% of the world's population (available in the World Health Organization [WHO] database at http://www.who.int/malaria/media/world_malaria_report_2013/en/index.htm). Despite advances in the development of malaria vaccines, no vaccine has shown high efficacy in protecting humans from Plasmodium infection or clinical malaria (1). Consequently, the use of antimalarial drugs will continue to be necessary for the management of this disease. Furthermore, morbidity and mortality due to malaria are exacerbated by underdiagnosed bacterial coinfections and severe pneumonia (2, 3). Thus, the coadministration of drugs with both antimalarial and antibiotic effects may be beneficial in certain field circumstances.
Malaria chemotherapy has relied on a limited number of drugs that include chloroquine, sulfas and pyrimethamine, and artemisinin-derived drugs, among others (4). Unfortunately, Plasmodium has developed mechanisms of resistance against these drugs, limiting their efficacy in controlling the disease (5, 6; also available in the WHO database [http://www.who.int/malaria/publications/atoz/9789241500838/en/]). The lack of an effective malaria vaccine and the continued development of drug-resistant parasites highlight the need for new antimalarial agents and strategies to treat and control the disease.
Novel, effective, and safe antimalarial agents are urgently needed, especially drugs that could be used in combination with existing medicines to prevent the development of drug resistance. Macrolides are of particular interest as antimalarial drugs because, in contrast to others, they can be used safely in children and pregnant women (7–11).
The objective of this study was to evaluate the anti-Plasmodium activity of tulathromycin during both early and advanced blood stages of infection in a murine model of malaria. Here, we show the antimalarial effect of tulathromycin, a semisynthetic antimicrobial macrolide with a large margin of safety approved by the United States Food and Drug Administration and the European Medicines Agency for use in veterinary species (12).
The novelty of tulathromycin lies in its unique structure, three nitrogen/amine functional groups, representing the first member of a subclass of macrolides known as triamilides (13). In addition, tulathromycin could be formulated for different routes of delivery, since it is readily bioavailable when administered orally (14), subcutaneously (15), and intramuscularly (16). Furthermore, the antibacterial activity of tulathromycin has the potential to target bacterial coinfections, making it ideal as a combinatorial therapy.
We evaluated the efficacy of tulathromycin during the early blood stage of infection using an infective inoculum of Plasmodium yoelii. For this, C57BL/6N female mice (n = 23) (6 to 8 weeks old, 18 to 20 g in body weight) from the National Cancer Institute (Frederick, MD) were infected with 105 P. yoelii 17XNL-parasitized red blood cells (RBCs) intravenously at time zero. Mice were treated subcutaneously in the interscapular region with tulathromycin (n = 8) (25 mg/kg of body weight) (Draxxin injectable solution [100 mg/ml], lot A201905; Zoetis, Kalamazoo, MI, USA) or azithromycin (n = 8) (25 mg/kg of body weight) (diluted in propylene glycol at 100 mg/ml, lot P500088; Sigma-Aldrich, St. Louis, MO, USA) at −1 h and 12, 24, 36, and 48 h post-P. yoelii infection. The selection of the dose and route of administration of tulathromycin and azithromycin used in this study was based on information available in the literature (17). Azithromycin was used as a positive-control antimalarial macrolide (18). In this study, all mice tolerated the treatments. The rest of the mice (n = 7) remained untreated, representing the control group. Parasitemia (percentage of red blood cells infected with P. yoelii) was assessed by evaluation of Giemsa-stained monolayer RBC smears. A sample was taken from each mouse every 2 days from 5 to 30 days postinfection by snipping the tail and collecting a drop of blood. We assessed at least 10 high-power (1,000×) fields (the detection limit was ∼1 parasite per 5,000 RBCs). The number (mean ± standard deviation [SD]) of RBCs evaluated for each infected mouse/time point was 427 ± 131. One-way analysis of variance (ANOVA) and Tukey's multiple-comparison test were used to compare statistically the parasitemia and area under the parasitemia-time curve (AUC) between groups. The AUC was estimated for each group according to the trapezoidal rule (19, 20).
Following infection with P. yoelii, untreated mice exhibited peak parasitemia reaching about 50% 17 days postinfection followed by gradual clearance (Fig. 1). In striking contrast, treatment with tulathromycin before and shortly after infection with P. yoelii completely prevented the progression of infection to detectable parasitemia (Fig. 1), demonstrating compelling activity at preventing P. yoelii infections. The positive-control group treated with azithromycin showed the same effect, confirming the antiplasmodium effect of tulathromycin.
FIG 1.
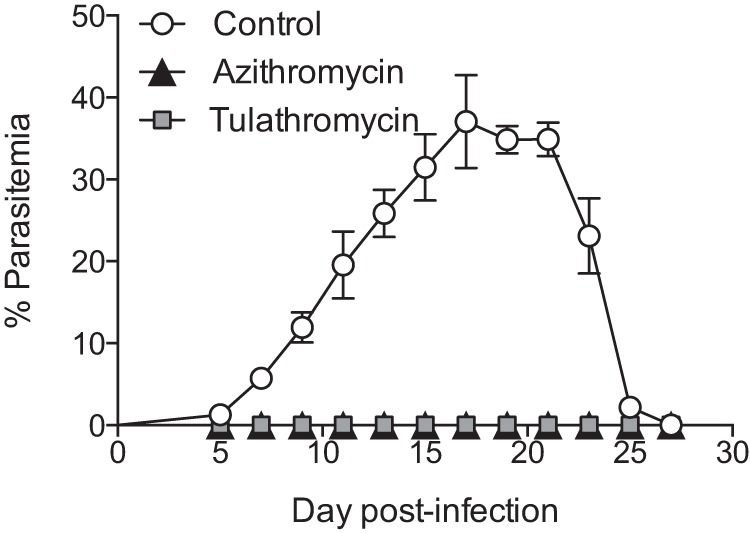
Tulathromycin exhibits strong activity against Plasmodium. Parasitemia in C57BL/6N mice infected with Plasmodium yoelii 17XNL (n = 23; n = 7 for control, n = 8 for azithromycin, and n = 8 for tulathromycin) at the indicated times. Mice in the azithromycin and tulathromycin groups were treated at −1, 12, 24, 36, and 48 h postinfection. Data (mean ± standard error of the mean) are cumulative data from two independent experiments.
We next tested the ability of tulathromycin to clear an established Plasmodium infection. C57BL/6N female mice (n = 38) were infected with 105 P. yoelii 17XNL-parasitized RBCs intravenously. Fifteen days postinfection, mice were treated with tulathromycin, azithromycin, or artemether alone or in combination. Artemether (lot F04107; USP, Rockville, MD, USA; diluted in olive oil at 10 mg/ml) was administered intraperitoneally at 25 mg/kg of body weight. Tulathromycin and azithromycin were administered subcutaneously, and parasitemia was tracked, as described above.
At the time of initial treatment, all mice had similar levels of parasitemia, about 50% (P > 0.05) (Fig. 2A). In contrast to the untreated group of mice, treatment with tulathromycin decreased the parasitemia in all mice, showing a delayed onset of action but a marked antimalarial effect, reflected in a lower AUC (P < 0.0001) in the tulathromycin group (Fig. 2B). Additionally, mice treated with tulathromycin cleared P. yoelii faster than did untreated animals (day 19 versus day 25 postinfection). The parasitemia levels in the tulathromycin and azithromycin groups were not different, confirming the antiplasmodium effect of tulathromycin. Of note, it is not possible to determine which drug is more active against Plasmodium, since factors such as type of formulation and rate and extent of drug absorption may confound the conclusions. However, research is warranted in this area.
FIG 2.
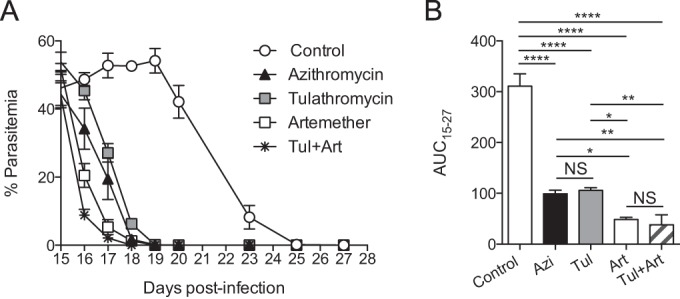
Tulathromycin efficiently controls high Plasmodium parasite burden. C57BL/6N mice were infected with Plasmodium yoelii at time zero (n = 37, 7 to 8 mice per group). Groups of mice were treated with azithromycin (Azin; n = 7), tulathromycin (Tuln; n = 8), artemether (Art; n = 7), or tulathromycin plus artemether (Tul+Art; n = 8). All the treatments were started at day 15 postinfection until resolution of the infection. A group of infected mice remained untreated, representing the control group (n = 7). (A) Percent parasitemia on the indicated days. (B) Area under the parasitemia-time curve (AUC) for each group during the period after drug administration until resolution of the infection. Data (mean ± standard error of the mean) are cumulative data from two independent experiments. *, P < 0.05; **, P < 0.01; ****, P < 0.0001; NS, not significant.
As expected, treatment with artemether resulted in antimalarial activity with the parasite cleared in all mice after 3 drug administrations (day 18 postinfection) (Fig. 2A and B). The coadministration of tulathromycin did not improve or impair the antimalarial activity of artemether, reflected in similar AUC15–18 days values (P > 0.05). These results demonstrate that tulathromycin exhibits robust antimalarial activity when administered at points of high parasitemia. Furthermore, they demonstrate that tulathromycin could be used in combination with artemether in treating Plasmodium. The possibility of combining these two drugs is an important advantage, considering that drug combinations increase the rates of clinical cures and overcome the selection pressure for the emergence of antimalarial resistance (21).
Due to its pharmacokinetic features, antimicrobial activity, and very promising anti-Plasmodium effect, tulathromycin warrants future research as a candidate for treatment of human malaria. Alternatively, its unique chemical structure may serve as a template to design new macrolide entities with antimalarial effects.
In this study, we discovered that tulathromycin is active against P. yoelii and is able to effectively eliminate the infection when used as a monotherapy. Whereas tulathromycin exhibits strong antimalarial activity as a monotherapy, this approach is strongly discouraged since it increases the likelihood that Plasmodium will develop drug resistance. Importantly, we also showed that tulathromycin could be combined with artemether without this combinatorial treatment interfering with the antimalarial activity of either. Since tulathromycin is in the same chemical family as azithromycin, it is possible that these drugs will exhibit the same mechanism of action and pharmacokinetics/pharmacodynamics, which may negate the need to develop tulathromycin as an antimalarial drug. However, given the key differences in the chemical structure between tulathromycin and other macrolides, it is equally likely that tulathromycin functions as an antimalarial through a novel mechanism and may exhibit important differences in pharmacokinetics/pharmacodynamics. Consequently, the novel findings on the antiplasmodium activity of tulathromycin certainly deserve further research. These could include an analysis of the causal prophylactic effect and pharmacokinetics/pharmacodynamics using different human Plasmodium species to explore the potential benefits of tulathromycin over other macrolides and/or antimalarial drugs. Furthermore, veterinary chemotherapy has made contributions to the treatment of other human parasitic diseases. For example, ivermectin has been used as an antiparasitic drug in both veterinary and human medicine (22, 23), which is one of many examples that support the potential of repurposing tulathromycin, or a derivative, as an antimalarial drug for human use.
ACKNOWLEDGMENTS
This work was supported by the University of Tennessee.
We thank Barry Rouse, Todd Reynolds, and Fabiana Landoni for reading the manuscript and providing feedback.
REFERENCES
- 1.Olotu A, Fegan G, Wambua J, Nyangweso G, Awuondo KO, Leach A, Lievens M, Leboulleux D, Njuguna P, Peshu N, Marsh K, Bejon P. 2013. Four-year efficacy of RTS,S/AS01E and its interaction with malaria exposure. N Engl J Med 368:1111–1120. doi: 10.1056/NEJMoa1207564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amexo M, Tolhurst R, Barnish G, Bates I. 2004. Malaria misdiagnosis: effects on the poor and vulnerable. Lancet 364:1896–1898. doi: 10.1016/S0140-6736(04)17446-1. [DOI] [PubMed] [Google Scholar]
- 3.Berkley J, Mwarumba S, Bramham K, Lowe B, Marsh K. 1999. Bacteraemia complicating severe malaria in children. Trans R Soc Trop Med Hyg 93:283–286. doi: 10.1016/S0035-9203(99)90024-X. [DOI] [PubMed] [Google Scholar]
- 4.Trape JF. 2001. The public health impact of chloroquine resistance in Africa. Am J Trop Med Hyg 64(1-2 Suppl):12–17. [DOI] [PubMed] [Google Scholar]
- 5.Maxmen A. 2013. Malaria: a race against resistance. Nature 503:186–188. doi: 10.1038/503186a. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. 2011. Global plan for artemisinin resistance containment. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 7.Bahat Dinur A, Koren G, Matok I, Wiznitzer A, Uziel E, Gorodischer R, Levy A. 2013. Fetal safety of macrolides. Antimicrob Agents Chemother 57:3307–3311. doi: 10.1128/AAC.01691-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin KJ, Mitchell AA, Yau WP, Louik C, Hernández-Díaz S. 2013. Safety of macrolides during pregnancy. Am J Obstet Gynecol 208:221.e1–221.e8. doi: 10.1016/j.ajog.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris JA, Kolokathis A, Campbell M, Cassell GH, Hammerschlag MR. 1998. Safety and efficacy of azithromycin in the treatment of community-acquired pneumonia in children. Pediatr Infect Dis J 17:865–871. doi: 10.1097/00006454-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Ruuskanen O. 2004. Safety and tolerability of azithromycin in pediatric infectious diseases: 2003 update. Pediatr Infect Dis J 23:S135–139. doi: 10.1097/01.inf.0000112528.75956.41. [DOI] [PubMed] [Google Scholar]
- 11.Kafetzis DA, Chantzi F, Tigani G, Skevaki CL. 2007. Safety and tolerability of clarithromycin administered to children at higher-than-recommended doses. Eur J Clin Microbiol Infect Dis 26:99–103. doi: 10.1007/s10096-006-0247-3. [DOI] [PubMed] [Google Scholar]
- 12.Norcia LJ, Silvia AM, Santoro SL, Retsema J, Letavic MA, Bronk BS, Lundy KM, Yang B, Evans NA, Hayashi SF. 2004. In vitro microbiological characterization of a novel azalide, two triamilides and an azalide ketal against bovine and porcine respiratory pathogens. J Antibiot (Tokyo) 57:280–288. doi: 10.7164/antibiotics.57.280. [DOI] [PubMed] [Google Scholar]
- 13.Letavic MA, Bronk BS, Bertsche CD, Casavant JM, Cheng H, Daniel KL, George DM, Hayashi SF, Kamicker BJ, Kolosko NL, Norcia LJ, Oberton VD, Rushing MA, Santoro SL. 2002. Synthesis and activity of a novel class of tribasic macrocyclic antibiotics: the triamilides. Bioorg Med Chem Lett 12:2771–2774. doi: 10.1016/S0960-894X(02)00526-7. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Tao YF, Huang LL, Chen DM, Yin SZ, Ihsan A, Zhou W, Su SJ, Liu ZL, Pan YH, Yuan ZH. 13 July 2011. Pharmacokinetics of tulathromycin and its metabolite in swine administered with an intravenous bolus injection and a single gavage. J Vet Pharmacol Ther doi: 10.1111/j.1365-2885.2011.01322.x. [DOI] [PubMed] [Google Scholar]
- 15.Nowakowski MA, Inskeep PB, Risk JE, Skogerboe TL, Benchaoui HA, Meinert TR, Sherington J, Sunderland SJ. 2004. Pharmacokinetics and lung tissue concentrations of tulathromycin, a new triamilide antibiotic, in cattle. Vet Ther 5:60–74. [PubMed] [Google Scholar]
- 16.Benchaoui HA, Nowakowski M, Sherington J, Rowan TG, Sunderland SJ. 2004. Pharmacokinetics and lung tissue concentrations of tulathromycin in swine. J Vet Pharmacol Ther 27:203–210. doi: 10.1111/j.1365-2885.2004.00586.x. [DOI] [PubMed] [Google Scholar]
- 17.Villarino N, Brown SA, Martín-Jiménez T. 2012. Pharmacokinetics of tulathromycin in healthy and neutropenic mice challenged intranasally with lipopolysaccharide from Escherichia coli. Antimicrob Agents Chemother 56:4078–4086. doi: 10.1128/AAC.00218-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puri SK, Singh N. 2000. Azithromycin: antimalarial profile against blood- and sporozoite-induced infections in mice and monkeys. Exp Parasitol 94:8–14. [DOI] [PubMed] [Google Scholar]
- 19.Méndez F, Muñoz A, Plowe CV. 2006. Use of area under the curve to characterize transmission potential after antimalarial treatment. Am J Trop Med Hyg 75:640–644. [PubMed] [Google Scholar]
- 20.Matthews JN, Altman DG, Campbell MJ, Royston P. 1990. Analysis of serial measurements in medical research. BMJ 300:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fidock DA. 2013. Microbiology. Eliminating malaria. Science 340:1531–1533. doi: 10.1126/science.1240539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell WC. 1982. Efficacy of the avermectins against filarial parasites: a short review. Vet Res Commun 3:251–262. [DOI] [PubMed] [Google Scholar]
- 23.Hoerauf A, Pfarr K, Mand S, Debrah AY, Specht S. 2011. Filariasis in Africa—treatment challenges and prospects. Clin Microbiol Infect 7:977–985. doi: 10.1111/j.1469-0691.2011.03586.x. [DOI] [PubMed] [Google Scholar]


