Abstract
Vancomycin is a renally excreted drug, and its body clearance correlates with creatinine clearance. However, the renal function estimation equation that best predicts vancomycin clearance has not been established yet. The objective of this study was to compare the abilities of different renal function estimation equations to describe vancomycin pharmacokinetics in elderly patients. The NPAG algorithm was used to perform population pharmacokinetic analysis of vancomycin concentrations in 78 elderly patients. Six pharmacokinetic models of vancomycin clearance were built, based on the following equations: Cockcroft-Gault (CG), Jelliffe (JEL), Modification of Diet in Renal Disease (MDRD), Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) (both in milliliters per minute per 1.73 m2), and modified MDRD and CKD-EPI equations (both in milliliters per minute). Goodness-of-fit and predictive performances of the six PK models were compared in a learning set (58 subjects) and a validation set (20 patients). Final analysis was performed to estimate population parameters in the entire population. In the learning step, the MDRD-based model best described the data, but the CG- and JEL-based models were the least biased. The mean weighted errors of prediction were significantly different between the six models (P = 0.0071). In the validation group, predictive performances were not significantly different. However, the use of a renal function estimation equation different from that used in the model building could significantly alter predictive performance. The final analysis showed important differences in parameter distributions and AUC estimation across the six models. This study shows that methods used to estimate renal function should not be considered interchangeable for pharmacokinetic modeling and model-based estimation of vancomycin concentrations in elderly patients.
INTRODUCTION
Renal function influences vancomycin elimination kinetics and its dosing in clinical practice. About 80 to 90% of a vancomycin dose is eliminated unchanged in the urine in subjects with normal renal function (1). Vancomycin body clearance is significantly reduced and half-life is prolonged in elderly patients compared with those in young adult patients. While reduced renal function is the major determinant, age has also been described as an independent descriptor of pharmacokinetic changes in the elderly (2).
The Bayesian approach is viewed as the most efficient method for vancomycin monitoring and dose adjustment (3, 4). This approach requires population information on the drug pharmacokinetics (PK), including the structural and covariate models, and parameter distributions. Population PK analysis can provide such information and identify quantitative relationships between pharmacokinetic parameters and clinical descriptors such as renal function.
In the 1980s and 1990s, several studies reported a positive linear correlation between vancomycin body clearance and creatinine clearance (CLCR) (5–10). In those studies, creatinine clearance was either estimated using the Cockcroft-Gault (CG) equation or measured from urine collection. For vancomycin and many other drugs, the CG equation has been the reference index for adjusting drug dosage regimens in the case of renal impairment. New equations for estimating the glomerular filtration rate (GFR) have been proposed in the last 15 years, notably the Modification of Diet in Renal Disease (MDRD) equation and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. It has been shown that these two equations provide more accurate estimation of the GFR than the CG equation in most patient populations (11–15). However, it remains to be proven that these new equations are better descriptors of drug clearance for renally excreted agents. The equation that best predicts vancomycin clearance has not been established yet.
The objective of this study was to compare the abilities of different renal function estimation equations to describe vancomycin clearance and estimate vancomycin concentrations in a population of elderly hospitalized patients.
(This work has been presented in part at the 9th French meeting of Physiology, Pharmacology and Therapeutics [P2T], 22 to 24 April 2014, Poitiers, France.)
MATERIALS AND METHODS
Study population.
This was a retrospective study performed with patients hospitalized in various geriatric units from October 2009 to January 2013. Most patients were hospitalized at the University Hospitals of Lyon. A few patients were hospitalized in other hospitals in the Lyon area. Vancomycin and serum creatinine assay were performed in the same laboratory, except for five patients. All patients benefited from routine therapeutic drug monitoring and Bayesian adaptive control of vancomycin dosage regimens using the BestDose (formerly MM-USC*PACK) software (Laboratory of Applied Pharmacokinetics, USC School of Medicine, Los Angeles, CA [http://www.lapk.org]). As this was a retrospective, noninterventional study based on data collected in routine clinical care, an Institutional Review Board (IRB) approval was not necessary, in accordance with French regulations for clinical research.
Data available for each patient were age, sex, height, weight, and serum creatinine levels, as well as vancomycin doses, infusion durations, dosing times, blood sampling times, and concentrations in plasma. Any changes in covariate observations during vancomycin therapy (e.g., body weight and serum creatinine) also were recorded precisely.
Serum creatinine was assayed by an enzymatic method on an Abbott Architect C8000 automated analyzer. This method was traceable to the reference isotope dilution mass spectrometry (IDMS) method. Serum vancomycin concentrations were measured by immunoturbidimetric assay on the same Abbott Architect automated system. The assay calibration data, expressed as coefficients of variation, were 6.56%, 2.20%, and 2.41% for control levels of 6.40, 22.26, and 36.08 mg/liter, respectively. The lower limit of quantification of the assay was 1.1 mg/liter.
Renal function estimation.
For each patient, renal function was estimated and updated for any change in serum creatinine by using six estimation equations: the original Cockcroft-Gault, the Jelliffe (JEL), the MDRD, the CKD-EPI, and the modified MDRD and CKD-EPI (MDRDm and CKD-EPIm, respectively) equations. Every serum creatinine available during the duration of vancomycin therapeutic drug monitoring was included in the analysis, even when vancomycin concentration was not measured on the same day. The modified MDRD and CKD-EPI equations consisted of adjusting the estimate provide by the equations to individual body surface area. The six equations are detailed below.
The Cockcroft and Gault (CG) formula is (16) CLCR (milliliters per minute) = [K × (140 − age) × weight]/SCr, where K is 1.23 if the subject is female and 1.04 if the subject is male, age is in years, actual body weight is in kilograms, and serum creatinine (SCr) is in micromoles per liter.
The Jelliffe equation (JEL), which is implemented in the BestDose software, is (17) CLCR (milliliters per minute per 1.73 m2) = [P − 0.4 × W × (C2 − C1)/T]/[(C1 + C2)/2 × 1,440] × (1.73/BSA), where P is the adjusted daily production of creatinine (in milligrams per day, estimated from previous works), W is the body weight in hundreds of grams, C1 and C2 are the first and the second serum creatinine levels in milligram/deciliter, and T is the time between the two serum creatinine levels in days; 1,440 is the number of minutes in 1 day. The estimate is adjusted to body surface area (BSA, in square meters) and expressed per 1.73 m2 of surface area. Body surface area is estimated using the Gehan and George equation (18).
The 4-variable simplified Modification of Diet in Renal Disease (MDRD) equation is (19) GFR (milliliters per minute per 1.73 m2) = 175 × SCr−1.154 × age−0.203 (with the result multiplied by 1.212 if the subject is black and 0.742 if the subject is female, and both coefficients apply to black females), where GFR is the glomerular filtration rate and SCr is serum creatinine in micromoles per liter.
The CKD-EPI equation is (13) GFR (milliliters per minute per 1.73 m2) = 141 × min(SCr/κ, 1)α × max(SCr/κ, 1)−1.209 × 0.993age (with the result multiplied by 1.159 if the subject is black and 1.018 if the subject is female, and both coefficients apply to black females), where SCr is serum creatinine (in micromoles per liter), κ is a constant equal to 0.7 if the subject is female and 0.9 if the subject is male, α is a constant equal to −0.329 if the subject is female and −0.411 if the subject is male, and min and max indicate the minimum and maximum of SCr/κ or 1, respectively.
The modified MDRD and CKD-EPI equations adjusted to individual body surface area are GFR (milliliters per minute) = GFR(MDRD or CKD-EPI) × (BSA/1.73 m2), where BSA is the patient's individual body surface area estimated by the Du Bois formula (20).
Pharmacokinetic model building.
The patient population was divided into two groups by random selection: a learning set of 58 patients and a validation set of 20 patients. The learning set was used for model selection and initial estimation of pharmacokinetic parameters. The validation set was then used for external validation of the models selected in the first step. The entire data set was used for final estimation of pharmacokinetic parameters (third step). The Non-Parametric Adaptive Grid (NPAG) algorithm was used as population approach for all analyses (21).
One-, two-, and three-compartment models without any covariates were fitted to vancomycin concentrations in the learning set to determine the best structural model. The influences of available covariates on PK parameters were then assessed in the selected structural model.
The influence of body weight on vancomycin clearance and volume of distribution was examined using various relationships, including linear equations and allometric scaling.
The influence of renal function on vancomycin clearance was modeled as follows:
| (1) |
where CLVANCO is vancomycin total body clearance (in liters per hour), CLNR is vancomycin nonrenal clearance (liters per hour), E is the estimated creatinine clearance or estimated glomerular filtration rate calculated with the CG, JEL, MDRD, MDRDm, CKD-EPI, or CKD-EPIm equation (estimates in milliliters per minute or milliliters per minute per 1.73 m2 were converted in liters per hour or liters per hour per 1.73 m2 by multiplying by 0.06 in the PK model file), and CLS is a slope parameter (in liters per hour per unit of CLCR or GFR).
Because a two-compartment model best fit the data (see Results), six two-compartment models were created and compared in subsequent analyses, each based on a renal function estimation equation (see equation 1). Each model had five parameters: the two clearance parameters described above (CLNR and CLS), volume of distribution (V, in liters), and intercompartment transfer rate constants from the central to the peripheral compartment (KCP, per hour) and in the opposite direction (KPC, per hour). These models are referred to as CG-, JEL-, MDRD-, MDRDm-, CKD-EPI-, and CKD-EPIm-based models in this article for ease of reading.
In the NPAG modeling procedure, the residual variability was described by a polynomial equation as follows:
| (2) |
where Yi is the predicted vancomycin assay standard deviation for the observed concentration Cobsi. The coefficients in the relationship between Yi and Cobsi are based on best-fit polynomial to the drug assay precision data. γ is a positive coefficient estimated by the NPAG algorithm which inflates the assay imprecision to take into account other sources of noise in the parameter estimation.
The goodness of fit of candidate models was evaluated using the Akaike information criterion (AIC = −2 log(L) + 2p, where L is the likelihood and p is the number of parameters in the model). When comparing two models, a lower AIC value indicates a better fit.
Bias and precision of population and individual Bayesian posterior predictions provided by the model were used to assess predictive performances of candidate models. The mean error (ME, in milligrams per liter) and the mean weighted error (MWE, in milligrams per liter) of prediction were used as measures of bias, while the mean weighted squared error (MWSE, in square milligrams per square liter) of prediction was used as a measure of precision. Weighted measures of bias and precision were used because outlier data (very high, unexplained observed vancomycin concentrations) were observed in the data set. Those measures are based on the individual error of prediction (Ei), according to the following equations:
where Cobsi and Cpredi represent the concentrations observed and predicted by the model, respectively (in milligrams per liter), and Yi is the standard deviation related to Cobsi and provided by equation 2. ME, MWE, and MWSE are the means of Ei, WEi, and WSEi, respectively.
Model validation.
The predictive performances of the final models were assessed in a subset of 20 patients who were not included in the learning step. The population nonparametric joint densities of pharmacokinetic parameters estimated by the NPAG algorithm in the learning set were used to calculate population predictions. In addition, those densities were used as priors for Bayesian estimation of individual parameters of the 20 patients and subsequent calculation of individual predictions. Bias and precision of both population and individual predictions from the six models were compared. Also, a cross-validation was performed for the models based on the CG, JEL, original MDRD, and original CKD-EPI equations, by calculating individual predictions from each model but replacing the corresponding renal function estimates with those provided by the other three equations for each patient in the validation data set. This cross-validation was performed to investigate whether the renal function estimation equations could be interchangeable in model-based prediction.
Final analysis.
Finally, the six final models were fitted to vancomycin data from the entire 78-patient data set in order to get final estimates of the PK parameters. The nonparametric population joint densities estimated in the 58-patient learning set were used as initial prior distributions for this calculation. The goodness-of-fit and predictive performances were assessed as described above for the learning set. In addition, Bayesian posterior parameters from each model were used to calculate the individual area under the concentration-time curves (AUCs). Because all 78 patients were treated for at least 48 h, AUC24–48 calculated over 24 h on the second day of therapy was used for between-model comparisons.
Statistical analysis.
The nonparametric Kruskal-Wallis test was used to compare bias and precision between the six models at each stage of the analysis. The same test was also used to compare the estimation of renal function provided by the six formulas. The nonparametric Friedman test for matched groups was used to compare the vancomycin AUC24–48 calculated from the six models. Statistical significance was set at 0.05 for all comparisons.
RESULTS
Patient characteristics are presented in Table 1. For the whole study population of 78 subjects, 376 measured vancomycin concentrations were available. The number of vancomycin concentrations per patient ranged from 1 to 16 (median, 4). Those concentrations were collected over the duration of vancomycin monitoring, which was variable. Most patients were sampled on several occasions, with one to three measurements taken on each occasion (i.e., a given dosing interval). There were 23.7% of concentrations measured at or near the peak (0.5 to 2 h after the end of the infusion, n = 89). About three-quarters of patients were administered intravenous vancomycin under intermittent dosing exclusively. The other patients received vancomycin by continuous intravenous (i.v.) treatment during the entire therapy or part of it. Two hundred sixteen serum creatinine measurements and the same number of renal function estimates from each prediction equation were available. The renal function estimates provided by the six equations were significantly different (P < 0.0001). The patients' characteristics were similar between the learning and the validation data sets.
TABLE 1.
Characteristics of the study population
| Characteristic | Value for groupa |
||
|---|---|---|---|
| Overall | Learning set | Validation set | |
| No. of patients | 78 | 58 | 20 |
| No. of females/males | 37/41 | 27/31 | 10/10 |
| Age (yrs) | 82.8 ± 6.8 | 83.2 ± 5.9 | 84.1 ± 9.0 |
| Wt (kg) | 62.7 ± 12.2 | 62.3 ± 11.8 | 63.9 ± 13.7 |
| Body surface area (m2) | 1.67 ± 0.19 | 1.67 ± 0.19 | 1.69 ± 0.19 |
| Serum creatinine (mg/dl)b | 1.15 ± 0.80 | 1.15 ± 0.89 | 1.17 ± 0.42 |
| Initial vancomycin dose (mg/24 h) | 1,649 ± 672 | 1,665 ± 691 | 1,602 ± 629 |
| Intermittent dosing (%) | 75.6 | 75.9 | 75.0 |
| No. of observed vancomycin concentrations | 376 | 289 | 87 |
| No. of peak levels (0.5–2 h postdose) | 89 | 68 | 21 |
| CLCR, CG (ml/min) | 52 ± 23c (6–126) | 52 ± 23 | 52 ± 23 |
| CLCR, JEL (ml/min/1.73 m2) | 58 ± 26c (9–152) | 60 ± 27 | 49 ± 21 |
| GFR, MDRD (ml/min/1.73 m2) | 70 ± 33c (10–193) | 72 ± 34 | 62 ± 26 |
| GFR, MDRDm (ml/min) | 68 ± 32c (8–188) | 69 ± 33 | 63 ± 27 |
| GFR, CKD-EPI (ml/min/1.73 m2) | 64 ± 24c (9–105) | 65 ± 24 | 58 ± 22 |
| GFR, CKD-EPIm (ml/min) | 61 ± 24c (7–114) | 62 ± 24 | 59 ± 24 |
Data are given as means ± SDs unless otherwise stated. Renal function estimates in the entire population are given as means ± SDs (min − max).
n = 216.
The estimates of renal function were significantly different in the entire population (P < 0.0001, global comparison with the Kruskal-Wallis test).
In the learning set, a two-compartment model best fit the data. Such a model was associated with a 291-point decrease in the AIC compared with a one-compartment model without any covariate. Body weight and age did not influence vancomycin clearance or volume of distribution. This was not surprising, as the ranges of those covariates were limited in this very homogenous population. Only renal function significantly influenced vancomycin pharmacokinetics. Values of the gamma parameter of the residual error model ranged from 1.23 (MDRDm model) to 1.39 (CKD-EPIm model), which indicates limited overall noise in the data. Goodness-of-fit and predictive performances of the six clearance models are shown in Table 2. The inclusion of estimated CLCR or GFR as a covariate on vancomycin body clearance significantly improved the model fit with each of the six renal function estimation equations. However, the relative decrease in the AIC compared with the simple two-compartment model varied substantially between the six models of vancomycin clearance: −43.6, −40.2, −58.5, −56.9, −12.2, and −6.7 for the models based on the CG, JEL, MDRD, MDRDm, CKD-EPI, and CKD-EPIm equations, respectively. The AIC criterion indicated that the MDRD- and MDRDm-based models best fit the data. The CG- and JEL-based models were close, while the CKD-EPI- and CKD-EPIm-based models showed poorer fits. Biases were significantly different between the six models (P = 0.0071). The JEL- and CG-based model showed the least mean weighted error. Precisions, as measured by the mean weighted squared error, were not significantly different (P = 0.80).
TABLE 2.
Goodness-of-fit and predictive performances of the six models in the learning set (n = 58 patients, 289 vancomycin concentrations)
| Model | AIC | AIC decreasea | Weighted error (mg/liter) | Weighted squared error (mg2/liter2) |
|---|---|---|---|---|
| CG | 1,606.7 | −43.6 | 0.82 ± 5.3 | 28.15 ± 52.6 |
| JEL | 1,610.1 | −40.2 | 0.50 ± 5.4 | 29.0 ± 67.3 |
| MDRD | 1,591.8 | −58.5 | 1.6 ± 5.6 | 34.1 ± 73.3 |
| MDRDm | 1,593.4 | −56.9 | 1.7 ± 5.7 | 35.1 ± 69.4 |
| CKDEPI | 1,638.1 | −12.2 | 1.4 ± 5.2 | 28.9 ± 62.7 |
| CKDEPIm | 1,643.6 | −6.7 | 1.4 ± 5.0 | 27.1 ± 54.6 |
| P valueb | 0.0071 | 0.80 |
Calculated as the AIC of the reference two-compartment model without covariate (1,650.3) minus the AIC of the selected model.
Probability that the errors do not differ among the models using the Kruskal-Wallis test.
In the analysis of the validation data set, bias and precision of both population and individual predictions from the six models were not significantly different (Table 3). In addition, a cross-validation of the CG-, JEL-, MDRD-, and CKD-EPI-based models was performed by fitting each model to the original renal function data and also to data with renal function estimated by each other equation. The results are shown in Table 4. For the CG- and JEL-based models, the use of GFR estimated by the MDRD or the CKD-EPI equation in place of the original equations (i.e., CG or JEL) resulted in greater negative biases for both population and individual predictions. In other words, using the MDRD or the CKD-EPI equations in place of the CG and JEL equations led to underestimation of vancomycin concentrations. The CG and JEL equations appear to be interchangeable, as the mean errors were very similar. For the MDRD- and CKD-EPI-based models, the use of CLCR estimated by the CG or the JEL equation in place of the original equation significantly altered population predictions but not individual predictions. For population predictions, the MDRD- and CKD-EPI-based models appear to overestimate vancomycin concentrations (greater positive biases) when CLCR estimated by the CG or the JEL equation was used. In contrast, the MDRD and CKD-EPI equations appear to be interchangeable for model-based prediction of vancomycin concentrations.
TABLE 3.
Predictive performance of the six models in the validation set (n = 20 patients, 87 vancomycin concentrations)
| Model | Population predictions |
Individual predictions |
||
|---|---|---|---|---|
| Weighted error (mg/liter) | Weighted squared error (mg2/liter2) | Weighted error (mg/liter) | Weighted squared error (mg2/liter2) | |
| CG | 1.9 ± 4.6 | 24.8 ± 63.8 | 0.38 ± 2.7 | 7.6 ± 12.9 |
| JEL | 1.6 ± 4.4 | 21.6 ± 57.7 | −0.15 ± 2.2 | 4.9 ± 7.5 |
| MDRD | 2.1 ± 5.1 | 30.0 ± 85.8 | −0.42 ± 2.0 | 4.2 ± 8.0 |
| MDRDm | 1.7 ± 4.5 | 22.6 ± 61.5 | 0.17 ± 2.4 | 5.6 ± 10.2 |
| CKDEPI | 2.1 ± 4.4 | 23.3 ± 61.6 | −0.24 ± 2.1 | 4.3 ± 7.6 |
| CKDEPIm | 1.4 ± 4.3 | 20.2 ± 56.9 | −0.23 ± 2.1 | 4.4 ± 7.6 |
| P valuea | 0.61 | 0.89 | 0.24 | 0.33 |
Probability that the errors do not differ among the models using the Kruskal-Wallis test.
TABLE 4.
Cross-validation of the vancomycin models (n = 20 patients, 87 vancomycin concentrations)a
| Model and type of prediction | Method used to estimate renal function in the validation data set |
P valueb | |||
|---|---|---|---|---|---|
| CG | JEL | MDRD | CKD-EPI | ||
| CG | |||||
| Population predictions | 0.64 | 0.66 | −2.1 | −1.36 | 0.0102 |
| Individual predictions | −1.5 | −2.6 | −3.1 | −3.36 | 0.038 |
| JEL | |||||
| Population predictions | 0.049 | 0.0037 | −2.7 | −2.0 | 0.0115 |
| Individual predictions | −2.8 | −2.6 | −4.1 | −3.8 | 0.0984 |
| MDRD | |||||
| Population predictions | 4.0 | 4.0 | 0.97 | 1.8 | 0.0157 |
| Individual predictions | −2.2 | −2.1 | −2.9 | −2.7 | 0.45 |
| CKD-EPI | |||||
| Population predictions | 3.4 | 3.4 | 0.48 | 1.2 | 0.0208 |
| Individual predictions | −1.7 | −1.5 | −2.6 | −2.46 | 0.25 |
Each value indicates the mean error of population or individual predictions provided by the CG-, JEL-, MDRD-, and CKD-EPI-based models in the analysis of data, including renal function estimated by each renal function estimation equation. Results in bold indicate the reference analysis based on the reference model and reference renal function estimation method.
Row-based probability that the biases do not differ among the methods to estimate renal function based on the reference model using the Kruskal-Wallis test.
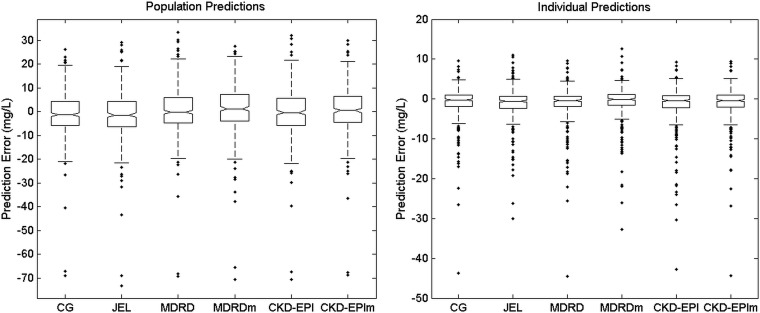
Figure 1 shows the distribution of the prediction errors from each model in the final analysis of the entire patient data sets (n = 78 patients) for both population and individual predictions (i.e., predicted minus observed vancomycin concentrations, also known as residuals). The corresponding plots of observed versus predicted concentrations are provided in the supplemental material. The six models predicted vancomycin concentrations well, although some high, unexplained vancomycin concentrations were underpredicted by all models, even after Bayesian estimation of individual parameters. Table 5 shows goodness of fit, predictive performance, and individual AUC24–48 from the six models in this final analysis. While the MDRDm-based model showed the lowest AIC value, it displayed the largest bias and imprecision. Biases were significantly different between the six models, the CG-based model being the least biased. Precisions were not significantly different. The six models provided significantly different estimates of the AUC in the 78 individuals.
FIG 1.
Box plots of prediction errors from the six vancomycin models. For each box, the central marker is the median, and the edges of the box are the 25th and 75th percentiles. The whisker length is 1.5 times the interquartile range. Dots represent outlier values. The left panel shows population predictions; the right panel shows individual predictions. For individual predictions, two outlier values less than −50 mg/liter are not shown, for ease of graphical display.
TABLE 5.
Goodness-of-fit, predictive performance (population predictions) and individual AUC24–48 from the six models in the final analysis (n = 78 patients, 376 vancomycin concentrations)
| Model | AIC | Weighted error (mg/liter) | Weighted squared error (mg2/liter2) | Individual AUC24–48 (mg · h · liter−1) |
|---|---|---|---|---|
| CG | 2,126.9 | 0.41 ± 4.6 | 21.6 ± 42.0 | 393 ± 167 |
| JEL | 2,097.2 | −0.49 ± 5.1 | 26.2 ± 61.3 | 373 ± 163 |
| MDRD | 2,101.2 | 1.1 ± 5.3 | 29.0 ± 67.3 | 391 ± 162 |
| MDRDm | 2,088.9 | 1.6 ± 5.2 | 29.8 ± 62.4 | 402 ± 169 |
| CKDEPI | 2,140.9 | 0.88 ± 4.6 | 22.2 ± 49.6 | 384 ± 162 |
| CKDEPIm | 2,126.9 | 1.3 ± 4.8 | 24.9 ± 52.4 | 385 ± 160 |
| P value | 0.0004a | 0.80a | 0.0002b |
Probability that the errors are not different among the models, using the Kruskal-Wallis test.
Probability that the individual AUCs calculated on the second day of therapy from each model are not significantly different, using the Friedman test.
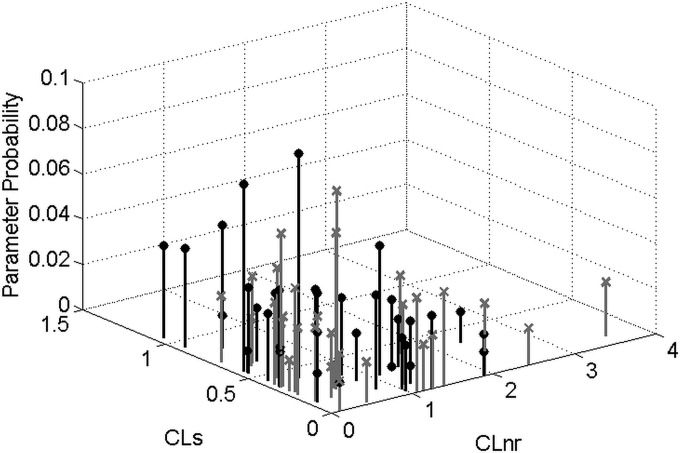
A summary of population PK parameters of vancomycin estimated in the final analysis is shown in Table 6. Except for volume of distribution, which estimation was quite consistent across the six models, typical parameter values as well as variability estimated by the different models varied substantially. So, estimates of renal function markedly influenced the estimation of vancomycin population PK parameters. As an example, Fig. 2 shows the population distributions of vancomycin clearance parameters estimated by the CG- and the MDRD-based models. Clearly, the two distributions did not fully overlap. The CG-based model showed a cluster of high probability points with high CLS and low CLNR, while the MDRD-based model displayed a cluster of low-CLS and high-CLNR pairs.
TABLE 6.
Population pharmacokinetic parameters of vancomycin estimated in the entire population study (n = 78 patients, 376 vancomycin concentrations)
| Model and type of resultb | CLNR (liters/h) | CLSa | KCP (h−1) | KPC (h−1) | V (liters) |
|---|---|---|---|---|---|
| CG | |||||
| Mean | 0.60 | 0.42 | 0.20 | 0.10 | 44.3 |
| Median (IQR) | 0.37 (0.23–1.08) | 0.39 (0.20–0.61) | 0.086 (0.031–0.21) | 0.074 (0.021–0.15) | 44.3 (33.4–56.4) |
| CV (%) | 89.1 | 67.6 | 141.7 | 104.8 | 48.4 |
| JEL | |||||
| Mean | 0.71 | 0.37 | 0.73 | 0.32 | 35.1 |
| Median (IQR) | 0.34 (0.17–1.15) | 0.32 (0.17–0.51) | 0.27 (0.047–0.90) | 0.13 (0.043–0.19) | 35.0 (14.6–47.9) |
| CV (%) | 103.4 | 69.7 | 205.5 | 314.9 | 64.4 |
| MDRD | |||||
| Mean | 0.67 | 0.27 | 0.28 | 0.14 | 41.1 |
| Median (IQR) | 0.46 (0.27–1.14) | 0.28 (0.085–0.4) | 0.12 (0.036–0.39) | 0.078 (0.012–0.17) | 38.2 (28.0–54.7) |
| CV (%) | 107.2 | 74.9 | 109.0 | 148.7 | 51.7 |
| MDRDm | |||||
| Mean | 0.72 | 0.29 | 0.43 | 0.52 | 40.7 |
| Median (IQR) | 0.42 (0.18–0.95) | 0.30 (0.10–0.41) | 0.14 (0.046–0.26) | 0.094 (0.040–0.23) | 41.2 (36.3–54.9) |
| CV (%) | 124.7 | 70.1 | 175.5 | 274.6 | 43.3 |
| CKD-EPI | |||||
| Mean | 0.55 | 0.34 | 0.17 | 0.11 | 44.6 |
| Median (IQR) | 0.36 (0.23–0.89) | 0.32 (0.19–0.44) | 0.075 (0.030–0.20) | 0.045 (0.017–0.14) | 43.5 (31.2–54.0) |
| CV (%) | 94.2 | 53.1 | 133.3 | 163.1 | 51.7 |
| CKD-EPIm | |||||
| Mean | 0.44 | 0.38 | 0.19 | 0.15 | 44.5 |
| Median (IQR) | 0.33 (0.22–0.60) | 0.35 (0.17–0.55) | 0.092 (0.027–0.24) | 0.055 (0.015–0.19) | 42.9 (29.9–57.8) |
| CV (%) | 116.7 | 68.1 | 136.7 | 163.8 | 49.1 |
The unit of CLS is liters per hour per unit of CLCR or GFR (liters/hour or liters/hour per1.73 m2), depending on the renal function estimation equation. For example, with the CG-based model, the mean vancomycin renal clearance for a subject with a creatinine clearance of 6 liters/h (100 ml/min) is: 0.42 × 6 = 2.52 liters/h.
IQR, interquartile range; CV, coefficient of variation.
FIG 2.
Discrete joint distribution of vancomycin nonrenal clearance (CLnr) and renal clearance coefficient (CLs) estimated in the 78 patients with the CG-based model (black lines and filled circles) and the MDRD-based model (gray lines and crosses).
DISCUSSION
Because renal function declines with age, drug dosage adjustment for renal function is often necessary for elderly patients. The Cockcroft-Gault equation has long been the reference equation for adjusting vancomycin as well as other drug dosage regimens to renal function. New renal function estimation equations have emerged in the last 15 years, notably the MDRD and CKD-EPI equations, which are now widely used to estimate the glomerular filtration rate in clinical routine practice. Numerous studies have compared the ability of old and newer estimation equations to estimate GFR in various patient populations, and newer equations usually performed better than older equations in estimating the true GFR in most patient groups (11, 15). However, the use of the MDRD or CKD-EPI equation for dose adjustment of renally excreted drugs has not been validated.
Individualization of vancomycin dosage regimens can be optimized by combining therapeutic drug monitoring and Bayesian adaptive control based on population PK models (3, 22). Most population PK models of vancomycin previously published for adult patients incorporated CLCR estimated by the CG equation as a covariate influencing vancomycin body clearance, as reviewed by Marsot et al. (23). More recently, it has been suggested that AUC should be used as a target criterion in place of trough level for vancomycin therapeutic drug monitoring, because it better correlates with the drug efficacy (24, 25). In routine clinical practice, it is not possible to get rich data and calculate the actual AUC, but one can estimate the AUC from a few concentration measurements using PK models and Bayesian estimation. Little is known about the influence of PK model covariates such as renal function on model-based vancomycin AUC estimation.
To our knowledge, this is the first population PK study which assessed six renal function estimation equations, including the CG, MDRD, and CKD-EPI equations, as covariates for prediction of vancomycin clearance, concentrations in serum, and AUC. Several important conclusions can be drawn from this study.
First, estimates of renal function provided by the CG, JEL, MDRD, MDRDm, CKD-EPI, and CKD-EPIm equations were significantly different. In particular, in this population of nonobese elderly patients with normal mean body weight, estimates provided by the CG equation were generally lower than those provided by the original MDRD and CKD-EPI equations, which is consistent with previous works from our group and many others (26–32). Because of this systematic difference, the use of the newer MDRD or CKD-EPI equation in place of the CG equation for drug dosing may have serious implications and cause overdosing in elderly patients for many drugs that are not monitored with concentration measurement (29, 32, 33).
Second, when those six renal function estimation equations were incorporated as a covariate in a two-compartment model, important differences were observed in the modeling of vancomycin pharmacokinetics. While all models fit data better than a two-compartment model without covariables, the goodnesses of fit of the models were not fully consistent, as large differences were observed in AIC values in both the learning step and the final analysis. The mean weighted errors of population predictions from the six models were also significantly different in those two steps. Overall, the CKD-EPI- and CKD-EPIm-based models appeared to be less appropriate in elderly patients, with AIC values greater than those of the MDRD and MDRDm-based models and mean weighted errors greater than those of the CG- and JEL-based models. The final analysis in the entire population of 78 patients also showed that parameter distributions and their descriptive statistics, as well as AUC estimation, were influenced by the renal covariate. As the equation used to estimate renal function significantly influenced data fit, parameter estimation, and AUC estimation, these results suggest that those six equations should not been considered interchangeable for PK modeling and prediction of vancomycin concentrations.
Third, when the six PK models were used to predict vancomycin kinetics in a validation data set with CLCR or GFR estimated with the reference equation, all models adequately described vancomycin concentrations and provided similar performances. This means that each model could be used for model-based, Bayesian pharmacokinetic monitoring and dose adjustment of vancomycin in elderly patients in clinical practice, provided that one used the same renal function estimation equation as was used for model building. Once serum concentration data are available and a Bayesian posterior model of drug behavior has been made for each individual patient, then an empirical relationship can be established between that patient's data of serum creatinine and the behavior of the drug, and it is likely that the differences between the various means of estimating creatinine clearance will be minimized.
However, when another equation is used to estimate renal function in place of the reference equation in the model, this could alter the model-based prediction of vancomycin levels. When GFR estimated by the MDRD or the CKD-EPI equation was used along with the CG- or JEL-based model, underestimation of vancomycin concentrations was observed on average, for both population and individual predictions. In contrast, population predictions based on the MDRD- or the CKD-EPI-based model overestimate vancomycin concentrations when the CG or the JEL equation was used to estimate renal function. Also, the cross-validation results suggest that the CG and JEL equations appear to be interchangeable, and so do the MDRD and CKD-EPI equations, for model-based prediction of vancomycin concentrations in the elderly. However, older CLCR estimation equations (CG and JEL) and the newer GFR estimation equations (MDRD and CKD-EPI) should not be interchanged for such a task. These observations are consistent with the differences observed in the estimation of renal function from each equation in the population (Table 1).
A few other studies examined the influence of renal function estimation on vancomycin PK modeling. A study conducted with Japanese elderly patients compared the performance of the CG equation based on enzymatic SCr, the CG equation based on Jaffe-converted SCr, and the MDRD equation in predicting vancomycin trough levels using a Bayesian approach (34). When the mean value of the population PK parameters was used to predict vancomycin concentrations (a priori prediction), the CG equation based on Jaffe-converted SCr provided the best predictive performance. The use of the MDRD equation was associated with the largest bias and imprecision. After Bayesian estimation of individual parameters, the three methods showed comparable performances. Our study results are consistent with those from that study, confirming that the performance of the CG-based model was comparable to or even better than that of the MDRD-based model.
Recently, Conil and colleagues compared the ability of measured CLCR, CLCR estimated by the CG equation, and GFR estimated by the MDRD and the CKD-EPI equations to predict vancomycin body clearance in intensive care unit (ICU) patients who received continuous i.v. vancomycin (35). Overall, significant but limited correlation was observed between vancomycin clearance and any index of renal function. In the Bland-Altman analysis, measured CLCR showed the least bias, and GFR estimated by the CKD-EPI provided the best precision. The authors concluded that the CKD-EPI equation was the best predictor of vancomycin clearance. It seems more difficult to compare results from Conil et al. with ours because the population, average renal function, PK estimation methods, and vancomycin use were different.
Sanchez et al. developed a population PK model of vancomycin in 141 subjects, including 40 subjects aged ≥64 years. The predictive performance of the model as well as that of 10 other PK methods was then evaluated in an independent data set of 95 patients with a mean age of 50 ± 17 years (36). The 10 other methods consisted of regression equations predicting vancomycin body clearance and, for some of them, volume of distribution. The results showed that the predictive performance was not consistent across all methods. All of them included CLCR in the prediction equation of vancomycin clearance, which was estimated using the CG equation for most of them. That study did not specifically address the influence of the estimation of renal function. The differences in regression coefficients, structural model, and influencing covariates between the methods may explain in part the difference in predictive performance.
The final parameter estimates from the six models (Table 6) are in agreement with those from previous studies. Significant nonrenal clearance of vancomycin was found in this study, with median values from the six final models ranging from 0.34 to 0.46 liter/h. This parameter varies widely in the literature, as reported by Murphy and colleagues (10). In a study performed with 10 anuric patients, nonrenal clearance of vancomycin ranged from 0.23 to 1.40 liters/h (37). Others parameter values are broadly consistent with population studies performed with senior patients (36, 38).
There are several limitations in this study. First, only a limited number of elderly Caucasian patients with normal body weight were included. As a consequence, this study result may not be able to be generalized to other patient populations.
While many other estimation equations and refinements have been proposed, we assessed only four original and two modified (MDRDm and CKD-EPIm, in milliliters per minute) renal function equations. We selected the CG and MDRD equations because they are the most widely used methods to estimate renal function in clinical practice. The CKD-EPI equation is an emerging equation which might replace the MDRD equation in the future (13). The Jelliffe equation was selected because it has been the reference equation in the MM-USC*PACK collection of programs. It also has the unique feature of using a pair of serum creatinine values, thus accommodating unstable renal function (17). In very large or small people, GFR estimated by the MDRD or the CKD-EPI equation in milliliters per minute per 1.73 m2 may be substantially different from GFR in milliliters per minute, and it has been suggested that GFR in milliliters per minute be used to account for individual body surface area in drug dosing (39, 40). This is why we evaluated modified versions of the MDRD and CKD-EPI equation to get GFR estimates in milliliters per minute. Such modification did not appear to significantly influence the estimation of renal function in the study population, as the mean estimates were similar between the original and modified MDRD and CKD-EPI equations (Table 1) in the study population. It also had limited influence on model-based prediction of vancomycin concentrations and parameter estimation.
We did not assess the influence of the serum creatinine assay method on the results. The CG equation was developed from serum creatinine measured with a colorimetric Jaffe method which is no longer in use today. The coefficients of the CG equation have never been revised for modern, IDMS traceable serum creatinine assays. Tsuji et al. have shown that converting enzymatic SCr values into corresponding Jaffe values may significantly improve the performance of the CG equation as a covariate to predict vancomycin concentrations (34). However, in our opinion, it is unlikely that clinicians will perform such conversion in routine clinical practice.
Other limitations are inherent to the clinical environment of this study. As data were collected during routine patient care, this may have affected the precision of drug dosing and sampling times, as well as weight, height, or BSA values.
Finally, we did not evaluate the dose requirements predicted by each pharmacokinetic model. Despite significant differences observed in data fitting and predictive performance, the six models might show little difference in the doses required to reach some predefined target serum levels in patients. It would be interesting to examine this point in future studies.
Because the MDRD and CKD-EPI equations were shown to estimate GFR better than the CG equation in most patients, some authors have recommended using those newer GFR estimation equations for drug dosing (41). In France, the national health authority (Haute Autorité de Santé) supports the use of the CKD-EPI equation for diagnosing chronic kidney disease and estimating renal function. This institution suggested that drug labels be revised in order to incorporate the CKD-EPI equation in place of the CG equation for drug dose adjustment (42).
However, there is no scientific rationale to support this paradigm shift. When it comes to estimating renal function for drug dosing, accurate estimation of GFR is not the ultimate goal. In pharmacology and therapeutics, the objective is to individualize the drug dosage regimen using the most appropriate index of renal function. So, one should determine and use the renal function index that best predicts the drug PK.
In this study, when used as covariate in PK models, the MDRD and CKD-EPI equations did not outperform the CG and Jelliffe equations. In a previous study conducted by our group with elderly patients who were administered gentamicin, the CG- and the JEL-based models provided better predictive performance than the MDRD-based models (26). These findings do not support a general switch from older to new renal function estimation equations for drug dosing. Further research is necessary to determine which marker of renal function is the most adequate for drug dose adjustment. It is likely that the results may vary across drugs and depend on the pharmacokinetic properties, especially the renal transport of each agent. Our approach based on population PK may serve as a template for such evaluation.
Conclusion.
In conclusion, this study conducted with elderly patients showed that the equation used to estimate renal function significantly influenced both concentration prediction and estimation of PK parameters of vancomycin. Pharmacokinetic models based on the MDRD and CKD-EPI equations did not outperform models based on the Cockcroft-Gault and the Jelliffe equations. These results suggest that the various renal function estimation equations should not be considered interchangeable for PK modeling and prediction of concentrations of renally excreted drugs such as vancomycin.
Supplementary Material
ACKNOWLEDGMENTS
This work was not supported by any institutional, company, or sponsor fund. We have no conflicts of interest that are relevant to the content of this study.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.04132-14.
REFERENCES
- 1.Capitano B, Frye RF, Matzke GR. 2008. Vancomycin, p 329–344. In Murphy JE. (ed), Clinical pharmacokinetics. American Society of Health-System Pharmacists, Bethesda, MD. [Google Scholar]
- 2.Guay DR, Vance-Bryan K, Gilliland S, Rodvold K, Rotschafer J. 1993. Comparison of vancomycin pharmacokinetics in hospitalized elderly and young patients using a Bayesian forecaster. J Clin Pharmacol 33:918–922. doi: 10.1002/j.1552-4604.1993.tb01922.x. [DOI] [PubMed] [Google Scholar]
- 3.Avent ML, Vaska VL, Rogers BA, Cheng AC, van Hal SJ, Holmes NE, Howden BP, Paterson DL. 2013. Vancomycin therapeutics and monitoring: a contemporary approach. Intern Med J 43:110–119. doi: 10.1111/imj.12036. [DOI] [PubMed] [Google Scholar]
- 4.Neely MN, Youn G, Jones B, Jelliffe RW, Drusano GL, Rodvold KA, Lodise TP. 2014. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother 58:309–316. doi: 10.1128/AAC.01653-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matzke GR, McGory RW, Halstenson CE, Keane WF. 1984. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother 25:433–437. doi: 10.1128/AAC.25.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moellering RC Jr, Krogstad DJ, Greenblatt DJ. 1981. Vancomycin therapy in patients with impaired renal function: a nomogram for dosage. Ann Intern Med 94:343–346. doi: 10.7326/0003-4819-94-3-343. [DOI] [PubMed] [Google Scholar]
- 7.Rodvold KA, Blum RA, Fischer JH, Zokufa HZ, Rotschafer JC, Crossley KB, Riff LJ. 1988. Vancomycin pharmacokinetics in patients with various degrees of renal function. Antimicrob Agents Chemother 32:848–852. doi: 10.1128/AAC.32.6.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ducharme MP, Slaughter RL, Edwards DJ. 1994. Vancomycin pharmacokinetics in a patient population: effect of age, gender, and body weight. Ther Drug Monit 16:513–518. doi: 10.1097/00007691-199410000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Birt JK, Chandler MH. 1990. Using clinical data to determine vancomycin dosing parameters. Ther Drug Monit 12:206–209. doi: 10.1097/00007691-199003000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Murphy JE, Gillespie DE, Bateman CV. 2006. Predictability of vancomycin trough concentrations using seven approaches for estimating pharmacokinetic parameters. Am J Health Syst Pharm 63:2365–2370. doi: 10.2146/ajhp060047. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. 1999. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130:461–470. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. 2006. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. 2009. A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilbride HS, Stevens PE, Eaglestone G, Knight S, Carter JL, Delaney MP, Farmer CK, Irving J, O'Riordan SE, Dalton RN, Lamb EJ. 2013. Accuracy of the MDRD (Modification of Diet in Renal Disease) study and CKD-EPI (CKD Epidemiology Collaboration) equations for estimation of GFR in the elderly. Am J Kidney Dis 61:57–66. doi: 10.1053/j.ajkd.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Froissart M, Rossert J, Jacquot C, Paillard M, Houillier P. 2005. Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol 16:763–773. doi: 10.1681/ASN.2004070549. [DOI] [PubMed] [Google Scholar]
- 16.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 17.Jelliffe R. 2002. Estimation of creatinine clearance in patients with unstable renal function, without a urine specimen. Am J Nephrol 22:320–324. doi: 10.1159/000065221. [DOI] [PubMed] [Google Scholar]
- 18.Gehan EA, George SL. 1970. Estimation of human body surface area from height and weight. Cancer Chemother Rep 54:225–235. [PubMed] [Google Scholar]
- 19.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F. 2007. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 20.Du Bois D, Du Bois EF. 1989. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 5:303–311; discussion, 312–313. [PubMed] [Google Scholar]
- 21.Tatarinova T, Neely M, Bartroff J, van Guilder M, Yamada W, Bayard D, Jelliffe R, Leary R, Chubatiuk A, Schumitzky A. 2013. Two general methods for population pharmacokinetic modeling: non-parametric adaptive grid and non-parametric Bayesian. J Pharmacokinet Pharmacodyn 40:189–199. doi: 10.1007/s10928-013-9302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurst AK, Yoshinaga MA, Mitani GH, Foo KA, Jelliffe RW, Harrison EC. 1990. Application of a Bayesian method to monitor and adjust vancomycin dosage regimens. Antimicrob Agents Chemother 34:1165–1171. doi: 10.1128/AAC.34.6.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marsot A, Boulamery A, Bruguerolle B, Simon N. 2012. Vancomycin: a review of population pharmacokinetic analyses. Clin Pharmacokinet 51:1–13. doi: 10.2165/11596390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, Dalovisio JR, Levine DP. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 66:82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 25.Pai MP, Neely M, Rodvold KA, Lodise TP. 5 June 2014. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev doi: 10.1016/j.addr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Charhon N, Neely MN, Bourguignon L, Maire P, Jelliffe RW, Goutelle S. 2012. Comparison of four renal function estimation equations for pharmacokinetic modeling of gentamicin in geriatric patients. Antimicrob Agents Chemother 56:1862–1869. doi: 10.1128/AAC.05634-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamb EJ, Webb MC, Simpson DE, Coakley AJ, Newman DJ, O'Riordan SE. 2003. Estimation of glomerular filtration rate in older patients with chronic renal insufficiency: is the modification of diet in renal disease formula an improvement? J Am Geriatr Soc 51:1012–1017. doi: 10.1046/j.1365-2389.2003.51330.x. [DOI] [PubMed] [Google Scholar]
- 28.Gill J, Malyuk R, Djurdjev O, Levin A. 2007. Use of GFR equations to adjust drug doses in an elderly multi-ethnic group—a cautionary tale. Nephrol Dial Transplant 22:2894–2899. doi: 10.1093/ndt/gfm289. [DOI] [PubMed] [Google Scholar]
- 29.Helldén A, Odar-Cederlof I, Nilsson G, Sjoviker S, Soderstrom A, Euler M, Ohlen G, Bergman U. 2013. Renal function estimations and dose recommendations for dabigatran, gabapentin and valaciclovir: a data simulation study focused on the elderly. BMJ Open 3:e002686. doi: 10.1136/bmjopen-2013-002686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maccallum PK, Mathur R, Hull SA, Saja K, Green L, Morris JK, Ashman N. 2013. Patient safety and estimation of renal function in patients prescribed new oral anticoagulants for stroke prevention in atrial fibrillation: a cross-sectional study. BMJ Open 3:e003343. doi: 10.1136/bmjopen-2013-003343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dowling TC, Wang ES, Ferrucci L, Sorkin JD. 2013. Glomerular filtration rate equations overestimate creatinine clearance in older individuals enrolled in the Baltimore longitudinal study on aging: impact on renal drug dosing. Pharmacotherapy 33:912–921. doi: 10.1002/phar.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moranville MP, Jennings HR. 2009. Implications of using modification of diet in renal disease versus Cockcroft-Gault equations for renal dosing adjustments. Am J Health Syst Pharm 66:154–161. doi: 10.2146/ajhp080071. [DOI] [PubMed] [Google Scholar]
- 33.Park EJ, Wu K, Mi Z, Dong T, Lawrence JP, Ko CW, Huang SM, Zhang L, Crentsil V, Zhang J, Xu NN. 2012. A systematic comparison of Cockcroft-Gault and modification of diet in renal disease equations for classification of kidney dysfunction and dosage adjustment. Ann Pharmacother 46:1174–1187. doi: 10.1345/aph.1Q757. [DOI] [PubMed] [Google Scholar]
- 34.Tsuji Y, Hiraki Y, Mizoguchi A, Sadoh S, Sonemoto E, Kamimura H, Karube Y. 2009. Effect of various estimates of renal function on prediction of vancomycin concentration by the population mean and Bayesian methods. J Clin Pharm Ther 34:465–472. doi: 10.1111/j.1365-2710.2008.01015.x. [DOI] [PubMed] [Google Scholar]
- 35.Conil JM, Georges B, Breden A, Ruiz S, Cougot P, Fourcade O, Saivin S. 2014. Estimation of glomerular filtration rate to adjust vancomycin dosage in critically ill patients: superiority of the Chronic Kidney Disease Epidemiology Collaboration equation? Anaesth Intensive Care 42:178–184. [DOI] [PubMed] [Google Scholar]
- 36.Sánchez JL, Dominguez AR, Lane JR, Anderson PO, Capparelli EV, Cornejo-Bravo JM. 2010. Population pharmacokinetics of vancomycin in adult and geriatric patients: comparison of eleven approaches. Int J Clin Pharmacol Ther 48:525–533. doi: 10.5414/CPP48525. [DOI] [PubMed] [Google Scholar]
- 37.Macias WL, Mueller BA, Scarim SK. 1991. Vancomycin pharmacokinetics in acute renal failure: preservation of nonrenal clearance. Clin Pharmacol Ther 50:688–694. doi: 10.1038/clpt.1991.208. [DOI] [PubMed] [Google Scholar]
- 38.Yasuhara M, Iga T, Zenda H, Okumura K, Oguma T, Yano Y, Hori R. 1998. Population pharmacokinetics of vancomycin in Japanese adult patients. Ther Drug Monit 20:139–148. doi: 10.1097/00007691-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Matzke GR, Aronoff GR, Atkinson AJ Jr, Bennett WM, Decker BS, Eckardt KU, Golper T, Grabe DW, Kasiske B, Keller F, Kielstein JT, Mehta R, Mueller BA, Pasko DA, Schaefer F, Sica DA, Inker LA, Umans JG, Murray P. 2011. Drug dosing consideration in patients with acute and chronic kidney disease—a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 80:1122–1137. doi: 10.1038/ki.2011.322. [DOI] [PubMed] [Google Scholar]
- 40.National Kidney Disease Education Program. 2010. Chronic kidney disease and drug dosing: information for providers. National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD: http://nkdep.nih.gov/resources/ckd-drug-dosing-508.pdf. [Google Scholar]
- 41.Stevens LA, Nolin TD, Richardson MM, Feldman HI, Lewis JB, Rodby R, Townsend R, Okparavero A, Zhang YL, Schmid CH, Levey AS. 2009. Comparison of drug dosing recommendations based on measured GFR and kidney function estimating equations. Am J Kidney Dis 54:33–42. doi: 10.1053/j.ajkd.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haute Autorité de Santé. 2011. Evaluation du débit de filtration glomérulaire et du dosage de la créatininémie dans le diagnostic de la maladie rénale chronique chez l'adulte. Saint-Denis La Plaine, France: http://www.has-sante.fr/portail/upload/docs/application/pdf/2011-12/rapport_dfg_creatininemie.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.