Abstract
Tenofovir (TFV) is eliminated by renal excretion, which is mediated through multidrug-resistant protein 2 (MRP2) and MRP4, encoded by ABCC2 and ABCC4, respectively. Genetic polymorphisms of these transporters may affect the plasma concentrations of tenofovir. Therefore, the aim of this study was to investigate the influence of genetic and nongenetic factors on tenofovir plasma concentrations. A cross-sectional study was performed in Thai HIV-infected patients aged ≥18 years who had been receiving tenofovir disoproxil fumarate at 300 mg once daily for at least 6 months. A middose tenofovir plasma concentration was obtained. Multivariate analysis was performed to investigate whether there was an association between tenofovir plasma concentrations and demographic data, including age, sex, body weight, estimated glomerular filtration rate (eGFR), hepatitis B virus coinfection, hepatitis C virus coinfection, duration of tenofovir treatment, concomitant use of ritonavir-boosted protease inhibitors, and polymorphisms of ABCC2 and ABCC4. A total of 150 Thai HIV-infected patients were included. The mean age of the patients was 43.9 ± 7.2 years. The mean tenofovir plasma concentration was 100.3 ± 52.7 ng/ml. In multivariate analysis, a low body weight, a low eGFR, the concomitant use of ritonavir-boosted protease inhibitors, and the ABCC4 4131T → G variation (genotype TG or GG) were independently associated with higher tenofovir plasma concentrations. After adjusting for weight, eGFR, and the concomitant use of ritonavir-boosted protease inhibitors, a 30% increase in the mean tenofovir plasma concentration was observed in patients having the ABCC4 4131 TG or GG genotype. Both genetic and nongenetic factors affect tenofovir plasma concentrations. These factors should be considered when adjusting tenofovir dosage regimens to ensure the efficacy and safety of a drug. (This study has been registered at ClinicalTrials.gov under registration no. NCT01138241.)
INTRODUCTION
Tenofovir disoproxil fumarate (TDF), an oral prodrug of tenofovir (TFV), is widely used for the treatment of human immunodeficiency virus (HIV) infection because of its high potency, good safety profile, limited drug interaction, and convenient once-daily dosing (1, 2). After absorption, TDF is rapidly converted to tenofovir. Tenofovir is then phosphorylated intracellularly to tenofovir diphosphate, an active analog, which inhibits HIV reverse transcriptase, resulting in a termination DNA chain elongation (1, 2).
Tenofovir is eliminated by renal excretion through glomerular filtration and active tubular secretion. It is transported into kidney tubular cells by organic anion transporter 1 (OAT1) and OAT3, encoded by the SLC22A6 and SLC22A8 genes, respectively, at the basolateral membrane. Subsequently, tenofovir is secreted to the tubular lumen by multidrug-resistant protein 2 (MRP2) and MRP4, encoded by the ABCC2 and ABCC4 genes, respectively, at the apical membrane (3). Therefore, genetic polymorphisms of these transporter genes may affect the transport of tenofovir at kidney tubular cells and may have an impact on tenofovir plasma concentrations.
Previous studies have shown that the polymorphisms of ABCC2 and ABCC4 are associated with higher tenofovir concentrations (4, 5) and a higher tenofovir plasma concentration is associated with renal impairment (6, 7). The cutoff values of the middose (12-h) tenofovir concentration (C12) and the trough (minimum) concentration (Cmin) (>160 ng/ml and >90 ng/ml, respectively) were proposed to discriminate a risk of kidney tubular dysfunction (KTD) (6, 7). These results suggest that genetic variation in tenofovir transporter genes may lead to overexposure to tenofovir, resulting in kidney tubular cell damage. Therefore, a study investigating the influence of genetic and nongenetic factors on tenofovir concentrations is crucial for the optimization of dosage regimens to prevent renal toxicity.
However, there are limited studies showing an association between genetic polymorphisms of drug transporters and tenofovir plasma concentrations. Therefore, the aim of this study was to investigate the influence of genetic variants of ABCC2 and ABCC4 and nongenetic factors on tenofovir plasma concentrations. The results of this study will be useful for the design of tenofovir dosage regimens to optimize drug concentrations and ensure the safety and efficacy of this drug.
MATERIALS AND METHODS
Study population.
A cross-sectional study was performed in Thai HIV-infected patients recruited from the HIV Netherlands Australia Thailand Research Collaboration (HIV-NAT), Bangkok, Thailand, from March 2012 to May 2013 (ClinicalTrials.gov registration no. NCT01138241). Patients aged 18 years and older who had been receiving TDF at 300 mg once daily for at least 6 months for the treatment of HIV infection were included in the study. Blood samples were obtained at middose (10 to 14 h after the last dose) for tenofovir concentration determination and genotyping assay. Demographic and laboratory data, including age, sex, body weight, serum creatinine concentration, hepatitis B virus coinfection, hepatitis C virus coinfection, duration of tenofovir treatment, and concomitant use of antiretroviral drugs, were recorded. The estimated glomerular filtration rate (eGFR) was calculated using the modification of diet in renal disease (MDRD) formula. The study was approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand. All patients provided written informed consent.
Determination of tenofovir plasma concentration.
Tenofovir plasma concentrations were determined at the HIV-NAT research laboratory by a validated high-performance liquid chromatography assay with a fluorescence detector using the modified method of Droste et al. (8) with a lower limit of quantification of 15 ng/ml. The tenofovir calibration curve was linear over the concentration range of from 15 to 1,500 ng/ml. The within-run and between-run coefficients of variation (precision) were less than 10%, and the accuracy of the tenofovir concentration was between 95 and 105%.
Genotyping assay.
Human genomic DNA was extracted from peripheral blood mononuclear cells by use of a QIAamp DNA blood minikit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. Five single nucleotide polymorphisms, ABCC2 −24C → T (rs717620), ABCC2 1249G → A (rs2273697), ABCC2 3972C → T (rs3740066), ABCC4 3463A → G (rs1751034), and ABCC4 4131T → G (rs3742106), were genotyped. These polymorphisms were chosen on the basis of the allele frequency, evidence of their association with tenofovir plasma concentrations or toxicity, and their influence on drugs excreted via glomerular filtration and active tubular secretion (4, 9–12).
The genotyping assay was performed by real-time PCR using a TaqMan allelic discrimination assay with a predesigned probe and primer (Applied Biosystems, CA, USA). The PCR conditions were as follows: 95°C for 10 min, followed by 92°C for 15 s and 60°C for 1 min.
Statistical analyses.
Statistical analyses in this study were performed using Statistical Package for the Social Sciences (SPSS; version 17; SPSS Co., Ltd., Bangkok, Thailand) software. The demographic characteristics of the patients are presented as the mean ± standard deviation (SD) for continuous data and the frequency (number and percentage of patients) for categorical data. The allele frequencies of ABCC2 and ABCC4 were calculated. The distribution of the observed genotype according to Hardy-Weinberg equilibrium was tested by the chi-square test. Due to the small number of patients in some genotype groups, the mean tenofovir plasma concentration was compared between 2 genotype groups, patients with a homozygous wild-type allele and patients with at least 1 variant allele, by Student's t test. A regression model was used to assess whether an association exists between tenofovir plasma concentrations and demographic data, including age, sex, body weight, eGFR, hepatitis B virus coinfection, hepatitis C virus coinfection, duration of tenofovir treatment, polymorphisms of ABCC2, polymorphisms of ABCC4, and concomitant use of ritonavir-boosted protease inhibitors. Any independent variables with a P value of <0.1 in the univariate analysis were entered into a model of multivariable regression analysis using the stepwise method. A P value of <0.05 was considered statistically significant.
RESULTS
Demographic data.
A total of 150 patients providing 150 blood samples were included in this study. A summary of the patient characteristics is presented in Table 1. Among the 150 patients, 101 (67.3%), 48 (32.0%), and 1 (0.7%) patients were receiving tenofovir in combination with nonnucleoside reverse transcriptase inhibitors (NNRTIs), ritonavir boosted-protease inhibitors, and an integrase inhibitor, respectively.
TABLE 1.
Demographic characteristics of study patientsa
| Characteristic | Value |
|---|---|
| No. (%) of patients of the following sex: | |
| Male | 85 (56.7) |
| Female | 65 (43.3) |
| Mean age ± SD (yr) | 43.9 ± 7.2 |
| Mean body wt ± SD (kg) | 60.3 ± 11.9 |
| Mean body mass index ± SD (kg/m2) | 22.5 ± 3.6 |
| Mean serum creatinine concn ± SD (mg/dl) | 0.9 ± 0.2 |
| Mean eGFRb ± SD (ml/min/1.73 m2) | 90.3 ± 18.0 |
| No. (%) of patients: | |
| Hepatitis B virus antigen positive | 60 (40.0) |
| Hepatitis C antibody positive | 12 (8.0) |
| Mean duration of tenofovir treatment ± SD (yr) | 3.7 ± 2.0 |
| Mean tenofovir sampling time after last dose ± SD (h) | 11.9 ± 0.8 |
| Mean tenofovir plasma concn ± SD (ng/ml) | 100.3 ± 52.7 |
| No. (%) of patients receiving the following comedications: | |
| Lamivudine | 94 (62.7) |
| Emtricitabine | 46 (30.7) |
| Zidovudine | 10 (6.7) |
| Efavirenz | 91 (60.7) |
| Nevirapine | 10 (6.7) |
| Lopinavir-ritonavir | 17 (11.3) |
| Atazanavir-ritonavir | 9 (6.0) |
| Darunavir-ritonavir | 3 (2.0) |
| Saquinavir-ritonavir | 19 (12.7) |
| Raltegravir | 1 (0.7) |
Data are for 150 patients.
Calculated by use of the MDRD formula.
The frequencies of genetic polymorphisms of ABCC2 and ABCC4 are shown in Table 2. All polymorphisms were in Hardy-Weinberg equilibrium (χ2, P ≥ 0.05).
TABLE 2.
Frequencies of ABCC2 and ABCC4 genotype polymorphismsa
| Genetic polymorphism | Genotype |
Allele |
|||
|---|---|---|---|---|---|
| Genotype | No. of patients | % of patients | Allele | % of patients | |
| ABCC2 −24C → T | CC | 98 | 65.3 | C | 80.7 |
| CT | 46 | 30.7 | T | 19.3 | |
| TT | 6 | 4.0 | |||
| ABCC2 1249G → A | GG | 123 | 82.0 | G | 90.7 |
| GA | 26 | 17.3 | A | 9.3 | |
| AA | 1 | 0.7 | |||
| ABCC2 3972C → T | CC | 94 | 62.7 | C | 78.3 |
| CT | 47 | 31.3 | T | 21.7 | |
| TT | 9 | 6.0 | |||
| ABCC4 3463A → G | AA | 96 | 64.0 | A | 80.7 |
| AG | 50 | 33.3 | G | 19.3 | |
| GG | 4 | 2.7 | |||
| ABCC4 4131T → G | TT | 34 | 22.7 | T | 49.3 |
| TG | 80 | 53.3 | G | 50.7 | |
| GG | 36 | 24.0 | |||
Data are for 150 patients.
Predictors of tenofovir plasma concentrations.
The mean tenofovir plasma concentration for each polymorphism is presented in Table 3. Tenofovir plasma concentrations between patients with a homozygous wild-type allele and patients with at least 1 variant allele were not significantly different for all genetic polymorphisms.
TABLE 3.
Association between tenofovir plasma concentrations and ABCC2 and ABCC4 genotypes
| Genetic polymorphism | Genotype | Mean tenofovir plasma concn ± SD (ng/ml) | P valuea |
|---|---|---|---|
| ABCC2 −24C → T | CC | 101.5 ± 57.0 | 0.706 |
| CT or TT | 98.1 ± 43.9 | ||
| ABCC2 1249G → A | GG | 100.3 ± 55.7 | 0.984 |
| GA or AA | 100.5 ± 37.0 | ||
| ABCC2 3972C → T | CC | 102.8 ± 57.1 | 0.455 |
| CT or TT | 96.1 ± 44.6 | ||
| ABCC4 3463A → G | AA | 104.7 ± 57.2 | 0.177 |
| AG or GG | 92.6 ± 43.0 | ||
| ABCC4 4131T → G | TT | 86.0 ± 30.7 | 0.072 |
| TG or GG | 104.5 ± 57.0 |
Determined by Student's t test.
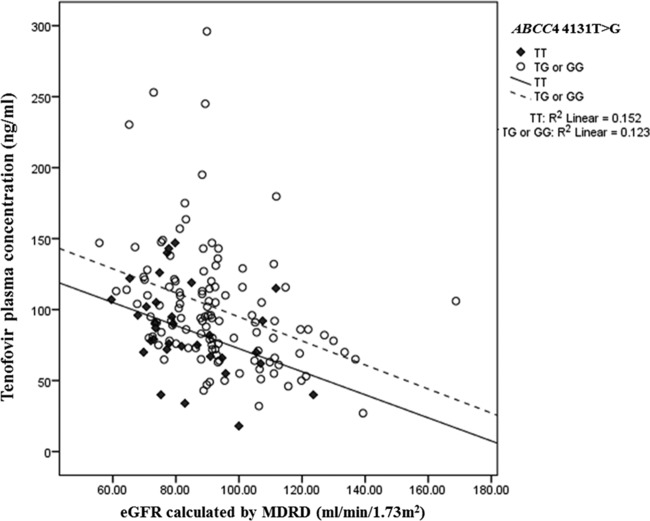
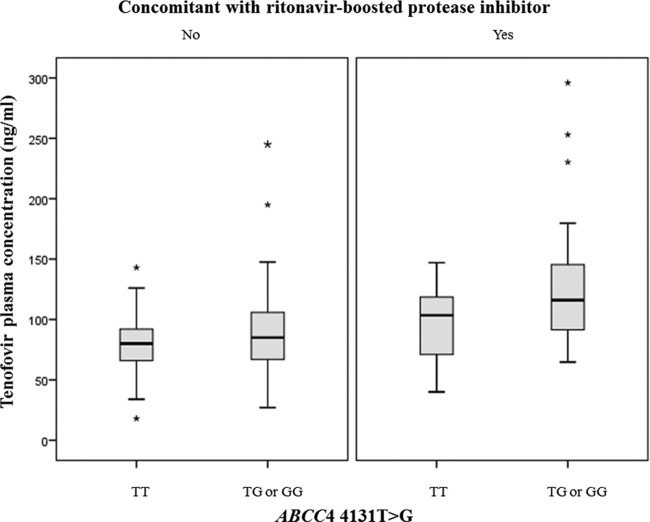
The influence of genetic and nongenetic factors on tenofovir plasma concentrations in univariate and multivariate analyses is presented in Table 4. Factors including low body weight, low eGFR, concomitant use of ritonavir-boosted protease inhibitors, and having an ABCC4 4131T → G variation (genotype TG or GG) were independently associated with higher tenofovir plasma concentration (P < 0.05) in the multivariate analysis. Figure 1 presents the relationship between tenofovir plasma concentrations and eGFR by ABCC4 4131T → G genotype. Patients having the ABCC4 4131 TG or GG genotype tended to have higher tenofovir plasma concentration than those having the TT genotype. On the basis of the results from multivariate analysis, it was shown that after controlling for body weight, eGFR, and concomitant use of a ritonavir-boosted protease inhibitor, patients having the ABCC4 4131 TG or GG genotype had, on average, 30% higher mean tenofovir plasma concentrations than patients having the ABCC4 4131 TT genotype (P = 0.007) (Fig. 2).
TABLE 4.
Univariate and multivariate analyses of genetic and nongenetic factors for tenofovir plasma concentrations
| Factor | Univariate analysis |
Multivariate analysisa |
||||
|---|---|---|---|---|---|---|
| B | 95% CIb | P valuec | B | 95% CI | P valuec | |
| Female | 13.986 | −3.079 to 31.052 | 0.107 | |||
| Age | 0.183 | −1.002 to 1.368 | 0.760 | |||
| Body wt | −0.918 | −1.622 to −0.214 | 0.011* | −0.861 | −1.494 to −0.229 | 0.008** |
| eGFRd | −0.970 | −1.420 to −0.520 | <0.001* | −0.934 | −1.375 to −0.492 | <0.001** |
| Hepatitis B virus positive | −12.241 | −29.541 to 5.059 | 0.164 | |||
| Hepatitis C virus positive | −0.275 | −31.721 to 31.171 | 0.986 | |||
| Duration of tenofovir treatment | −0.385 | −4.677 to 3.907 | 0.860 | |||
| Concomitant RTV-boosted PIe | 40.088 | 22.998 to 57.177 | <0.001* | 29.231 | 12.649 to 45.813 | 0.001** |
| ABCC2 −24 CT or TT | −3.435 | −21.352 to 14.482 | 0.706 | |||
| ABCC2 1249 GA or AA | 0.217 | −21.989 to 22.422 | 0.984 | |||
| ABCC2 3972 CT or TT | −6.675 | −24.279 to 10.930 | 0.455 | |||
| ABCC4 3463 AG or GG | −12.127 | −29.791 to 5.536 | 0.177 | |||
| ABCC4 4131 TG or GG | 18.464 | −1.690 to 38.619 | 0.072* | 25.180 | 7.049 to 43.310 | 0.007** |
Factors with P values of <0.1 in the univariate analysis were entered into the multivariate analysis.
CI, confidence interval.
*, P < 0.1; **, P < 0.05.
Calculated by use of the MDRD formula.
RTV, ritonavir; PI, protease inhibitor.
FIG 1.
Relationship between tenofovir plasma concentrations and eGFR, calculated by use of the MDRD formula, subgrouped by ABCC4 4131 TT genotype and ABCC4 4131 TG or GG genotype.
FIG 2.
Box plots of tenofovir plasma concentrations in patients having the ABCC4 4131 TT genotype versus those having the TG or GG genotype by whether the patients were using ritonavir-boosted protease inhibitors as a comedication. ABCC4 4131T → G genotypes are shown on the x axis. The medians and interquartile ranges of tenofovir plasma concentrations are shown on the y axis. Among patients who did not use a ritonavir-boosted protease inhibitor, the median tenofovir plasma concentration was 85.0 ng/ml in patients with the ABCC4 4131 TG/GG genotype, whereas it was 80.0 ng/ml in those with the TT genotype. Among patients using a ritonavir-boosted protease inhibitor, the median tenofovir plasma concentration in patients with the ABCC4 4131 TG/GG genotype was 118.0 ng/ml, whereas it was 103.5 ng/ml in those with the TT genotype. After controlling for body weight, eGFR, and concomitant use of a ritonavir-boosted protease inhibitor, patients having the ABCC4 4131 TG/GG genotype had, on average, a 30% higher mean tenofovir plasma concentration than those having the TT genotype (P = 0.007).
DISCUSSION
Although tenofovir is well tolerated, the induction of nephrotoxicity by tenofovir has been reported (13). There is evidence of an association between tenofovir plasma concentrations and renal toxicity (4, 6, 7, 14). A study by Rodríguez-Nóvoa et al. has shown that patients with a tenofovir plasma concentration of more than 160 ng/ml at middose (10 to 14 h after the last dose) were at a 4.8 times higher risk of experiencing KTD than patients with a tenofovir plasma concentration below this cutoff value (6). Moreover, a previous study by Poizot-Martin et al. suggested that a threshold tenofovir trough concentration of >90 ng/ml is a predictor of a risk of KTD (7). Therefore, tenofovir dose adjustment is crucial in decreasing the risk of renal toxicity when tenofovir is prescribed.
The pharmacokinetics of tenofovir are highly variable between individuals (4, 15, 16). Thus, identifying factors that contribute to this high variability would be beneficial for tenofovir dose adjustment. In this study, we investigated the influence of both genetic and nongenetic factors on tenofovir plasma concentrations. The results from multivariate analysis showed that tenofovir plasma concentrations are associated with body weight, eGFR, concomitant use of a ritonavir-boosted protease inhibitor, and the polymorphism ABCC4 4131T → G. A lower patient body weight was associated with higher tenofovir plasma concentrations in our study. This finding is consistent with the results from previous studies which demonstrated that body weight is one of the important predictors of tenofovir's pharmacokinetics (4, 15, 17).
Tenofovir is mainly eliminated by renal excretion. It is effluxed across renal proximal tubule cells by MRP2 and MRP4, encoded by the ABCC2 and ABCC4 genes, respectively. Genetic polymorphisms of these transporters have been reported to be associated with higher levels of tenofovir exposure and KTD (4, 5, 9, 10, 18). A study by Kiser et al. found that patients carrying the ABCC4 3463A → G variation had lower tenofovir renal clearance than those carrying the wild type, leading to an approximately 32% increase in the tenofovir area under the curve in the ABCC4 3463A → G variant group (9). A more recent study in a Thai HIV-infected population reported that the ABCC2 −24 CC genotype was associated with a higher tenofovir plasma concentration than the CT or TT genotype (114 ng/ml for the CC genotype and 93 ng/ml for the CT or TT genotype) (4). However, that study investigated only a limited number of genes (ABCC2 −24C → T and ABCB1 3435C → T), and it is possible that the influence of polymorphisms of other transporter genes may have not been detected. Interestingly, we could not confirm the influence of the ABCC4 3463A → G and ABCC2 −24C → T polymorphisms on tenofovir plasma concentrations in our study. This could be due to the small number of patients enrolled in previous studies and the different ethnicities and the different genetic polymorphisms of the patients investigated among the studies. However, it could be postulated that several transporter genes may play a role in tenofovir elimination. The present study is the first to report an association between the ABCC4 4131T → G variation and tenofovir plasma concentrations. The results from multivariate analysis showed that after controlling for body weight, eGFR, and concomitant use of a ritonavir-boosted protease inhibitor, patients carrying the ABCC4 4131 TG or GG genotype had, on average, a 30% higher mean tenofovir plasma concentration than patients carrying the TT genotype. Although an association between the ABCC4 4131T → G polymorphism and the tenofovir plasma concentrations has never been found, the influence of this polymorphism on the intracellular concentrations of lamivudine has been reported (12). A study by Anderson et al. found a 20% increase in the intracellular concentrations of lamivudine in patients carrying the ABCC4 4131 TG or GG genotype than those carrying the TT genotype (12). A potential mechanism of this interaction was proposed. The ABCC4 4131T → G variation may reduce MRP4 protein expression and decrease the transportation of drugs in kidney tubular cells (12).
As the elimination of tenofovir requires drug transporters, tenofovir may be susceptible to a drug transporter-mediated interaction (1, 19). Previous reports showed that ritonavir-boosted protease inhibitors, including lopinavir-ritonavir, atazanavir-ritonavir, and darunavir-ritonavir, can increase the level of tenofovir exposure by approximately 17 to 37% (1, 9, 20). These interactions were confirmed in our study. The concomitant use of a ritonavir-boosted protease inhibitor resulted in a 35% increase in the tenofovir plasma concentration. Even though the exact mechanism of the interaction between a ritonavir-boosted protease inhibitor and tenofovir has not been conclusively defined, possible mechanisms have been proposed. Ritonavir was shown to be a potent inhibitor of P glycoprotein (P-gp) and MRP2 (21). Inhibition of P-gp by a ritonavir-boosted protease inhibitor could lead to increased absorption in the gut (22). On the other hand, an increase in the level of tenofovir exposure due to inhibition of MRP2, an efflux transporter from the renal proximal tubule cells, resulting in decreased renal excretion, was also speculated (21).
Some study limitations should be noted. First, the polymorphisms of other transporter genes involving tenofovir influx transport, such as SLC22A6 and SLC22A8, were not investigated in this study. However, there is evidence that genetic polymorphisms of these transporters are not associated with the pharmacokinetics of several drugs, including tenofovir, adefovir, pravastatin, and torsemide (9, 23–25). Therefore, the transportation of drugs across the apical membrane (from cell to tubular lumen) by multidrug-resistant proteins may be a rate-limiting step for drug secretion (23, 25). Thus, it is likely that the polymorphisms of organic anion transporters may not be associated with tenofovir's pharmacokinetics. Furthermore, we studied a selected number of efflux transporter polymorphisms. A more comprehensive investigation of various polymorphisms should be performed. Second, the overall effect of ritonavir-boosted protease inhibitors was quantified in this study. Due to the small number of patients using atazanavir-ritonavir and darunavir-ritonavir, the influence of each ritonavir-boosted protease inhibitor was not identified. Third, due to the cross-sectional design of the study, an association between a higher tenofovir plasma concentration and renal toxicity cannot be confirmed. However, it is worth mentioning that if a middose concentration of tenofovir of >160 ng/ml were used as the cutoff for a risk of renal toxicity, all of the patients having a tenofovir concentration at middose of >160 ng/ml in our study would have the ABCC4 4131 TG or GG genotype. Therefore, it is possible that patients having the ABCC4 4131T → G variation could be at higher risk of renal toxicity because of a high tenofovir plasma concentration. Finally, due to the inhibitory effect of ritonavir-boosted protease inhibitors on the renal tubular secretion of serum creatinine, a rise in the serum creatinine concentration could be observed in a group of patients using ritonavir-boosted protease inhibitors. This may lead to an underestimate of the eGFR in this group of patients. However, with the small increase in the serum creatinine concentration reported in a previous study (0.08 mg/dl) (26), this would result in a negligible decrease in the eGFR and should not affect the results of this study.
In summary, this study showed that both genetic and nongenetic factors influence tenofovir plasma concentrations, which could be associated with tenofovir-induced renal toxicity. Patients who had a low body weight and a low eGFR, who concomitantly used a ritonavir-boosted protease inhibitor, and who had the ABCC4 4131 TG or GG genotype were at risk of a higher tenofovir concentration. Therefore, the tenofovir concentration should be closely monitored in these groups of patients.
ACKNOWLEDGMENTS
This work was supported by the Ratchadaphiseksomphot Endowment Fund, Chulalongkorn University; the National Research Council of Thailand (NRCT) under grant numbers PorKor/2554-136 and PorKor/2553-112; the Thailand Research Fund (TRF) under grant number RSA5380002; and the Aligning Care and Prevention of HIV/AIDS with Government Decentralization to Achieve Coverage and Impact: ACHIEVED Project (Global Fund Thailand).
We thank all patients participating in this study and the HIV-NAT staffs for their support.
REFERENCES
- 1.Kearney BP, Flaherty JF, Shah J. 2004. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin Pharmacokinet 43:595–612. doi: 10.2165/00003088-200443090-00003. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez E, Morello J, Soriano V, Labarga P, Rodriguez-Novoa S. 2011. Critical appraisal and update on tenofovir in management of human immunodeficiency virus infection. Virus Adapt Treat 3:55–69. doi: 10.2147/VAAT.S12708. [DOI] [Google Scholar]
- 3.Rodriguez-Novoa S, Labarga P, Soriano V. 2009. Pharmacogenetics of tenofovir treatment. Pharmacogenomics 10:1675–1685. doi: 10.2217/pgs.09.115. [DOI] [PubMed] [Google Scholar]
- 4.Manosuthi W, Sukasem C, Thongyen S, Nilkamhang S, Sungkanuparph S. 2014. ABCC2*1C and plasma tenofovir concentration are correlated to decreased glomerular filtration rate in patients receiving a tenofovir-containing antiretroviral regimen. J Antimicrob Chemother 69:2195–2201. doi: 10.1093/jac/dku129. [DOI] [PubMed] [Google Scholar]
- 5.Kiser JJ, Aquilante CL, Anderson PL, King TM, Carten ML, Fletcher CV. 2008. Clinical and genetic determinants of intracellular tenofovir diphosphate concentrations in HIV-infected patients. J Acquir Immune Defic Syndr 47:298–303. doi: 10.1097/QAI.0b013e31815e7478. [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez-Nóvoa S, Labarga P, D'Avolio A, Barreiro P, Albalate M, Vispo E, Solera C, Siccardi M, Bonora S, Perri GD, Sariano V. 2010. Impairment in kidney tubular function in patients receiving tenofovir is associated with higher tenofovir plasma concentrations. AIDS 24:1064–1066. doi: 10.1097/QAD.0b013e32833202e2. [DOI] [PubMed] [Google Scholar]
- 7.Poizot-Martin I, Solas C, Allemand J, Obry-Roguet V, Pradel V, Bregigeon S, Faucher O, Lacarelle B. 2013. Renal impairment in patients receiving a tenofovir-cART regimen: impact of tenofovir trough concentration. J Acquir Immune Defic Syndr 62:375–380. doi: 10.1097/QAI.0b013e31827ce4ee. [DOI] [PubMed] [Google Scholar]
- 8.Droste JA, Verweij-van Wissen CP, Kearney BP, Buffels R, Vanhorssen PJ, Hekster YA, Burger DM. 2005. Pharmacokinetic study of tenofovir disoproxil fumarate combined with rifampin in healthy volunteers. Antimicrob Agents Chemother 49:680–684. doi: 10.1128/AAC.49.2.680-684.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiser JJ, Carten ML, Aquilante CL, Anderson PL, Wolfe P, King TM, Delahunty T, Bushman LR. 2008. The effect of lopinavir/ritonavir on the renal clearance of tenofovir in HIV-infected patients. Clin Pharmacol Ther 83:265–272. doi: 10.1038/sj.clpt.6100269. [DOI] [PubMed] [Google Scholar]
- 10.Izzedine H, Hulot JS, Villard E, Goyenvalle C, Dominguez S, Ghosn J, Valantin MA, Lechat P, Deray G. 2006. Association between ABCC2 gene haplotypes and tenofovir-induced proximal tubulopathy. J Infect Dis 194:1481–1491. doi: 10.1086/508546. [DOI] [PubMed] [Google Scholar]
- 11.Hagleitner MM, Coenen MJ, Schrauwen M, Vermeulen SH, deBont ES, Hoogerbrugge P, TeLoo DM. 2010. Association of a genetic variant in the ABCC2 gene with high methotrexate plasma concentrations in pediatric malignancies. J Clin Oncol 28:15s. [Google Scholar]
- 12.Anderson PL, Lamba J, Aquilante C, Schuetz E, Fletcher C. 2006. Pharmacogenetic characteristics of indinavir, zidovudine, and lamivudine therapy in HIV-infected adults: a pilot study. J Acquir Immune Defic Syndr 42:441–449. doi: 10.1097/01.qai.0000225013.53568.69. [DOI] [PubMed] [Google Scholar]
- 13.Hall AM. 2013. Update on tenofovir toxicity in the kidney. Pediatr Nephrol 28:1011–1023. doi: 10.1007/s00467-012-2269-7. [DOI] [PubMed] [Google Scholar]
- 14.Ezinga M, Wetzels JF, Bosch ME, van der Ven AJ, Burger DM. 2014. Long-term treatment with tenofovir: prevalence of kidney tubular dysfunction and its association with tenofovir plasma concentration. Antivir Ther 19:765–771. doi: 10.3851/IMP2761. [DOI] [PubMed] [Google Scholar]
- 15.Gagnieu M, Barkil ME, Livrozet JM, Cotte L, Miailhes P, Boibieux A, Guitton J, Tod M. 2008. Population pharmacokinetics of tenofovir in AIDS patients. J Clin Pharmacol 48:1282–1288. doi: 10.1177/0091270008322908. [DOI] [PubMed] [Google Scholar]
- 16.Jullien V, Treluyer JM, Rey E, Jaffray P, Krivine A, Moachon L, Louet AL, Lescoat A, Dupin N, Salmon D, Pons G, Urien S. 2005. Population pharmacokinetics of tenofovir in human immunodeficiency virus-infected patients taking highly active antiretroviral therapy. Antimicrob Agents Chemother 49:3361–3366. doi: 10.1128/AAC.49.8.3361-3366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baxi SM, Greenblatt RM, Bacchetti P, Scherzer R, Minkoff H, Huang Y, Anastos K, Cohen M, Gange SJ, Young M, Shlipak MG, Gandhi M. 2014. Common clinical conditions—age, low BMI, ritonavir use, mild renal impairment—affect tenofovir pharmacokinetics in a large cohort of HIV-infected women. AIDS 28:59–66. doi: 10.1097/QAD.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez-Novoa S, Labarga P, Soriano V, Egan D, Albalater M, Morello J, Cuenca L, Gonzalez-Pardo G, Khoo S, Back D, Owen A. 2009. Predictors of kidney tubular dysfunction in HIV-infected patients treated with tenofovir: a pharmacogenetic study. Clin Infect Dis 48:e108–e116. doi: 10.1086/598507. [DOI] [PubMed] [Google Scholar]
- 19.James AM, Ofotokun I, Sheth A, Acosta EP, King JR. 2012. Tenofovir: once-daily dosage in the management of HIV infection. Clin Med Insights Ther 4:201–216. doi: 10.4137/CMT.S8316. [DOI] [Google Scholar]
- 20.Kearney BP, Mathias A, Mittan A, Sayre J, Ebrahimi R, Cheng AK. 2006. Pharmacokinetics and safety of tenofovir disoproxil fumarate on coadministration with lopinavir/ritonavir. J Acquir Immune Defic Syndr 43:278–283. doi: 10.1097/01.qai.0000243103.03265.2b. [DOI] [PubMed] [Google Scholar]
- 21.Gutmann H, Fricker G, Drewe J, Toeroek M, Miller DS. 1999. Interactions of HIV protease inhibitors with ATP-dependent drug export proteins. Mol Pharmacol 56:383–389. [DOI] [PubMed] [Google Scholar]
- 22.Tong L, Phan TK, Robinson KL, Babusis D, Strab R, Bhoopathy S, Hidalgo IJ, Rhodes GR, Ray AS. 2007. Effects of human immunodeficiency virus protease inhibitors on the intestinal absorption of tenofovir disoproxil fumarate in vitro. Antimicrob Agents Chemother 51:3498–3504. doi: 10.1128/AAC.00671-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujita T, Brown C, Carlson EJ, Taylor T, Cruz M, Johns SJ, Stryke D, Kawamoto M, Fujita K, Castro R, Chen C, Lin ET, Brett CM, Burchard EG, Ferrin TE, Huang CC, Leabman MK, Giacomini KM. 2005. Functional analysis of polymorphisms in the organic anion transporter, SLC22A6 (OAT1). Pharmacogenet Genomics 15:201–209. doi: 10.1097/01213011-200504000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Nishizato Y, Ieiri I, Suzuki H, Kimura M, Kawabata K, Hirota T, Takane H, Irie S, Kusuhara H, Urasaki Y, Urae A, Higuchi S, Otsubo K, Sugiyama Y. 2003. Polymorphisms of OATP-C (SLC21A6) and OAT3 (SLC22A8) genes: consequences for pravastatin pharmacokinetics. Clin Pharmacol Ther 73:554–565. doi: 10.1016/S0009-9236(03)00060-2. [DOI] [PubMed] [Google Scholar]
- 25.Vormfelde SV, Schirmer M, Hagos Y, Toliat MR, Engelhardt S, Meineke I, Burckhardt G, Nurnnerg P, Brockmoller J. 2006. Torsemide renal clearance and genetic variation in luminal and basolateral organic anion transporters. Br J Clin Pharmacol 62:323–335. doi: 10.1111/j.1365-2125.2006.02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.German P, Liu HC, Szwarcberg J, Hepner M, Andrews J, Kearney BP, Mathias A. 2012. Effect of cobicistat on glomerular filtration rate in subjects with normal and impaired renal function. J Acquir Immune Defic Syndr 61:32–40. doi: 10.1097/QAI.0b013e3182645648. [DOI] [PubMed] [Google Scholar]