Abstract
Rifapentine is a potent antituberculosis drug currently in phase III trials. Bioavailability decreases with increasing dose, yet high daily exposures are likely needed to improve efficacy and shorten the tuberculosis treatment duration. Further, the limits of tolerability are poorly defined. The phase I multicenter trial in healthy adults described here investigated two strategies to increase rifapentine exposures: dividing the dose or giving the drug with a high-fat meal. In arm 1, rifapentine was administered at 10 mg/kg of body weight twice daily and 20 mg/kg once daily, each for 14 days, separated by a 28-day washout; the dosing sequence was randomized. In arm 2, 15 mg/kg rifapentine once daily was given with a high-fat versus a low-fat breakfast. Sampling for pharmacokinetic analysis was performed on days 1 and 14. Population pharmacokinetic analyses were performed. This trial was stopped early for poor tolerability and because of safety concerns. Of 44 subjects, 20 discontinued prematurely; 11 of these discontinued for protocol-defined toxicity (a grade 3 or higher adverse event or grade 2 or higher rifamycin hypersensitivity). Taking rifapentine with a high-fat meal increased the median steady-state area under the concentration-time curve from time zero to 24 h (AUC0–24ss) by 31% (relative standard error, 6%) compared to that obtained when the drug was taken with a low-fat breakfast. Dividing the dose increased exposures substantially (e.g., 38% with 1,500 mg/day). AUC0–24ss was uniformly higher in our study than in recent tuberculosis treatment trials, in which toxicity was rare. In conclusion, two strategies to increase rifapentine exposures, dividing the dose or giving it with a high-fat breakfast, successfully increased exposures, but toxicity was common in healthy adults. The limits of tolerability in patients with tuberculosis remain to be defined. (AIDS Clinical Trials Group study A5311 has been registered at ClinicalTrials.gov under registration no. NCT01574638.)
INTRODUCTION
In 2013, there were an estimated 9.0 million new cases of tuberculosis (TB) and 1.5 million TB-related deaths worldwide (1). Although effective treatment is available, standard short-course therapy with isoniazid, rifampin, pyrazinamide, and ethambutol must be given for 6 months to be effective. Rifampin, a rifamycin antibiotic, is a cornerstone of modern first-line regimens because rifamycins have unique sterilizing activity against TB. In the absence of rifampin, treatment must be prolonged to 12 to 24 months, as no drug has proven clinical activity sufficient to replace it. Current dosing of rifampin yields concentrations that are on the steep slope of the dose-response curve (2–5). Optimization of rifamycins represents a promising path toward TB treatment shortening.
The rifamycin antibiotic rifapentine (RPT) has a longer half-life and a lower MIC against Mycobacterium tuberculosis than rifampin. It is being investigated as a potent anti-TB drug that may help shorten the treatment duration. In mouse models of TB, RPT is about four times more potent than rifampin, and its activity is dose dependent; daily RPT-containing regimens cure TB in 3 months or less (6, 7). In immune-deficient mice, RPT renders mice culture negative more rapidly than the standard dose of rifampin and better protects against the emergence of resistance to companion drugs (8).
In previous trials that evaluated different RPT dose schedules for pulmonary TB, daily doses up to 20 mg/kg of body weight given with food were effective and well tolerated; however, the maximum tolerated dose (MTD) has not been defined (9, 10). Notably, in those studies, dose escalations did not proportionally increase the mean steady-state RPT concentrations, which plateaued in the 15-mg/kg arm. Clinical trials aimed at testing the highest well-tolerated dose of RPT are thus justified by preclinical data that demonstrate the concentration-dependent treatment-shortening activity of the drug, its activity in immune deficiency, and its ability to protect against the development of resistance to companion drugs. Since strategies with doses higher than 10 mg/kg daily are needed to optimize RPT efficacy but concentrations may plateau with a dose of 15 mg/kg, we designed this trial to evaluate the safety and pharmacokinetics (PKs) of two strategies intended to increase RPT plasma exposures without increasing the total daily dose: dividing the dose and giving RPT with a globally available, inexpensive food (a boiled egg) to increase absorption (11).
MATERIALS AND METHODS
Study population.
Healthy adults 18 to 65 years of age were recruited at four AIDS Clinical Trials Group (ACTG) sites in the United States between June 2012 and May 2013. Inclusion criteria were a weight of 50 to 100 kg, alanine aminotransferase and total bilirubin concentrations ≤1.2 times the upper limit of normal, a serum creatinine concentration of ≤1.5 mg/dl, a platelet count of ≥125,000/mm3, an absolute neutrophil count of ≥1,250/mm3, a serum albumin concentration of ≥3.5 g/dl, negative HIV and hepatitis C virus antibody tests, and no active TB or history of TB. The study was approved by the institutional review boards of the participating sites; all participants provided written informed consent. ACTG study A5311 has been registered at ClinicalTrials.gov under registration no. NCT01574638.
Experimental protocol. (i) Study design.
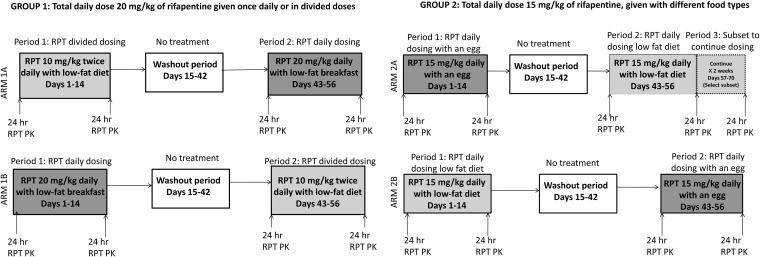
This was a phase I, open-label, two-arm, crossover study. In group 1 we evaluated divided dosing, and in group 2 we evaluated meal type (Fig. 1). In arm 1A, participants received RPT at 10 mg/kg twice daily for 14 days, followed by a 28-day washout, and then RPT at 20 mg/kg once daily for 14 days. Participants in arm 1B received the same regimens but in reverse order: RPT at 20 mg/kg once daily, followed by a washout, and then RPT at 10 mg/kg twice daily. In arm 1, doses were taken with a low-fat meal. Participants in arm 2A received RPT at 15 mg/kg once daily for 14 days with an egg, which served as a proxy for a high-fat meal, followed by a 28-day washout, and then RPT at 15 mg/kg once daily for 28 days with a low-fat breakfast. In arm 2B, participants received RPT at 15 mg/kg once daily for 14 days with a low-fat breakfast, followed by a 28-day washout, and then RPT at 15 mg/kg once daily for 14 days with an egg. Individuals available to participate in prolonged dosing were assigned to arm 2A. All other participants were randomized 1:1:1 to arm 1A, 1B, or 2B. Intensive sampling for analysis of the PKs of RPT and its less active deacetyl metabolite (desacetyl-rifapentine [desRPT]) was performed following the 1st and 14th doses in each dosing period. In arm 2A, sampling was performed on days 1, 14, and 28 during the final dosing period (to assess the time to steady state). For twice-daily dosing, sampling times were predose; at 0.5, 1, 2, 4, 5, 8, and 12 h after the a.m. dose; and at 2, 4, and 12 h following the p.m. dose. For once-daily dosing, samples were collected predose and at 0.5, 1, 2, 4, 5, 8, 12, and 24 h postdose.
FIG 1.
Study schema.
(ii) Safety monitoring.
Participants underwent safety evaluations approximately weekly. Signs, symptoms, and laboratory events were graded according to the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 1.0 (December 2004, August 2009 clarification) (12).
(iii) Criteria for premature treatment discontinuation.
The study drug was discontinued for grade 3 or higher drug-related adverse events (AEs) or for grade 2 or higher rifamycin hypersensitivity syndrome (RHS). Consistent with other RPT trials, RHS was defined as either (i) hypotension, urticaria, angioedema, acute bronchospasm, or conjunctivitis that occurred in relation to study drug or (ii) more than 4 of the following (with one being grade 2 or higher) occurring in relation to study drug: weakness, fatigue, nausea, vomiting, headache, fever, aches, sweats, dizziness, shortness of breath, flushing, or chills (10, 13, 14).
Measurement of RPT and desRPT in plasma.
The liquid chromatography-tandem mass spectrometry (LC-MS/MS) method used to quantify drug was based on a previously described method (9). Briefly, RPT and desRPT were eluted under reversed-phase conditions with 5 mM ammonium formate in water (mobile phase A) and 3% dimethyl sulfoxide in acetonitrile (mobile phase B). Ascorbic acid (0.5 mg/ml) in extraction solvents prevented the drugs from becoming oxidized to form quinones. RPT and desRPT were detected over a 4.0-min run using an AB-Sciex QTRAP 5500 mass analyzer (Foster City, CA) interfaced with a Waters Acquity ultraperformance liquid chromatography (UPLC) system (Milford, MA). The ion transitions monitored for RPT, desRPT,and isotopically labeled rifampin (internal standard) were m/z 877.6 → m/z 845.5, m/z 835.5 → m/z 803.5, and m/z 826.6 → m/z 749.5, respectively. Analytical ranges for both RPT and desRPT were 50 to 80,000 ng/ml. Intraday precision and accuracy for RPT and desRPT were ≤8.81% and ≤±13.5%, respectively, and interday precision and accuracy were ≤10.8% and ≤±11.3%, respectively.
Pharmacogenetic testing.
Genotyping of two targeted polymorphisms in the membrane transporter gene SLCO1B1, rs4149056 521T → C and rs4149032 C → T, was performed by the TaqMan assay with an ABI Prism 7900 HT sequence detection system (Applied Biosystems, Inc., Foster City, CA). The former polymorphism was found to be associated with increased plasma concentrations of statins and other drugs (15), and the latter was found to be associated with decreased plasma rifampin concentrations in patients with TB in South Africa (16).
PK and statistical analyses. (i) Sample size.
We estimated that 20 evaluable volunteers in each group would provide at least a 90% power to detect at least a 25% mean difference in the RPT steady-state area under the concentration-time curve from time zero to 24 h (AUC0–24ss) using a nonparametric paired-sample Wilcoxon test with a type I error rate of 0.05. We aimed to enroll at least 24 volunteers per group (12 persons each completing study arms 1A, 1B, 2A, and 2B) to ensure 20 PK-evaluable participants per group.
(ii) PK and statistical evaluation.
PK parameters for RPT and desRPT, including AUC0–24ss, the maximum concentration in plasma (Cmax), the time to the maximum concentration in plasma (Tmax), half-life (t1/2), and oral clearance (CL/F), were determined using standard noncompartmental analysis (NCA) methods performed in SAS (SAS Institute Inc., Cary, NC). Statistical analyses for PK comparisons were based on nonparametric tests.
(iii) Population PKs and PK-toxicity analyses.
In addition to NCA, population PK modeling was planned a priori to strengthen and expand knowledge of RPT PKs. The population PK model was developed using nonlinear mixed effect modeling, implemented in NONMEM software. Previously described model structure and model-building procedures were used (17). In brief, parent and metabolite data were fitted simultaneously. A one-compartment disposition model was used for both the parent drug and the metabolite. RPT absorption was described using a transit compartment model. The relationship between relative bioavailability and dose was quantified to describe the change in bioavailability with increased dose. Since the numbers of individuals that received the same dose level were small, a separate bioavailability parameter was estimated for dose groups classified as low (600 and 750 mg), medium (900, 1,050, and 1,200 mg), and high (1,350, 1,500, and 1,800 mg). The parameter describing the increase in bioavailability when RPT was dosed with an egg compared to that when it was dosed with a low-fat breakfast was estimated separately. An autoinduction model describing the increase in clearance (CL) over time was implemented. Individual parameters were assumed to be log-normally distributed, and residual error was assumed to be proportional. All data were analyzed using the nonlinear mixed effects approach available in NONMEM (version 7.3; Icon Development Solutions, Ellicott City, MD). The first-order conditional estimation with interaction (FOCEI) method was employed throughout the analyses. The model-building procedure was guided by the likelihood ratio test, diagnostic plots, and internal model validation techniques, including visual and numerical predictive checks.
RESULTS
Study subjects.
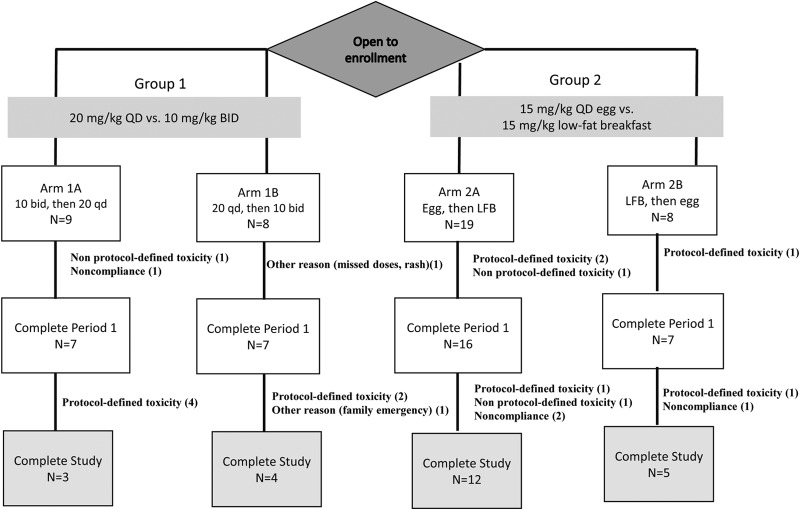
Forty-four participants were enrolled. More participants opted for longer-duration trial participation, so more were assigned to arm 2A than were randomized to arm 1A, 1B, or 2B (Fig. 2). The median age was 35 years, and the median weight was 83 kg; 27% were women, and 32% were black non-Hispanic (Table 1). The median total daily RPT dose was 1,350 mg (range, 900 to 1,800 mg).
FIG 2.
Consort diagram. BID or bid, twice daily; QD or qd, once daily; LFB, low-fat breakfast.
TABLE 1.
Characteristics of study participantsa
| Characteristic | Value |
|---|---|
| Median (range) age (yr) | 35 (20–59) |
| No. (%) of participants of the following gender: | |
| Male | 32 (73) |
| Female | 12 (27) |
| No. (%) of participants of the following race/ethnicity: | |
| Black non-Hispanic | 14 (32) |
| White non-Hispanic | 20 (45) |
| Hispanic | 6 (14) |
| Asian, Pacific Islander | 2 (5) |
| Not reported | 2 (4) |
| Median (range) wt (kg) | 83 (60–99) |
| Median (range) BMIb (kg/m2) | 27 (20–35) |
| No. (%) of participants with the following SLCO1B1 rs4149056 521T → C genotype: | |
| TT | 35 (80) |
| CT | 7 (15) |
| CC | 2 (5) |
| No. (%) of participants with the following SLCO1B1 rs4149032 genotype: | |
| TT | 12 (27) |
| CT | 16 (36) |
| CC | 16 (36) |
Data are for 44 participants.
BMI, body mass index.
Safety and tolerability.
Of 44 participants, 20 discontinued prematurely; 11 of these discontinued for protocol-defined toxicity (a grade 3 or higher AE or grade 2 or higher RHS) (Fig. 2). There were no serious adverse events (SAEs). Reasons for discontinuation by arm and by dose (in milligrams) are presented in Table 2. Of 16 participants receiving RPT at 10 mg/kg twice daily at any time, 7 (44%) discontinued early. For doses of 15 mg/kg with a low-fat breakfast, 15 mg/kg with an egg, and 20 mg/kg once daily, rates of discontinuation were 5/23 (22%), 5/25 (20%), and 3/13 (23%), respectively. Of 7 participants receiving 1,800 mg, 5 (71%) discontinued because of toxicity. There was no apparent association between period (i.e., before versus after washout) and the risk of discontinuation (data not shown), and most protocol-defined toxicities occurred early in the dosing period (the first 1 to 3 days). While there were no prespecified halting rules, tolerability was poor, and the study was stopped early because of safety concerns. A summary of protocol-defined toxicities is provided in Table 3.
TABLE 2.
Off-treatment information, by regimen and dose
| Regimen or dosea | No. of participantsb | No. (%) of discontinuationsc | No. of participants with the following reason for discontinuation: |
|||
|---|---|---|---|---|---|---|
| Protocol-defined toxicity | Non-protocol-defined toxicity | Noncompliant | Other | |||
| Regimen | ||||||
| 10 mg/kg BID | 16 | 7 (44) | 4 | 1 | 1 | 1 |
| 15 mg/kg QD with an LFB | 23 | 5 (22) | 3 | 1 | 1 | 0 |
| 15 mg/kg QD with an egg | 25 | 5 (20) | 2 | 1 | 2 | 0 |
| 20 mg/kg QD | 13 | 3 (23) | 2 | 0 | 0 | 1 |
| Total daily dose (mg) | ||||||
| 900 mg | 4 | 4 (100) | 2 | 0 | 2 | 0 |
| 1,200 | 17 | 6 (35) | 3 | 1 | 1 | 1 |
| 1,350 | 12 | 3 (25) | 1 | 1 | 1 | 0 |
| 1,500 | 4 | 1 (25) | 1 | 0 | 0 | 0 |
| 1,800 | 7 | 6 (86) | 4 | 1 | 0 | 1 |
BID, twice daily; QD, once daily; LFB, low-fat breakfast.
Number of participants who received this regimen at any time.
Discontinuations among participants receiving this regimen.
TABLE 3.
Summary of protocol-defined toxicities, defined as grade 3 or higher drug-related AEs or grade 2 or higher RHS
| Arm | Total daily dose (mg) | Adverse event | Highest AE grade |
|---|---|---|---|
| 1A | 1,800 | Neutropenia | 3 |
| 1A | 1,500 | RHS | 2 |
| 1A | 1,800 | RHS | 3 |
| 1A | 1,800 | Elevated alanine aminotransferase | 4 |
| 1B | 1,200 | RHS | 3 |
| 1B | 1,800 | RHS | 2 |
| 2A | 1,350 | Lymphopenia | 3 |
| 2A | 1,200 | RHS | 3 |
| 2A | 1,200 | Neutropenia | 3 |
| 2B | 900 | RHS, elevated alanine aminotransferase | 3 |
| 2B | 900 | Headache with nausea, vomiting, fatigue | 3 |
Pharmacokinetics of RPT.
Participants who completed visits for PK analysis were included in the PK analyses even if they did not complete the entire study. Median (interquartile ratio [IQR]) steady-state RPT PK parameters are shown in Table 4. We did not meet the target sample size required to evaluate dosing strategies using intraparticipant comparisons of NCA-derived AUC0–24ss, so those analyses were not performed. Rather, results from prespecified modeling analyses are reported.
TABLE 4.
Pharmacokinetic parameters for RPT and desRPT among ACTG study A5311 participants using NCAa
| Pharmacokinetic parameter | Dosing cohort | No. of participants | RPT |
desRPT |
||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | |||
| AUC0–24ss (μg · h/ml) | 20 mg/kg QD | 9 | 580 | 521–637 | 467 | 256–689 |
| 10 mg/kg BID | 10 | 756 | 681–898 | 918 | 666–1,014 | |
| 15 mg/kg with an LFB | 19 | 689 | 611–860 | 556 | 487–803 | |
| 15 mg/kg with an egg | 19 | 715 | 577–1,037 | 687 | 537–932 | |
| Cmin (μg/ml) | 20 mg/kg QD | 9 | 13 | 1–17 | 10 | 0.5–23 |
| 10 mg/kg BID | 10 | 22 | 19–30 | 27 | 19–35 | |
| 15 mg/kg with an LFB | 19 | 14 | 12–25 | 13 | 11–21 | |
| 15 mg/kg with an egg | 19 | 18 | 13–23 | 22 | 15–29 | |
| Cmax (μg/ml) | 20 mg/kg QD | 9 | 39 | 32–45 | 25 | 14–37 |
| 10 mg/kg BID | 10 | 39 | 35–44 | 44 | 34–48 | |
| 15 mg/kg with an LFB | 19 | 42 | 34–49 | 30 | 24–40 | |
| 15 mg/kg with an egg | 19 | 44 | 36–61 | 38 | 28–46 | |
QD, once daily; BID, twice daily; LFB, low-fat breakfast; Cmin, minimum concentration in plasma.
Population PK modeling.
Model-based estimates of steady-state RPT exposures are presented in Table 5. Full model parameters are provided in Table S1 in the supplemental material. Taking RPT with an egg increased RPT bioavailability by 31% (relative standard error [RSE], 6%). Bioavailability decreased with increased dose; the bioavailabilities of medium doses (900 and 1,200 mg) and high doses (1,350 mg, 1,500 mg, and 1,800 mg) were estimated to be 0.84 and 0.64, respectively, confirming that if a high dose is required, dividing the dose is a reasonable strategy to capitalize on the better bioavailability of lower dose levels. For example, for a dose of 1,500 mg, dividing the dose increased the AUC0–24ss by 38%. Covariates that affected exposures included diet and dose (each affected bioavailability). In the model, the SLCO1B1 rs4149056 and rs4149032 C alleles were each associated with decreased clearance, but statistical significance was not reached (P ≥ 0.25 for each). With single-dose data, the rs4149056 CT and CC genotypes were associated with 10% and 21% lower median RPT clearances, respectively, versus the clearance for the rs4149056 TT genotype, while the rs4149032 CT and CC genotypes were associated with 7% and 15% lower clearances, respectively, versus the clearance for the rs4149032 TT genotype. With multiple-dose data, the rs4149056 CT and CC genotypes were associated with 14% and 21% lower mean RPT clearances, respectively, versus the clearance for the rs4149056 TT genotype, but for rs4149032 there was a <5% apparent difference in the mean clearance between the CC, CT, and TT genotypes. Steady state appeared to be reached by the end of the second week, when autoinduction seemed to be complete; estimates of CL did not significantly increase after 14 days of daily treatment in arm 2A participants receiving prolonged daily dosing.
TABLE 5.
Model-based estimates of steady-state RPT exposures for different dosing schemes
| Regimena | Median AUC0–24ss (μg · h/ml) |
|---|---|
| 900 mg QD with a low-fat breakfast | 552 |
| 900 mg QD with an egg | 718 |
| 1,500 mg QD with a low-fat breakfast | 589 |
| 750 mg BID with a low-fat breakfast | 920 |
QD, once daily; BID, twice daily.
DISCUSSION
Two dosing strategies, dividing the dose and giving the dose with a boiled egg, successfully increased RPT exposures. However, these high exposures were poorly tolerated in this trial, which enrolled healthy volunteers without TB. Protocol-defined toxicities, including RHS, were common, and the trial was terminated early for safety concerns. We identified a possible association between the SLCO1B1 rs4149056 521T → C allele and decreased RPT clearance, consistent with the known association of this polymorphism with plasma exposure of statins and other drugs (15), but the small sample size limited our ability to demonstrate statistical significance or to stratify analyses by race/ethnicity.
Rifamycins drive the treatment response for TB, and with the doses currently used or tested clinically, higher doses are more efficacious (5, 18, 19). This appears to be the case for both rifampin and RPT. For rifampin, increasing the dose results in supraproportional increases in plasma drug concentrations (4). In contrast, RPT bioavailability decreases with increasing dose (17), so alternative strategies to increase exposures are needed. To maximize the likelihood of success with RPT-containing shorter-duration regimens, the relationships between exposure and microbiologic response must be better understood and fully characterized. PK/pharmacodynamic (PD) analyses from recent phase II clinical trials are helping to define this target for RPT (10, 14, 18). Studies such as the present one are valuable because they provide strategies for achieving these target concentrations in the largest possible number of patients.
A shortened TB treatment duration benefits patients and public health systems by reducing the logistical burdens of treatment delivery. However, strategies such as providing a boiled egg or giving a drug twice daily (or other interventions designed to maximize the success of a shortened regimen) add logistical concerns. Models suggest that a regimen of 4 months or shorter would be cost-effective in most settings (20), but we know little about the value that health care workers and patients place on treatment shortening and acceptable trade-offs in exchange for reduced treatment time.
Higher doses of rifamycins are desirable to improve efficacy, but their impact on safety and tolerability is unknown. In Tuberculosis Trials Consortium (TBTC) study 29X, a recently completed phase II trial of RPT given at doses ranging from 10 to 20 mg/kg daily with food and concomitant isoniazid, pyrazinamide, and ethambutol during the first 8 weeks of TB treatment (14), participants with TB receiving 15 mg/kg once daily had a median AUC0–24ss of 406 μg · h/ml, and participants receiving 20 mg/kg had a median exposure of 580 μg · h/ml, lower than the exposures in the current study of healthy volunteers (18). RPT was very well tolerated in TBTC study 29X, with only 3 discontinuations for toxicity in the 15- and 20-mg/kg arms (because of grade 2 nausea in 1 of 81 participants receiving 15 mg/kg and because of hepatitis and drug allergy 2 of 81 participants receiving 20 mg/kg) (14). The difference in exposures in our study compared with those in TBTC study 29X may be due to differences in participants' weights. Weight does not significantly impact RPT oral clearance (17), which means that higher-weight individuals (such as the healthy volunteers in our trial) receive higher doses (in milligrams) and have higher exposures than lower-weight individuals (such as patients with TB) for the same milligram-per-kilogram dose (the median dose in TBTC study 29X was 900 mg; in ACTG study A5311 it was 1,350 mg). In addition, in our study the intention was to increase exposures by dividing the dose or giving the dose with a meal type known to enhance absorption (11). High-fat meals may improve the absorption of lipophilic drugs by increasing their solubility, thus enhancing their passive diffusion through enterocytes, and drugs may also associate with triglyceride-rich lipoproteins in high-fat meals with eggs, which facilitates absorption through the lymphatics system. Also, in TBTC study 29X, rifapentine was given with companion drugs, which may have affected the absorption of rifapentine.
There are several possible reasons for the higher rates of adverse events (AEs) in our study than the rates observed in TBTC study 29X. These include higher drug exposures, a lower threshold for defining a dose-limiting toxicity (drug was stopped in any participant who met the case definition of RHS and had a grade 2 or higher AE), the easier attribution of drug-related adverse events in healthy volunteers than in patients with TB-related symptoms, geographic or genetic differences in patient populations, or, perhaps, more robust immunologic responses. It is unclear whether RHS or other rifamycin-related toxicities (e.g., cytopenia or hepatotoxicity) are more common among healthy volunteers than among persons with TB and/or HIV infection (21, 22) or if patients with TB would have similar toxicities if they had exposures similar to those for the participants in this study. Further, an underlying mechanism(s) for rifamycin-induced cytopenias, hepatotoxicity, and RHS remains elusive (23, 24). In ACTG study A5311, RHS symptoms resolved quickly, and no individuals with RHS required hospitalization.
This study had limitations. It was stopped early, so target accrual was not achieved. Therefore, NCA estimates of PK values were imprecise and the sample size was insufficient to make reliable within-arm, intraindividual comparisons; however, population modeling analyses that used data from all participants in all arms provided valuable information about the effects of food type and dosing frequency on PK parameters. This study exemplifies how incorporating model-based PK and PK/PD analyses into the study design can provide unforeseen benefits. The fact that more participants opted for arm 2A because of its higher remuneration may have introduced some bias. However, protocol-defined toxicity was objectively assessed and graded, and PK evaluations should not be subject to bias.
In conclusion, dividing the RPT dose or giving RPT with an egg increased RPT exposures among healthy volunteers, but frequent toxicities, including RHS, led to early study termination. A possible reason for the poorer tolerability in our study than in recent phase II studies in patients with TB was the much higher plasma RPT exposures. The limits of tolerability among patients with TB who achieve RPT exposures similar to those in this trial are unknown and must be assessed prospectively.
Supplementary Material
ACKNOWLEDGMENTS
The research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers UM1 AI068634, UM1 AI068636, and UM1 AI106701. Other support was provided by the following: awards 2UM1 AI069465, UL1 TR001079, and K23AI080842 (to K.E.D.); 2UM1 AI069439-08 and UL1 TR000445 (to D.W.H.); UM1 AI069432 (to C.A.B.); AI069423, 1UL1 TR001111, and P30 AI50410 (to K.P.); and U01 AI068634 (to J.-G.P. and Y.C.). Sanofi provided the rifapentine for this study.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
The study team is thankful to the individuals who volunteered for and participated in this study. We acknowledge the support of the analytical laboratory, particularly Teresa Parsons, in the Division of Clinical Pharmacology at Johns Hopkins University School of Medicine. We also thank Meghan Ames and Susan Oh, study dieticians, for their assistance. We also appreciate the following team members for their contributions to the success of this study: Michelle Wildman, Geraint Davies, Michelle Saemann, Antoine Simmons, Fran Hyc, and Sharon Williams. We appreciate the efforts of the investigators and research staff at the following ACTG clinical trial units (CTUs) and clinical research sites (CRSs): Linda Meixner and Paula Potter, University of California, San Diego, CRS (Site 701); Becky Straub and Miriam Chicurel-Bayard, University of North Carolina Global CTU, Chapel Hill CRS (Site 3201); Marcia Free and Michael Leonard, Vanderbilt Therapeutics CRS (Site 3652); and Andi Weiss and Ilene Wiggins, Johns Hopkins University CRS (Site 201).
Footnotes
This is AIDS Clinical Trials Group study A5311.
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.05128-14.
REFERENCES
- 1.World Health Organization. 2014. Global tuberculosis report 2014. Report WHO/HTM/TB/2014.08 World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Jayaram R, Gaonkar S, Kaur P, Suresh BL, Mahesh BN, Jayashree R, Nandi V, Bharat S, Shandil RK, Kantharaj E, Balasubramanian V. 2003. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother 47:2118–2124. doi: 10.1128/AAC.47.7.2118-2124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boeree MJ, Plemper van Balen G, Aarnoutse RA. 2011. High-dose rifampicin: how do we proceed? Int J Tuberc Lung Dis 15:1133. doi: 10.5588/ijtld.11.0113,10.5588/ijtld.11.0198. [DOI] [PubMed] [Google Scholar]
- 4.Boeree M, Diacon A, Dawson R, Venter A, du Bois J, Narunsky K, Hoelscher M, Gillespie S, Phillips P, Aarnoutse R, PanACEA Consortium . 2013. What is the “right” dose of rifampin?, paper 148LB. abstr 128 Abstr 20th Conf Retrovir Opportunist Infect, Atlanta, GA. [Google Scholar]
- 5.Diacon AH, Patientia RF, Venter A, van Helden PD, Smith PJ, McIlleron H, Maritz JS, Donald PR. 2007. Early bactericidal activity of high-dose rifampin in patients with pulmonary tuberculosis evidenced by positive sputum smears. Antimicrob Agents Chemother 51:2994–2996. doi: 10.1128/AAC.01474-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenthal IM, Zhang M, Williams KN, Peloquin CA, Tyagi S, Vernon AA, Bishai WR, Chaisson RE, Grosset JH, Nuermberger EL. 2007. Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model. PLoS Med 4:e344. doi: 10.1371/journal.pmed.0040344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenthal IM, Tasneen R, Peloquin CA, Zhang M, Almeida D, Mdluli KE, Karakousis PC, Grosset JH, Nuermberger EL. 2012. Dose-ranging comparison of rifampin and rifapentine in two pathologically distinct murine models of tuberculosis. Antimicrob Agents Chemother 56:4331–4340. doi: 10.1128/AAC.00912-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang M, Li SY, Rosenthal IM, Almeida DV, Ahmad Z, Converse PJ, Peloquin CA, Nuermberger EL, Grosset JH. 2011. Treatment of tuberculosis with rifamycin-containing regimens in immune-deficient mice. Am J Respir Crit Care Med 183:1254–1261. doi: 10.1164/rccm.201012-1949OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dooley KE, Bliven-Sizemore EE, Weiner M, Lu Y, Nuermberger EL, Hubbard WC, Fuchs EJ, Melia MT, Burman WJ, Dorman SE. 2012. Safety and pharmacokinetics of escalating daily doses of rifapentine, an antituberculosis drug, in healthy volunteers. Clin Pharmacol Ther 91:881–888. doi: 10.1038/clpt.2011.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorman SE, Goldberg S, Stout JE, Muzanyi G, Johnson JL, Weiner M, Bozeman L, Heilig CM, Feng PJ, Moro R, Narita M, Nahid P, Ray S, Bates E, Haile B, Nuermberger EL, Vernon A, Schluger NW, Tuberculosis Trials Consortium. 2012. Substitution of rifapentine for rifampin during intensive phase treatment of pulmonary tuberculosis: study 29 of the Tuberculosis Trials Consortium. J Infect Dis 206:1030–1040. doi: 10.1093/infdis/jis461. [DOI] [PubMed] [Google Scholar]
- 11.Zvada SP, Van Der Walt JS, Smith PJ, Fourie PB, Roscigno G, Mitchison D, Simonsson US, McIlleron HM. 2010. Effects of four different meal types on the population pharmacokinetics of single-dose rifapentine in healthy male volunteers. Antimicrob Agents Chemother 54:3390–3394. doi: 10.1128/AAC.00345-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Division of AIDS. 2011. Table for grading the severity of adult and pediatric adverse events, version 1.0. Division of AIDS, National Institutes of Health, Bethesda, MD. [Google Scholar]
- 13.Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, Hackman J, Hamilton CD, Menzies D, Kerrigan A, Weis SE, Weiner M, Wing D, Conde MB, Bozeman L, Horsburgh CR Jr, Chaisson RE, TB Trials Consortium PREVENT TB Study Team . 2011. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med 365:2155–2166. doi: 10.1056/NEJMoa1104875. [DOI] [PubMed] [Google Scholar]
- 14.Dorman SE, Savic RM, Goldberg S, Stout JE, Schluger N, Muzanyi G, Johnson JL, Nahid P, Hecker EJ, Heilig CM, Bozeman L, Feng PJ, Moro RN, MacKenzie W, Dooley KE, Nuermberger EL, Vernon A, Weiner M, Tuberculosis Trials Consortium . 2014. Daily rifapentine for treatment of pulmonary tuberculosis. A randomized, dose-ranging trial. Am J Respir Crit Care Med 19:333–343. doi: 10.1164/rccm.201410-1843OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung JY, Cho JY, Yu KS, Kim JR, Oh DS, Jung HR, Lim KS, Moon KH, Shin SG, Jang IJ. 2005. Effect of OATP1B1 (SLCO1B1) variant alleles on the pharmacokinetics of pitavastatin in healthy volunteers. Clin Pharmacol Ther 78:342–350. doi: 10.1016/j.clpt.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Chigutsa E, Visser ME, Swart EC, Denti P, Pushpakom S, Egan D, Holford NH, Smith PJ, Maartens G, Owen A, McIlleron H. 2011. The SLCO1B1 rs4149032 polymorphism is highly prevalent in South Africans and is associated with reduced rifampin concentrations: dosing implications. Antimicrob Agents Chemother 55:4122–4127. doi: 10.1128/AAC.01833-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savic RM, Lu Y, Bliven-Sizemore E, Weiner M, Nuermberger E, Burman W, Dorman SE, Dooley KE. 2014. Population pharmacokinetics of rifapentine and desacetyl rifapentine in healthy volunteers: nonlinearities in clearance and bioavailability. Antimicrob Agents Chemother 58:3035–3042. doi: 10.1128/AAC.01918-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savic RM, Weiner M, MacKenzie W, Heilig C, Dooley K, Engle M, Nsubuga P, Phan H, Peloquin C, Dorman S, for the Tuberculosis Trials Consortium of the Centers for Disease Control and Prevention. 2013. PK/PD analysis of rifapentine in patients during intensive phase treatment for tuberculosis from Tuberculosis Trials Consortium Studies 29 and 29X, Denver, CO. Abstr 6th Int Workshop Clin Pharmacol Tuberc Drugs. [Google Scholar]
- 19.Ruslami R, Ganiem AR, Dian S, Apriani L, Achmad TH, van der Ven AJ, Borm G, Aarnoutse RE, van Crevel R. 2013. Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open-label, randomised controlled phase 2 trial. Lancet Infect Dis 13:27–35. doi: 10.1016/S1473-3099(12)70264-5. [DOI] [PubMed] [Google Scholar]
- 20.Owens JP, Fofana MO, Dowdy DW. 2013. Cost-effectiveness of novel first-line treatment regimens for tuberculosis. Int J Tuberc Lung Dis 17:590–596. doi: 10.5588/ijtld.12.0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterling T, Benson C, Shang N, Miro J, Grinsztejn B, Chaisson R, Lucchetti A, Sanchez J, Scott N, Villarino E, Clinical Trials Group AIDS, Tuberculosis Trials Consortium . 2014. Tolerability among HIV-positive persons of three months of once-weekly rifapentine + INH (3HP) versus 9 months of daily INH (9H) for treatment of latent tuberculosis infection: the PREVENT TB study (TBTC study 26/ACTG 5259), abstr MOAB0302 Abstr 19th Int AIDS Conf. [Google Scholar]
- 22.Centers for Disease Control and Prevention (CDC) and American Thoracic Society. 2003. Update: adverse event data and revised American Thoracic Society/CDC recommendations against the use of rifampin and pyrazinamide for treatment of latent tuberculosis infection—United States, 2003. MMWR Morb Mortal Wkly Rep 52:735–739. mm5231a4. [PubMed] [Google Scholar]
- 23.Grosset J, Leventis S. 1983. Adverse effects of rifampin. Rev Infect Dis 5(Suppl 3):S440–S450. [DOI] [PubMed] [Google Scholar]
- 24.Martinez E, Collazos J, Mayo J. 1999. Hypersensitivity reactions to rifampin. Pathogenetic mechanisms, clinical manifestations, management strategies, and review of the anaphylactic-like reactions. Medicine (Baltimore) 78:361–369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.