Abstract
Acanthamoeba is a protist pathogen that can cause serious human infections, including blinding keratitis and a granulomatous amoebic encephalitis that almost always results in death. The current treatment for these infections includes a mixture of drugs, and even then, a recurrence can occur. Photochemotherapy has shown promise in the treatment of Acanthamoeba infections; however, the selective targeting of pathogenic Acanthamoeba has remained a major concern. The mannose-binding protein is an important adhesin expressed on the surface membranes of pathogenic Acanthamoeba organisms. To specifically target Acanthamoeba, the overall aim of this study was to synthesize a photosensitizing compound (porphyrin) conjugated with mannose and test its efficacy in vitro. The synthesis of mannose-conjugated porphyrin was achieved by mixing benzaldehyde and pyrrole, yielding tetraphenylporphyrin. Tetraphenylporphyrin was then converted into mono-nitrophenylporphyrin by selectively nitrating the para position of the phenyl rings, as confirmed by nuclear magnetic resonance (NMR) spectroscopy. The mono-nitrophenylporphyrin was reduced to mono-aminophenylporphyrin in the presence of tin dichloride and confirmed by a peak at m/z 629. Finally, mono-aminoporphyrin was conjugated with mannose, resulting in the formation of an imine bond. Mannose-conjugated porphyrin was confirmed through spectroscopic analysis and showed that it absorbed light of wavelengths ranging from 425 to 475 nm. To determine the antiacanthamoebic effects of the derived product, amoebae were incubated with mannose-conjugated porphyrin for 1 h and washed 3 times to remove extracellular compound. Next, the amoebae were exposed to light of the appropriate wavelength for 1 h. The results revealed that mannose-conjugated porphyrin produced potent trophicidal effects and blocked excystation. In contrast, Acanthamoeba castellanii incubated with mannose alone and porphyrin alone did not exhibit an antiamoebic effect. Consistently, pretreatment with mannose-conjugated porphyrin reduced the A. castellanii-mediated host cell cytotoxicity from 97% to 4.9%. In contrast, treatment with porphyrin, mannose, or solvent alone had no protective effects on the host cells. These data suggest that mannose-conjugated porphyrin has application for the targeted photodynamic therapy of Acanthamoeba infections and may serve as a model in the development of therapeutic interventions against other eukaryotic infections.
INTRODUCTION
Pathogenic Acanthamoeba spp. are well known to produce serious infections, including fatal granulomatous amebic encephalitis (GAE) and a painful sight-threatening keratitis (reviewed in references 1 and 2). The most distressing aspect is that the mortality associated with GAE due to pathogenic Acanthamoeba has remained significant (>90%) despite the advances made in antimicrobial chemotherapy and supportive care (1, 2). Similarly, diagnosis of Acanthamoeba keratitis is currently difficult due to lack of awareness (1, 2), and the available treatments are lengthy and not fully effective against all strains (3); this is in part due to the ability of the amoebae to transform into a resistant cyst form (4), resulting in infection recurrence. A complete understanding of the pathogenesis and pathophysiology of these infections will undoubtedly lead to the development of diagnostic advances and therapeutic interventions (5, 6). Recent studies have identified a mannose-binding protein (MBP) as an important adhesin expressed on the surface membranes of Acanthamoeba spp., as free exogenous mannose blocks the adhesion of the parasites to primary human brain microvascular endothelial cells and corneal epithelial cells (7, 8). Notably, oral immunization with recombinant mannose-binding protein ameliorates Acanthamoeba keratitis in the Chinese hamster model (9). The Acanthamoeba mbp gene contains 6 exons and 5 introns that span 3.6 kbp. The 2.5-kbp cDNA codes for an 833-amino-acid precursor protein with a signal sequence (amino acid residues 1 to 21), an N-terminal extracellular domain (amino acid residues 22 to 733) with five N- and three O-glycosylation sites, a transmembrane domain (amino acid residues 734 to 755), and a C-terminal intracellular domain (amino acid residues 756 to 833) (7).
Photochemotherapy is a promising technology that can target pathogens and thus has the potential for use in therapeutic interventions. The basis of this technology is that visible light (or light of an appropriate wavelength) activates the photosensitizing compound, resulting in the production of singlet oxygen and other reactive oxygen species (ROS) that induce cell death in the target pathogen/tissue (10, 11). However, it is possible that the photosensitizing compound may bind to host cells, and subsequent irradiation thus might lead to their destruction. In this context, the selective targeting of pathogenic Acanthamoeba has remained a major concern (12). To specifically target Acanthamoeba, the overall aim of this study was to synthesize the photosensitizing compound (porphyrin) conjugated with mannose and test its efficacy in vitro.
MATERIALS AND METHODS
Acanthamoeba castellanii cultures.
All chemicals were purchased from Sigma Laboratories (Poole, Dorset, England), unless otherwise stated. A clinical isolate of A. castellanii belonging to the T4 genotype was obtained from the American Type Culture Collection (strain ATCC 50492). Amoebae were routinely grown in 10 ml of PYG medium (0.75% [wt/vol] proteose peptone, 0.75% [wt/vol] yeast extract, and 1.5% [wt/vol] glucose) in a T-75-cm2 tissue culture flask at 37°C, as previously described (12). The medium was refreshed 15 to 20 h prior to the experiments. The amoebae that adhered to the flask represented the trophozoite form and were collected by placing the flask on ice for 20 min with gentle agitation; these were used in all subsequent experiments.
Human brain microvascular endothelial cell cultures.
The primary brain microvascular endothelial cells were of human origin and were isolated and cultured as previously described (8, 13). The endothelial cells were purified by fluorescence-activated cell sorting and their purities tested using endothelial markers, such as the expression of factor VIII (F-VIII), carbonic anhydrase IV, and the uptake of acetylated low-density lipoprotein (DiI-AcLDL) (8, 13), resulting in >99% pure endothelial cultures. The human brain microvascular endothelial cells (BMEC) were routinely grown in rat tail collagen-coated tissue culture dishes in RPMI 1640 containing 10% heat-inactivated fetal bovine serum, 10% Nu serum, 2 mM glutamine, 1 mM Na pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, nonessential amino acids, and vitamins and incubated at 37°C in a 5% CO2 incubator. For the cytotoxicity assays, human BMEC were grown in 24-well plates by inoculating 106 cells/ml/well. At this cell density, confluent monolayers were formed within 24 h and used in the experiments.
Synthesis of mannose-conjugated porphyrin.
Mannose-conjugated porphyrin was synthesized as follows: initially, 40 ml of propionic acid was heated at 90°C. A solution was prepared by mixing 1.9 ml of 18.85 mM benzaldehyde in 2.5 ml of propionic acid. Benzaldehyde was added very slowly in hot propionic acid. After continuous heating of the solution at reflux temperature, 1.3 ml of 18.85 mM pyrrole dissolved in 2.5 ml of propionic acid was added to the abovementioned mixture in a dropwise manner for 30 min. The reaction was refluxed and monitored by thin-layer chromatography (TLC) (14). After completion (about 1.5 h), the reaction mixture was cooled, and crude tetraphenylporphyrin in ethyl acetate (50 ml used three times) was extracted from 50 ml of a 2.5 M NaOH aqueous solution. The solvent was evaporated, and the solid product was passed from a silica column (ethyl acetate to hexane in a 1:9 ratio) to obtain the pure product in a 7% yield, as described previously (14). Next, 160 mg of 0.26 mM tetraphenylporphyrin was nitrated using 90 mg of 1.3 mM sodium nitrite (NaNO2) in 10 ml of trifluoroacetic acid (TFA), and the reaction mixture was stirred for 1 h. By controlling the amount of TFA and the reaction time, mono-nitrated phenylporphyrin was obtained as a major product, along with di-nitrophenylporphyrin. The reaction mixture was quenched with an excess of water and extracted in dichloromethane (DCM) (25 ml used three times). Next, it was evaporated in a vacuum, and mono-nitrated phenylporphyrin was isolated by using column chromatography (DCM to hexane in ratios of 3:1 to 1:1) to obtain a 30% yield. After this, 100 mg of 0.15 mM mono-nitrophenylporphyrin, together with 100 mg of 0.44 mM tin dichloride dihydrate, was added in 0.5 ml of concentrated HCl and heated at 60°C for 2.5 h. The progress of the reaction was monitored by TLC. After completion, ammonium hydroxide was added to neutralize the reaction mixture and was extracted with DCM (25 ml used three times) and purified with column chromatography (DCM to hexane in ratios of 1:3 to 3:1) to obtain mono-aminophenylporphyrin at a 53% yield. Finally, 15 mg of 0.079 mM mannose along with 50 mg of 0.079 mM mono-aminophenylporphyrin was dissolved in 8 ml of ethanol. Three drops of glacial acetic acid were added, and the reaction mixture was refluxed overnight. The precipitates that formed were filtered and washed with DCM and methanol separately, and the purity was confirmed by spectroscopic analysis. The residue was dissolved in dimethyl sulfoxide (DMSO) and was subjected to nuclear magnetic resonance (NMR) to determine the product purity. The synthesized product absorbed photons of 450 nm, which is within the range of blue light.
Amoebicidal assays.
To determine the killing effects of mannose-conjugated porphyrin, amoebicidal assays were performed, as described previously but with slight modifications (12). Briefly, A. castellanii was incubated with 10 μM (4-μl volume) and 50 μM (20-μl volume) concentrations of mannose-conjugated porphyrin, as well as porphyrin and mannose alone in RPMI 1640 (106 amoebae/ml/well) in 24-well plates for 1 h. The amoebae were collected by centrifugation at 1,000 × g for 5 min. The supernatant was aspirated and the pellet resuspended in 0.5 ml of RPMI 1640. This process was repeated 3 times to remove any residual mannose-conjugated porphyrin. Finally, the amoeba pellet was resuspended in PYG. Next, the lids were removed, and the plates were exposed to blue light for 1 h in a biosafety hood at room temperature using a blue round light-emitting diode (LED), which is lead-free and at 20 mA emits a minimum wavelength of 465 nm and maximum wavelength of 480 nm; the typical dominant wavelength is 470 nm at a pulse width of ≤0.1 ms, achieved by placing the 5-mm LED approximately 15 cm above the 24-well plate (Cree, Inc., Durham, NC, USA). Following this, the plates were incubated for 24 h at 37°C. The next day, the amoeba counts were determined using a hemocytometer, and images were obtained using a phase-contrast inverted microscope. For the solvent controls, amoebae were incubated in the same amounts of DMSO (4-μl and 20-μl volumes), 50% chloroform–50% methanol (1.34-μl and 6.7-μl volumes), and RPMI 1640 alone. For the positive controls, the amoebae were incubated with an antiacanthamoebic drug, chlorhexidine (at 20 μM in RPMI 1640 as a solvent), which exhibited 100% killing.
Host cell cytotoxicity assays.
To determine the antiamoebic effects of mannose-conjugated porphyrin in vitro, the assays were performed using human BMEC. A. castellanii was pretreated with mannose-conjugated porphyrin and exposed to light, as described above. Next, the amoebae were collected by centrifugation at 1,000 × g for 5 min. The supernatant was aspirated and the pellet resuspended in 0.5 ml of RPMI 1640. This process was repeated 3 times to remove any residual mannose-conjugated porphyrin. Finally, the amoebae were inoculated on human BMEC monolayers, grown in 24-well plates. The plates were incubated at 37°C in a 5% CO2 incubator. The next day, cell-free conditioned medium was collected, and host cell cytotoxicity was determined by measuring lactate dehydrogenase (LDH) release using the Cytotoxicity detection kit (Roche, Indianapolis, IN). The principle of this assay is that cell supernatant containing LDH catalyzes the conversion of lactate (solution from kit) to pyruvate, generating NADH and H+. In the second step, the catalyst (diaphorase, in a solution from the kit) transfers H and H+ from NADH and H+ to the tetrazolium salt p-iodonitrotetrazolium violet (INT), which is reduced to formazan (dye), and the absorbance at 492 nm is read. The human BMEC incubated alone were used as negative controls, whereas the monolayers lysed with 1% Triton X-100 for 30 min at 37°C were used as 100% cell death (high value). The absorbance of a sample was converted to percent cytotoxicity as follows: (sample value − negative value)/(high value − negative value) × 100 = % cytotoxicity.
In addition to the amoeba pretreatment, the antiamoebic effects of mannose-conjugated porphyrin were determined by coincubating A. castellanii, human BMEC, and mannose-conjugated porphyrin and exposing them to light as described above. Next, the plates were incubated at 37°C in a 5% CO2 incubator for 24 h, and cell-free conditioned medium was collected and the host cell cytotoxicity determined by measuring the LDH release.
Excystation assays.
For the excystation assays, A. castellanii cysts were prepared using nonnutrient agar plates (3% Oxoid agar technical using distilled water), as previously described (12). A. castellanii trophozoites (2 × 106 amoebae) were inoculated on nonnutrient agar plates and incubated at 37°C for up to 14 days to allow trophozoite differentiation into cysts. Following this incubation, no trophozoites were seen when observed under a phase-contrast inverted microscope. Next, the agar plates were flooded with 15 ml of phosphate-buffered saline (PBS) and placed on a shaker for 30 min at room temperature. After this incubation, the cysts were gently scraped off the agar surface using a rubber scraper. The cysts were collected in a 50-ml tube, followed by centrifugation at 1,500 × g for 10 min. The supernatant was discarded and the pellet resuspended in 10 ml of PBS. This process was repeated 3 times to wash the A. castellanii cysts, which were counted using a hemocytometer. To determine the effects of mannose-conjugated porphyrin on A. castellanii cysts, the excystation assays were performed as described previously (15). Briefly, A. castellanii cysts (5 × 105 cysts/ml/well) were incubated with 10 μM and 50 μM mannose-conjugated porphyrin, as well as porphyrin and mannose alone for 1 h. The amoebae were collected, washed 3 times with PBS, and resuspended in RPMI 1640 in 24-well plates. Next, the lids were removed, and the plates were exposed to blue light for 1 h, as described above. Finally, the cysts were collected and resuspended in growth medium (0.5 ml of PYG medium/well) in 24-well plates. The plates were incubated at 37°C for 72 h, and the emergence of actively growing trophozoites was observed microscopically. The trophozoite counts were determined using a hemocytometer.
RESULTS
Synthesis of mannose-conjugated porphyrin confirmed using NMR spectroscopy.
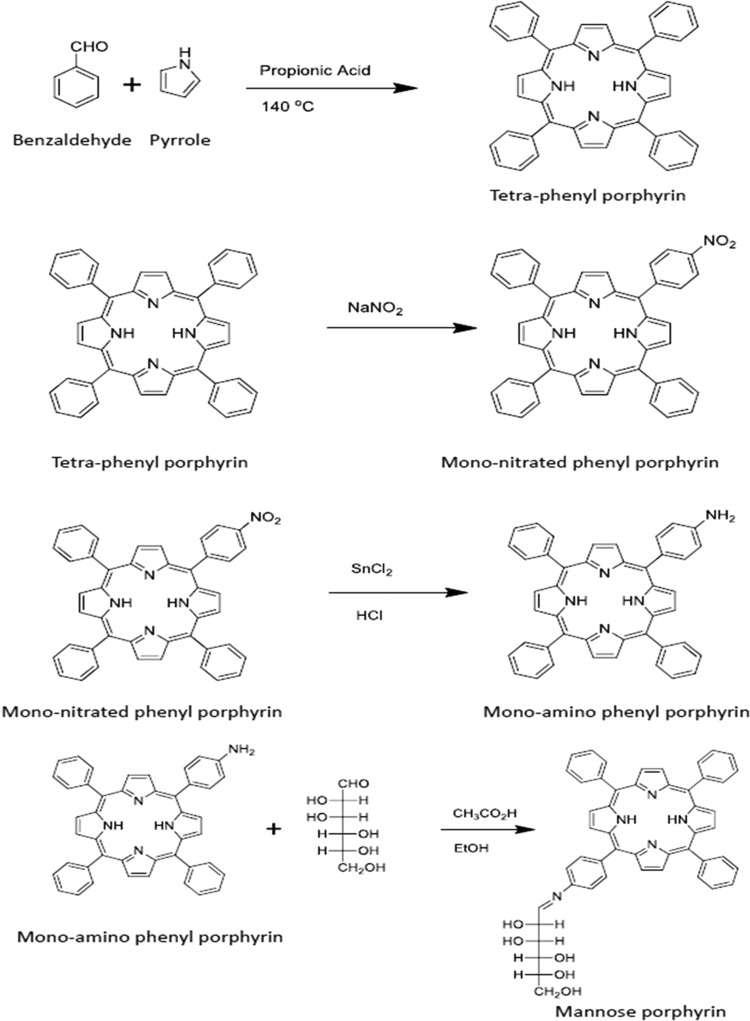
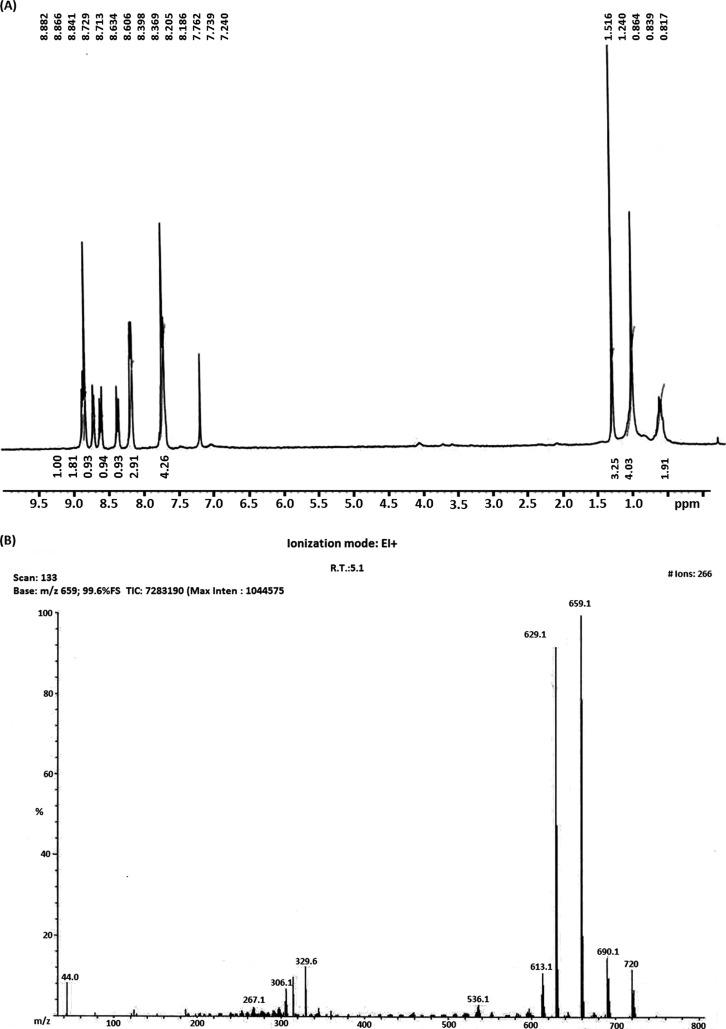
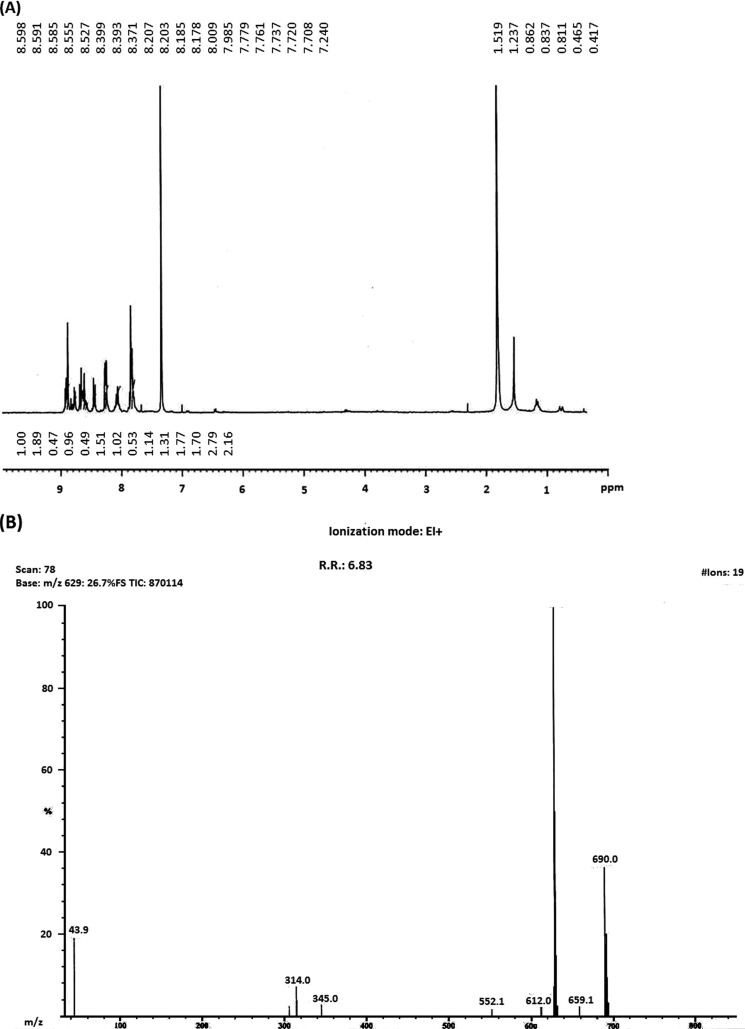
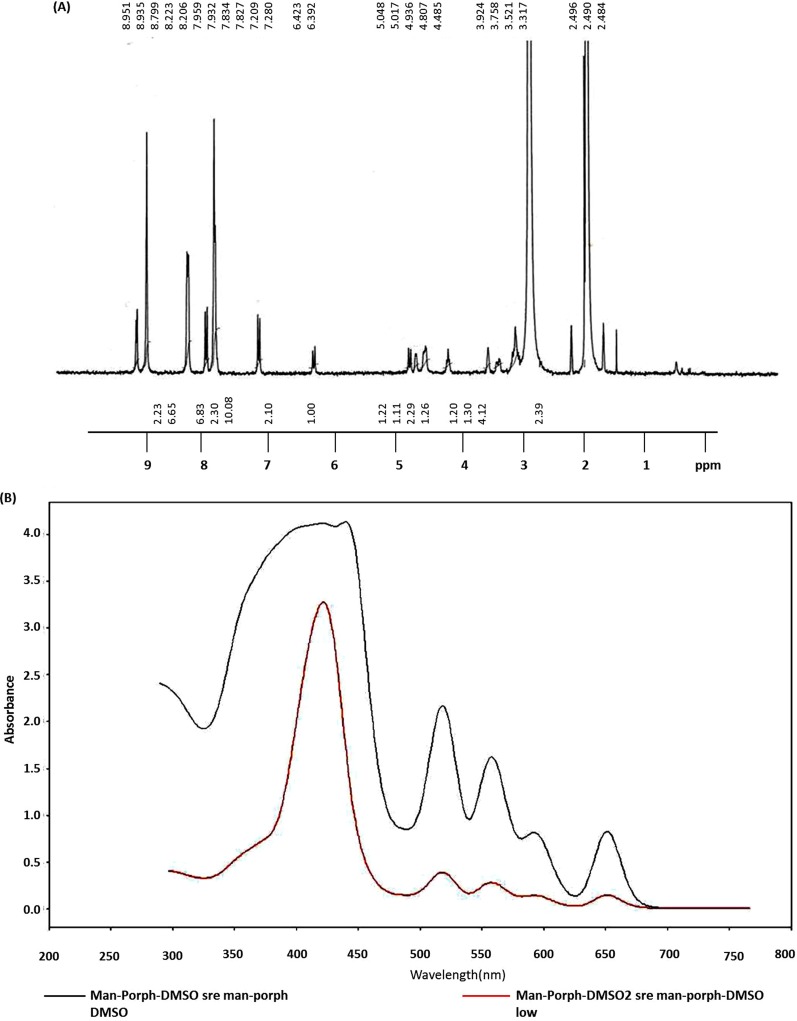
The synthesis of mannose-conjugated porphyrin was achieved in four linear steps (Fig. 1). Initially, tetraphenylporphyrin was obtained by mixing benzaldehyde and pyrrole in the presence of propionic acid. Tetraphenylporphyrin was then converted into mono-nitrophenylporphyrin by selectively nitrating the para position of the phenyl rings. As a result, a mixture of mono-nitrophenylporphyrin and di-nitrophenylporphyrin was obtained, which was separated by column chromatography. By controlling the equivalents of nitrating mixture, the mono-nitro product was obtained at a higher yield and checked using NMR spectroscopy (Fig. 2). Next, mono-aminophenylporphyrin was obtained by reducing mono-nitrophenylporphyrin in the presence of tin dichloride in hydrochloric acid. The reaction yielded mono-aminoporphyrin that resulted in a slight shifting of signals in the NMR spectrum (Fig. 3A); however, the mass spectra of the product revealed the disappearance of a mono-nitrophenylporphyrin mass peak, and the formation of the desired mono-aminoporphyrin was confirmed by a peak at m/z 629 (Fig. 3B). Finally, mannose-conjugated porphyrin was obtained by conjugating mono-aminoporphyrin with mannose, resulting in the formation of an imine bond. Mannose-conjugated porphyrin was obtained as a solid product, which was then washed with dichloromethane and methanol several times to remove excess starting materials and confirmed through spectroscopic analysis. The purity of mannose-conjugated porphyrin was 75%, as determined by NMR analysis (Fig. 4A). The spectrophotometric analysis of the obtained product showed that it absorbed light at wavelengths ranging from 425 to 475 nm (Fig. 4B).
FIG 1.
Steps involved in the synthesis of mannose porphyrin, as described in Materials and Methods. Briefly, benzaldehyde and pyrrole were mixed in propionic acid to yield tetraphenylporphyrin. It was then nitrated in the presence of sodium nitrite, and mono-nitrated phenylporphyrin was formed. Mono-nitrated phenylporphyrin, when neutralized in the presence of ammonium hydroxide, yielded mono-aminophenylporphyrin. Finally, the product was mixed with mannose and yielded mannose-conjugated porphyrin. The purity of intermediate compound synthesized at each step was checked using NMR and mass spectrometry. For mannose-conjugated porphyrin, mass spectrometry was not performed, due to its high polarity.
FIG 2.
(A) Results of NMR spectroscopy performed after the synthesis of mono-nitrated phenylporphyrin, as described in Fig. 1. (B) Results of mass spectrometry performed after the synthesis of mono-nitrated phenylporphyrin. FS, full scan; TIC, total ion chromatogram; R.T., retention time; EI, electron ionization.
FIG 3.
(A) Results of NMR spectroscopy performed after the synthesis of mono-aminophenylporphyrin, as described in Fig. 1. (B) Results of mass spectrometry performed after the synthesis of mono-aminophenylporphyrin.
FIG 4.
(A) Results of NMR spectroscopy performed after the synthesis of mannose-conjugated porphyrin (man-porph), as described in Fig. 1. Mass spectrometry of mannose-conjugated porphyrin was not performed due to its high polarity. (B) UV-visible absorption spectrum of mannose-conjugated porphyrin showed that it absorbed light of wavelengths ranging from 425 to 450 nm.
Mannose-conjugated porphyrin inhibited viability of A. castellanii.
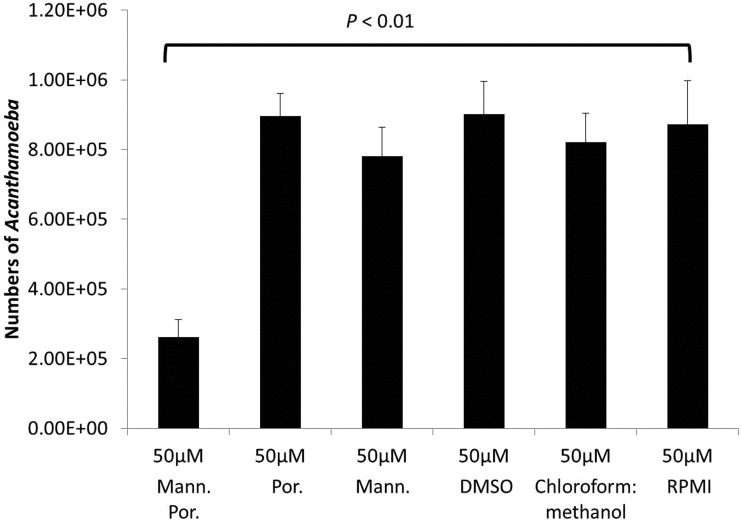
To determine the effects of mannose-conjugated porphyrin on A. castellanii trophozoites, amoebae were incubated with 10 μM and 50 μM mannose-conjugated porphyrin, and the amoebicidal effects were determined. The results revealed that 50 μM mannose-conjugated porphyrin exhibited significant amoebicidal effects (P < 0.01 using a 2-sample t test with a two-tailed distribution) (Fig. 5). Additionally, amoebicidal effects were confirmed by observation under a microscope (Fig. 6). In contrast, A. castellanii incubated with 50 μM mannose alone and porphyrin alone did not exhibit any killing effects. Also, solvent controls, such as RPMI 1640, DMSO, and 50% chloroform–50% methanol had no effect on the viability of A. castellanii (Fig. 5 and 6).
FIG 5.
Mannose-conjugated porphyrin (mann. por.) exhibited amoebicidal effects against A. castellanii. Briefly, amoebae (106) were incubated with and without 10 μM and 50 μM mannose-conjugated porphyrin and exposed to blue light, as described in Materials and Methods. Following this, the plates were incubated for 24 h, and the amoebae were enumerated using a hemocytometer. The treatment of A. castellanii with mannose-conjugated porphyrin exhibited significant amoebicidal effects (P < 0.01, using a 2-sample t test with a two-tailed distribution) compared with amoebae incubated alone. In contrast, the controls did not show any effect. The data are presented as the means ± standard errors from at least three independent experiments performed in duplicate.
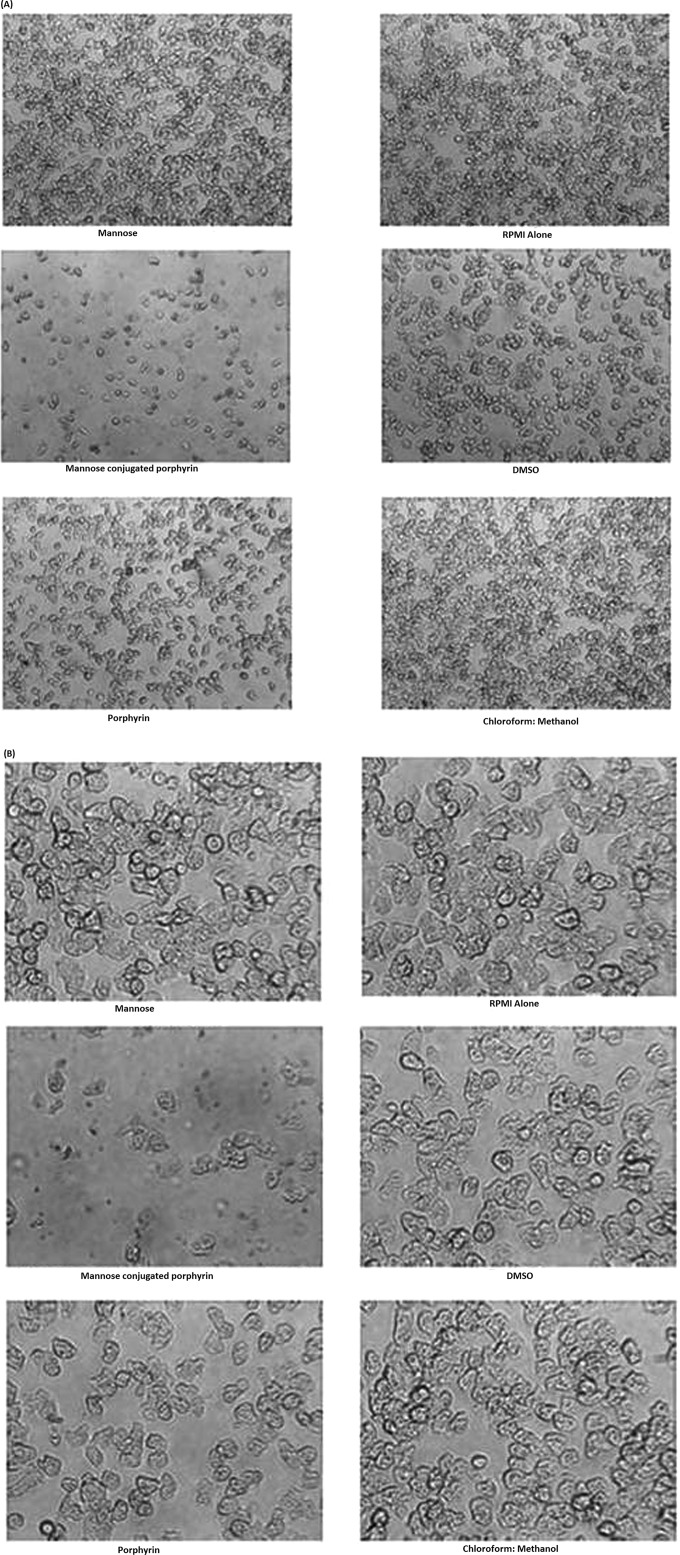
FIG 6.
A. castellanii was incubated with and without 50 μM mannose-conjugated porphyrin, and representative images were obtained at 100× (A) and at 400× (B) magnification using a phase-contrast inverted microscope.
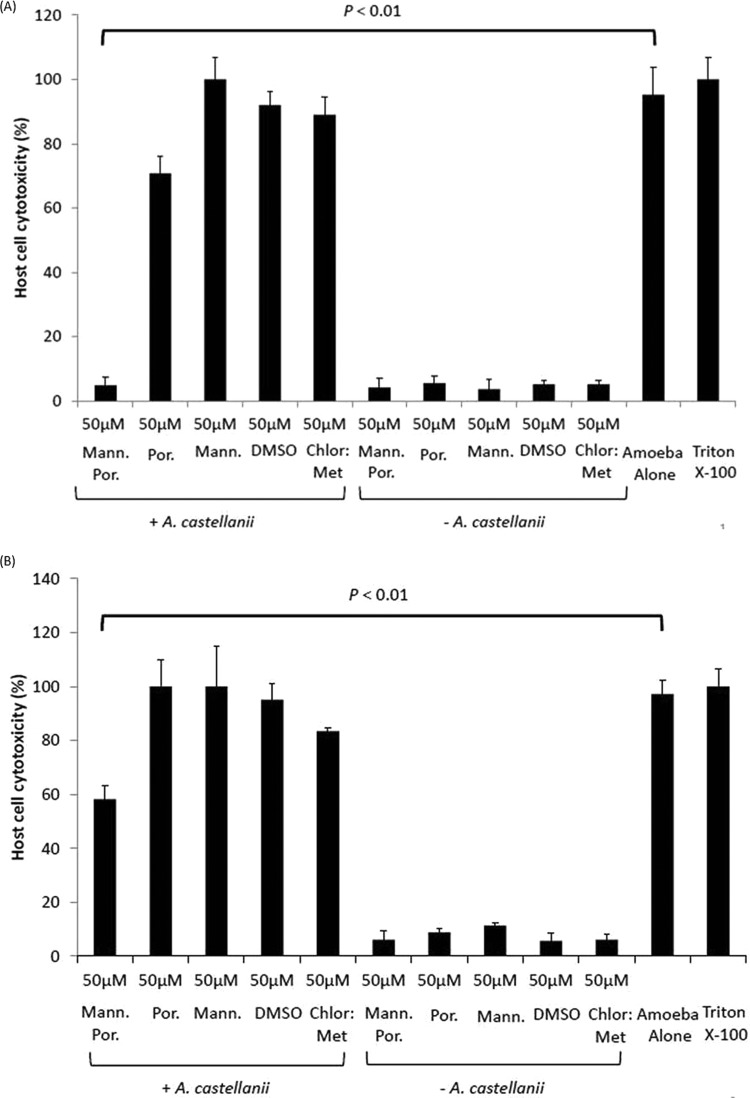
Mannose-conjugated porphyrin inhibited A. castellanii-mediated host cell cytotoxicity.
A. castellanii was pretreated with mannose-conjugated porphyrin, which was followed by the performance of host cell cytotoxicity assays. Without mannose-conjugated porphyrin, A. castellanii produced 95% ± 8.5% BMEC deaths within 24 h. The pretreatment of A. castellanii with mannose-conjugated porphyrin reduced host cell cytotoxicity significantly to 4.9% ± 2.5% (P < 0.01, using a 2-sample t test with a two-tailed distribution) compared to that with amoebae alone (Fig. 7A). In contrast, the incubation of A. castellanii with porphyrin, mannose, or solvent alone had no protective effects on the host cells (Fig. 7A). Similarly, solvent alone had no effect on BMEC cytotoxicity (Fig. 7A).
FIG 7.
(A) A. castellanii (5 × 105) preincubated with and without 50 μM mannose-conjugated porphyrin (mann. por.) and exposed to light; the cytotoxic potential was determined using human brain microvascular endothelial cells (BMEC), as described in Materials and Methods. When pretreated with mannose-conjugated porphyrin, A. castellanii showed limited host cell cytotoxicity (P < 0.01, using a 2-sample t test with two-tailed distribution) compared with that with nontreated amoebae. A. castellanii incubated with porphyrin, mannose, DMSO, and chloroform-methanol (chlor:met) also induced host cell cytotoxicity. However, the compounds and corresponding solvents alone had no effect on human BMEC cytotoxicity. The data are presented as the means ± standard errors from at least three independent experiments performed in duplicate. (B) A. castellanii (5 × 105) was coincubated with human BMEC and 50 μM mannose-conjugated porphyrin and exposed to light, as described in Materials and Methods. The coincubation of 50 μM mannose-conjugated porphyrin with amoebae plus human BMEC reduced cell cytotoxicity, albeit at lower levels than those with the pretreatment assays. The results represent the means ± standard errors from three independent experiments performed in duplicate.
In the coincubation assays, A. castellanii plus human BMEC plus 50 μM mannose-conjugated porphyrin also reduced host cell cytotoxicity, but to a lesser extent, at 58% ± 4% (Fig. 7B) (P < 0.01, using a 2-sample t test with a two-tailed distribution). In the absence of mannose-conjugated porphyrin, A. castellanii produced 97% ± 5% host cell cytotoxicity. Similarly, other compounds and solvents alone did not show any effect on A. castellanii-mediated host cell cytotoxicity (Fig. 7B).
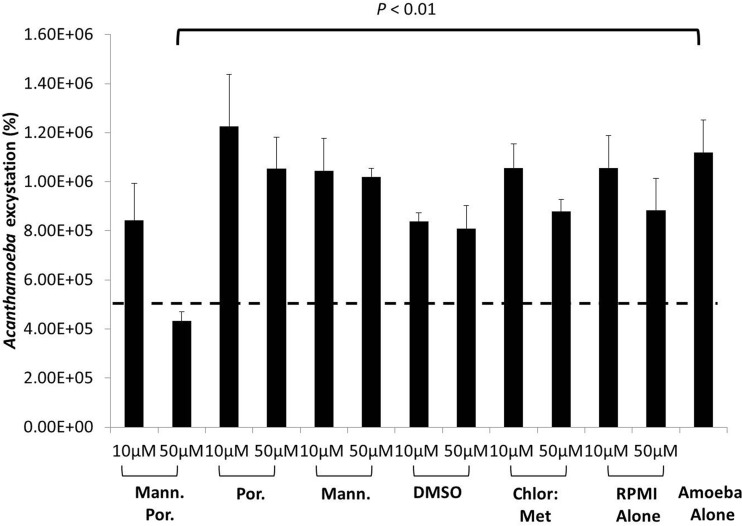
Mannose-conjugated porphyrin blocked excystation in A. castellanii.
The cysts were pretreated with and without mannose-conjugated porphyrin and inoculated in fresh growth medium. The findings revealed that A. castellanii cysts incubated with PYG alone emerged as viable trophozoites (1.2 × 106 amoebae) (Fig. 8). However, 50 μM mannose-conjugated porphyrin significantly inhibited A. castellanii excystation within 72 h (P < 0.01, using a 2-sample t test with a two-tailed distribution) compared with that with cysts inoculated in PYG alone. In contrast, viable trophozoites emerged in the wells containing A. castellanii cysts treated with mannose alone, porphyrin alone, and solvents alone, such as DMSO and 50% chloroform–50% methanol, within 72 h (Fig. 8).
FIG 8.
Mannose-conjugated porphyrin inhibited excystation of A. castellanii cysts. A. castellanii cysts were scraped from nonnutrient agar plates, incubated with 50 μM mannose porphyrin, and exposed to light, as described in Materials and Methods. Following this incubation, the cysts were incubated in fresh growth medium, PYG, for up to 72 h. After this incubation, A. castellanii trophozoites were enumerated using a hemocytometer. The dashed line indicates the original inoculum. When treated with 50 μM mannose-conjugated porphyrin, A. castellanii cysts did not reemerge as actively growing viable trophozoites. Viable A. castellanii trophozoites emerged in the control wells, as well as with all other compounds and solvents tested. The results are presented as the means ± standard errors from at least three independent experiments performed in duplicate.
DISCUSSION
The successful treatment of Acanthamoeba infections is challenging. For example, the current treatment of Acanthamoeba keratitis is problematic and involves hourly topical applications of a mixture of drugs, including chlorhexidine, polyhexamethylene biguanide, propamidine isethionate, and neomycin, for up to a year; even then, recurrence can occur (3). An intriguing report made by Ficker et al. (16) showed the development of propamidine resistance during the course of therapy for Acanthamoeba keratitis, which led to a recurrence of the infection. Of concern, several studies have shown the resistance of Acanthamoeba to antimicrobial chemotherapy (2, 4, 5). This is of particular concern in the absence of available alternative chemotherapeutic agents. Our recent studies have shown the potential of photochemotherapy, namely, the application of photodynamic compounds, followed by exposure to a suitable source of UV-visible (Vis) radiation (17); however, the selective targeting of Acanthamoeba has remained a major concern. By synthesizing a photoactivated compound, porphyrin, and its examining conjugation with mannose to selectively target Acanthamoeba, the potential of photochemotherapy for use against Acanthamoeba infections was investigated in the present study. The results showed that mannose-conjugated porphyrin exhibited potent amoebicidal effects and blocked excystation in vitro. The basis of this technology involves the activation of a photosensitizing molecule by exposure to visible light (of a certain wavelength). This in turn releases the singlet oxygen or reactive oxygen species that causes cell death (10, 11, 16). However, a major challenge in the use of photochemotherapeutic compounds in treating infections caused by eukaryotic pathogens is the specific delivery of the photoactivated agent to the pathogen. Previous studies have shown that pathogenic Acanthamoeba specifically binds to mannose but not to other sugars, and these studies have identified MBP as an important adhesin expressed on the surface membranes of Acanthamoeba (7–9). In the present study, a mannose sugar was attached to a porphyrin ring to synthesize mannose-conjugated porphyrin for use in the targeted delivery of this photoactivated compound to Acanthamoeba, which proved highly effective in killing the parasite and both exhibited trophicidal effects and abolished excystation against A. castellanii in vitro.
Notably, the pretreatment and cotreatment of A. castellanii with mannose-conjugated porphyrin showed varied host cell cytotoxicities, at 4% versus 58%, respectively. In contrast, no treatment resulted in >95% host cell cytotoxicity. A likely explanation of the higher cell cytotoxicity in the cotreatment assays is the production of reactive oxygen species produced by the photosensitizing compound in the presence of human cells that may have contributed to increased host cell cytotoxicity. It is also interesting to note that mannose-conjugated porphyrin blocked excystation. At 50 μM mannose-conjugated porphyrin, a complete inhibition of excystation was observed. This was unexpected, as previous studies showed that cysts do not exhibit adhesion to the host cells (18), and it was presumed that MBP is not expressed on the external cell wall membranes; however, the expression of MBP seems to occur in Acanthamoeba cysts, and if this is so, its precise localization has not been investigated. The inhibition of excystation as observed in this study might be due to lipid-soluble material or prolonged exposure to the photoactivated compound (19, 20), affecting trophozoite emergence from the dormant cyst stage. However, the underlying molecular mechanisms of the accumulation of photodynamic compound at the cyst wall and its possible traversal across the cell wall and/or through the operculum membranes to target trophozoites residing inside the shell remain to be investigated, as well as its efficacy in vivo; these will be the subjects of further studies. Future studies are also needed to conjugate porphyrin with Acanthamoeba-specific antibody and to test its potential for enhanced targeted therapy against Acanthamoeba infections. Additionally, there is a need to test the possibility of using various photosensitive molecules with high-intensity light but at a shorter duration (pulsed interval treatments), which may prove to be effective and more feasible for clinical application. If successful, this technology will be particularly useful in treating Acanthamoeba keratitis cases due to the ease of visible light usage and the fact that the current mode of visual inspection is the exposure to visible light; this can also be used to treat disseminated/tissue infections. For the disseminated/tissue infections, several lines of evidence suggest that photodynamic therapy has proven efficacy against a range of malignant and other diseased cells and tissues (21–26). With the targeted killing of pathogenic Acanthamoeba, as proposed in this study, photodynamic therapy can be recognized as a treatment strategy that is both minimally invasive and minimally toxic, and it should be investigated further in vivo.
ACKNOWLEDGMENTS
This work was funded by Aga Khan University, Karachi, as well as the Higher Education Commission, Pakistan.
We declare no conflicts of interest.
REFERENCES
- 1.Marciano-Cabral F, Cabral G. 2003. Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev 16:273–307. doi: 10.1128/CMR.16.2.273-307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Visvesvara GS, Moura H, Schuster FL. 2007. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol 50:1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 3.Pérez-Santonja JJ, Kilvington S, Hughes R, Tufail A, Metheson M, Dart JKG. 2003. Persistently culture positive Acanthamoeba keratitis; in vivo resistance and in vitro sensitivity. Ophthalmology 110:1593–1600. doi: 10.1016/S0161-6420(03)00481-0. [DOI] [PubMed] [Google Scholar]
- 4.Turner NA, Russell AD, Furr JR, Lloyd D. 2004. Resistance, biguanide sorption and biguanide-induced pentose leakage during encystment of Acanthamoeba castellanii. J Appl Microbiol 96:1287–1295. doi: 10.1111/j.1365-2672.2004.02260.x. [DOI] [PubMed] [Google Scholar]
- 5.Clarke DW, Niederkorn JY. 2006. The pathophysiology of Acanthamoeba keratitis. Trends Parasitol 22:175–180. doi: 10.1016/j.pt.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Panjwani N. 2010. Pathogenesis of Acanthamoeba keratitis. Ocul Surf 8:70–79. doi: 10.1016/S1542-0124(12)70071-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garate M, Cao Z, Bateman E, Panjwani N. 2004. Cloning and characterization of a novel mannose-binding protein of Acanthamoeba. J Biol Chem 279:29849–29856. doi: 10.1074/jbc.M402334200. [DOI] [PubMed] [Google Scholar]
- 8.Alsam S, Kim KS, Stins M, Rivas AO, Sissons J, Khan NA. 2003. Acanthamoeba interactions with human brain microvascular endothelial cells. Microb Pathog 35:235–241. doi: 10.1016/j.micpath.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Garate M, Cubillos I, Marchant J, Panjwani N. 2005. Biochemical characterization and functional studies of Acanthamoeba mannose-binding protein. Infect Immun 73:5775–5781. doi: 10.1128/IAI.73.9.5775-5781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamblin MR, Hasan T. 2004. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci 3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang L, Dai T, Hamblin MR. 2010. Antimicrobial photodynamic inactivation and photodynamic therapy for infections. Methods Mol Biol 635:155–173. doi: 10.1007/978-1-60761-697-9_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baig AM, Iqbal J, Khan NA. 2013. In vitro efficacy of clinically available drugs against growth and viability of Acanthamoeba castellanii keratitis isolate belonging to the T4 genotype. Antimicrob Agents Chemother 57:3561–3567. doi: 10.1128/AAC.00299-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stins MF, Gilles F, Kim KS. 1997. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J Neuroimmunol 76:81–90. doi: 10.1016/S0165-5728(97)00036-2. [DOI] [PubMed] [Google Scholar]
- 14.Adler AD, Longo FR, Finarelli JD, Goldmacher J, Assour J, Korsakoff L. 1967. A simplified synthesis for meso-tetraphenylporphine. J Org Chem 32:476. doi: 10.1021/jo01288a053. [DOI] [Google Scholar]
- 15.Lakhundi S, Khan NA, Siddiqui R. 2014. Inefficacy of marketed contact lens disinfection solutions against keratitis-causing Acanthamoeba castellanii belonging to the T4 genotype. Exp Parasitol 141:122–128. doi: 10.1016/j.exppara.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Ficker L, Seal D, Warhurst D, Wright P. 1990. Acanthamoeba keratitis–resistance to medical therapy. Eye (Lond) 4:835–838. doi: 10.1038/eye.1990.132. [DOI] [PubMed] [Google Scholar]
- 17.Siddiqui R, Khan NA. 2012. Photochemotherapeutic strategies against Acanthamoeba keratitis. AMB Express 2:47. doi: 10.1186/2191-0855-2-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudley R, Matin A, Alsam S, Sissons J, Mahsood AH, Khan NA. 2005. Acanthamoeba isolates belonging to T1, T2, T3, T4 but not T7 encyst in response to increased osmolarity and cysts do not bind to human corneal epithelial cells. Acta Trop 95:100–108. doi: 10.1016/j.actatropica.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z, Xuguang S, Zhiqun W, Ran L. 2008. In vitro amoebacidal [sic] activity of photodynamic therapy on Acanthamoeba. Br J Ophthalmol 92:1283–1286. doi: 10.1136/bjo.2007.134288. [DOI] [PubMed] [Google Scholar]
- 20.Ferro S, Coppellotti O, Roncucci G, Ben Amor T, Jori G. 2006. Photosensitized inactivation of Acanthamoeba palestinensis in the cystic stage. J Appl Microbiol 101:206–212. doi: 10.1111/j.1365-2672.2006.02893.x. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Keltner L, Christophersen J, Zheng F, Krouse M, Singhal A, Wang SS. 2002. New technology for deep light distribution in tissue for phototherapy. Cancer J 8:154–163. doi: 10.1097/00130404-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Park S. 2007. Delivery of photosensitizers for photodynamic therapy. Korean J Gastroenterol 49:300–313. (In Korean.) [PubMed] [Google Scholar]
- 23.Selbo PK, Høgset A, Prasmickaite L, Berg K. 2002. Photochemical internalisation: a novel drug delivery system. Tumour Biol 23:103–112. doi: 10.1159/000059713. [DOI] [PubMed] [Google Scholar]
- 24.Silva JN, Filipe P, Morliere P, Maziere JC, Freitas JP, Cirne de Castro JL, Santus R. 2006. Photodynamic therapies: principles and present medical applications. Biomed Mater Eng 16(4 Suppl):S147–S154. [PubMed] [Google Scholar]
- 25.Chen B, Pogue BW, Hoopes PJ, Hasan T. 2006. Vascular and cellular targeting for photodynamic therapy. Crit Rev Eukaryot Gene Expr 16:279–305. doi: 10.1615/CritRevEukarGeneExpr.v16.i4.10. [DOI] [PubMed] [Google Scholar]
- 26.Krammer B. 2001. Vascular effects of photodynamic therapy. Anticancer Res 21:4271–4277. [PubMed] [Google Scholar]