Abstract
Ceftolozane is a new cephalosporin with activity against Gram-negative and Gram-positive microorganisms. However, the compound is susceptible to degradation by extended-spectrum beta-lactamases (ESBLs). Tazobactam is an ESBL inhibitor and is combined with ceftolozane to broaden its activity. Surprisingly, although tazobactam has been available for over 20 years, few if any reliable data exist on the tazobactam pharmacokinetic (PK) properties in mice. To evaluate the PK and pharmacodynamic (PD) relationships in mice, the PK properties of tazobactam and ceftolozane were extensively investigated. Thigh-infected neutropenic CD-1 mice were injected intraperitoneally with a single 0.1-ml dose containing ceftolozane, tazobactam, or both compounds. Ceftolozane was applied in 2-fold-increasing doses of 4 mg/kg of body weight to 64 mg/kg alone or in combination. Tazobactam was combined in reverse doses (thus, 64/4 mg/kg, 32/8 mg/kg, etc.) (n = 2 per time point). In separate validation experiments, ceftolozane-tazobactam was given alone or in combination at 32/8 mg/kg and 8/32 mg/kg (n = 4 per time point). Plasma samples (one per mouse) and bronchoalveolar lavage samples were collected at up to 12 time points until 6 h after administration. There were no significant differences in the ceftolozane and tazobactam PK alone versus combined, indicating no PK interaction. The PKs were linear and dose proportional for both compounds and showed a good penetration in the epithelial lining fluid. The estimated mean (standard deviation) half-life of ceftolozane was 0.287 h (0.031 h), and that of tazobactam was 0.176 h (0.026), and the V was 0.43 liter/kg and 1.14 liter/kg, respectively. The estimates of tazobactam parameters can also be used to (re)interpret PD data.
INTRODUCTION
Ceftolozane is a novel cephalosporin with activity against Pseudomonas aeruginosa and other Gram-negative microorganisms. However, the compound is susceptible to degradation by extended-spectrum beta-lactamases (ESBLs); therefore, the compound is combined with tazobactam, a potent beta-lactamase inhibitor, to broaden its activity. The combination had been shown to be active in vitro against a wide variety of microorganisms with different resistance mechanisms (1–5). The combination has also been shown to be active in vivo, in particular in animal models, against both P. aeruginosa and Enterobacteriaceae (6, 7). However, the pharmacodynamics of tazobactam in vivo have not been fully identified, despite being available for over 20 years in the combination piperacillin-tazobactam. Indeed few, if any, reliable data exist on the tazobactam pharmacokinetic (PK) properties in mice, including its penetration in lung epithelial lining fluid (ELF), precluding pharmacodynamic analyses. In addition, it is not clear whether pharmacokinetic interactions exist between cephalosporins and tazobactam. In one earlier, limited study, such a pharmacokinetic interaction was observed between these two compounds (8) in mice, although it was not observed in men (9). Thus, the primary objectives of this study were to evaluate the pharmacokinetic profiling of ceftolozane and tazobactam in the mouse plasma and ELF and to assess potential drug interactions using a neutropenic murine thigh or lung infection model. Additionally, the protein binding and the pulmonary penetration of both agents were determined.
MATERIALS AND METHODS
Animals and setting.
Outbred female CD-1 mice obtained from Charles River (The Netherlands) and weighing 18 to 22 g at arrival were used in the experiments. Mice were rendered neutropenic by intraperitoneal (i.p.) injections of cyclophosphamide, 150 mg/kg of body weight at day −4 and 100 mg/kg at day −1 (10). The experiments were carried out in the Central Animal Facility (Centraal Dierenlab) at Radboud University Nijmegen Medical Centre. The animal studies were conducted in accordance with the recommendations of the European Community Directive 86/609/EEC, 24 November 1986. Studies were approved by the animal welfare committee of the Radboud University, no. RU-DEC 2011-102 and RU-DEC 2012-147.
Drugs.
Ceftolozane and tazobactam were supplied by Cubist Pharmaceuticals. Drugs were reconstituted in normal sterile saline (0.9% NaCl), according to the manufacturer's instructions, to a concentration of 200 mg/ml for ceftolozane and 100 mg/ml for tazobactam. Solutions were stored at −80°C until use and were combined with and/or diluted in normal saline to the final concentration needed for the experiments.
Infection and dosing.
On the day of the experiment, animals were infected with an inoculum of approximately 5 × 106 bacteria (normal or washed) per thigh, a different strain in each thigh. Klebsiella pneumoniae ATCC 700603 and a clinical Escherichia coli isolate were used in all experiments. Mice (n = 458) were injected i.p. with a 0.1-ml single dose containing ceftolozane, tazobactam, or both compounds combined 2 h after infection. Ceftolozane was applied in 2-fold increasing doses of 4 mg/kg to 64 mg/kg alone or in combination with tazobactam. Tazobactam was combined in reverse doses (thus, 64/4 mg/kg, 4/64 mg/kg, 32/8 mg/kg, and 8/32 mg/kg) (n = 2 mice per time point). In separate validation experiments to show indifference in pharmacokinetic profiling of the compounds alone or in combination, as well as to determine the ELF penetration, ceftolozane and tazobactam were given alone or in combination at ceftolozane-tazobactam doses of 32/8 mg/kg and 8/32 mg/kg (n = 4 mice per time point). For the 32/8-mg/kg dose, a second, separate inoculum control experiment was performed by washing the inoculum through centrifugation for 5 min and resuspending it in saline to the appropriate number of CFU/ml to determine whether free beta-lactamase in the inoculum has an impact on the pharmacokinetic profile of ceftolozane (11).
Sampling and analysis.
Blood samples of at least 0.5 ml for PK analyses were collected in 1-ml K3EDTA tubes through orbital sinus bleeding under isoflurane sedation following each single dose, just prior to dosing (hour 0) and at 0.083, 0.167, 0.33, 0.5, 0.75, 1, 1.5, 2, 3, 4, and 6 h after administration (one sample per mouse). Samples were immediately put on ice, and the blood subsequently was centrifuged in a precooled centrifuge and stored at −80°C until analysis. Bronchoalveolar lavage (BAL) samples were obtained immediately after mice were killed humanely, subsequent to previous blood collection. The ELF was obtained using a technique described previously (12). In short, after being killed under isoflurane anesthesia by cervical dislocation, mice were secured on a plastic platform, and the trachea was exposed by a 1-cm incision on the ventral neck skin for insertion of the cannula that was sutured in place. Lungs were instilled 4 times with 0.5 ml of sterile 0.9% saline, and the fluid was aspirated immediately. The aspirates thus recovered were pooled, directly placed on ice, and subsequently stored at −80°C.
Concentrations of ceftolozane and tazobactam in the ELF were determined by using the ratio of the urea concentration in the BAL fluid to that in the plasma, as measured with a modified enzymatic assay (QuantiChrom urea assay kit, DIUR-500; BioAssay Systems). The standard curve was linear, with a detection limit from 0.08 mg/dl to 100 mg/dl.
Protein binding in plasma was determined by the ultrafiltration technique. To obtain enough volume, plasma samples were pooled to obtain a concentration range of 2 to 35 mg/liter for ceftolozane and 0.1 to 7 mg/liter for tazobactam.
Concentrations were determined in plasma as well as in the ultrafiltrate. The ultrafiltrate was obtained using a Centrifree cartridge (30,000 MWCO; Millipore) and centrifuging at 1,300 × g for 15 min. During validation of the drug assessment protocol, no drug was lost during ultrafiltration due to nonspecific binding to components of the Centrifree device. The quantitative analysis of the ceftolozane and tazobactam concentrations in the blood and ELF was performed in a separate facility (MicroConstants, Inc., San Diego, CA). Briefly, samples containing ceftolozane and tazobactam, with FR259647 (Calixa Therapeutics, Inc.) and sulbactam as their respective internal standards and sodium heparin as the anticoagulant, were precipitated with a methanol-acetonitrile solution. The supernatant was further diluted as appropriate, and the sample extract was divided for analysis on two separate liquid chromatography-tandem mass spectrometry (LC/MS) systems. Ceftolozane was analyzed using electrospray positive ionization on a Supelco Discovery HS F5 column. Tazobactam was analyzed using electrospray negative ionization and was further prepared by online solid-phase extraction coupled to a Thermo Prism RP column. The coefficient of variation (CV) for the ceftolozane and tazobactam quality control samples ranged from 4.92% to 6.42% and 4.64% to 13.3%, respectively. The limit of detection for ceftolozane was 0.1 mg/liter and was 0.02 mg/liter for tazobactam.
Pharmacokinetic analysis.
The PK analysis was performed using noncompartmental modeling in GraphPad Prism 5.0 and WinNonlin 2.1 (Pharsight Corp., St. Louis, MO). The area under the concentration-time curve (AUC) was measured using the log-linear trapezoidal rule from the pooled data set for each curve (n = 2 or n = 4 per data point). The penetration in ELF was calculated as the AUC in the ELF divided by that in the plasma. Dose proportionality was determined following the standard methods by determining the relationship between the log(dose) and the log(AUC) following the power model approach (13).
RESULTS
Concentrations in plasma.
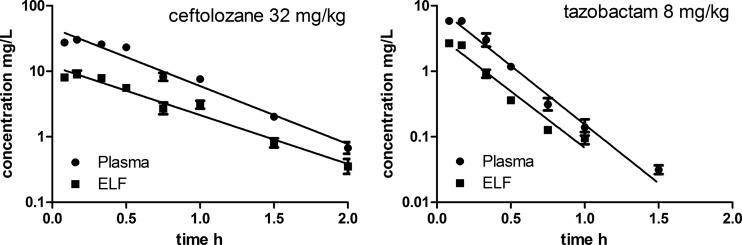
Figure 1 (left panel) shows an example of the concentration-time curve of ceftolozane for the 32-mg/kg dose with the pooled data. Likewise, Fig. 1 (right panel) shows an example of the concentration-time curve of tazobactam for the 8-mg/kg dose. There was a fast absorption phase and subsequent elimination for both compounds. The pharmacokinetics of both compounds in the plasma appears to be linear and dose proportional, as demonstrated by the dose proportionality in the plasma, as shown in Fig. 2. For ceftolozane (Fig. 2, left panel), the regression line for dose proportionality is shown both with and without tazobactam. There appears to be no significant difference between the two lines (P = 0.50). The overall pooled relationship can be described by log(AUC) = 0.208 + 0.742 × log(dose ceftolozane). Only two concentration-time curves were performed for tazobactam without ceftolozane; therefore, these are displayed as dots only (Fig. 2, right panel). The relationship of tazobactam with ceftolozane can be described by log(AUC) = −0.865 + 1.156 × log(dose tazobactam). Table 1 shows the pharmacokinetic profiling of ceftolozane and tazobactam. There was no significant difference in clearance (P = 0.99), half-life (P = 0.1), or V (P = 0.13) of ceftolozane with or without tazobactam. There was no clear effect observed of washing the inoculum.
FIG 1.
Concentration-time profiles in plasma and epithelial lining fluid (ELF) of neutropenic mice after a 32-mg/kg ceftolozane dose (left panel; n = 12 per time point) or an 8-mg/kg tazobactam dose (right panel; n = 10 or 12 per time point). Error bars indicate the standard error of the mean (SEM) (for some data points overlapping with the symbol).
FIG 2.
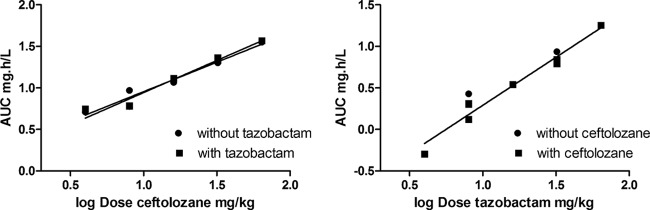
Dose proportionality of ceftolozane (left panel) and tazobactam (right panel) in plasma of infected mice after single doses. AUC, area under the concentration-time curve; dose, a single dose, administered intraperitoneally.
TABLE 1.
Pharmacokinetic parameter estimates of ceftolozane and tazobactam in thigh-infected neutropenic mice
| Drug | No. of curvesa | Pharmacokinetic parametersb |
|||||
|---|---|---|---|---|---|---|---|
| CL (liter/h/kg) |
t1/2 (min) |
V (liter/kg) |
|||||
| Estimate | SD | Estimate | SD | Estimate | SD | ||
| Ceftolozane (overall) | 15 | 1.33 | 0.32 | 17.2 | 1.86 | 0.43 | 0.15 |
| Without tazobactam | 7 | 1.32 | 0.38 | 18.1 | 2.36 | 0.47 | 0.18 |
| With tazobactam | 8 | 1.33 | 0.29 | 16.5 | 0.80 | 0.39 | 0.10 |
| Tazobactam | 10 | 4.64 | 1.46 | 10.6 | 1.57 | 1.14 | 0.56 |
Drug doses of 4 to 64 mg/kg.
CL, clearance; t1/2, half-life; V, volume of distribution.
Protein binding.
Protein binding was performed on pooled samples at concentrations ranging from 2 to 35 mg/liter for ceftolozane and 0.1 to 7 mg/liter for tazobactam. The overall estimates were 5.1% (standard error [SE], 0.85%; n = 10) for ceftolozane and 25.1% (SE, 2.45%; n = 14) for tazobactam. There appeared to be no concentration-dependent protein binding over the concentration range measured.
Concentrations in ELF.
Figure 1 shows examples of the concentration-time profiles of ceftolozane and tazobactam in the ELF together with the plasma for the two doses studied. Like in the plasma, the concentrations at the last time point may have been slightly overestimated, because a limited number of samples had concentrations below the lower limit of quantification (LLQ). However, this did not affect the pharmacokinetic parameter estimates.
Table 2 shows the penetration of ceftolozane and tazobactam in the ELF for the two different doses. Both compounds followed the concentrations in plasma and appeared to penetrate well in the ELF. For unbound concentrations (in ELF and plasma), the mean penetration ratio was 0.33 for ceftolozane and was 0.77 for tazobactam.
TABLE 2.
Penetration of ceftolozane and tazobactam in ELF for total drug and protein-unbound drug
| Drug | Dose, mg/kg | Total plasma AUC (mg · h/liter) | Total ELF AUC (mg · h/liter) | Total ELF/plasma ratio | Free ELF/plasma ratioa |
|---|---|---|---|---|---|
| Ceftolozane | 8 | 6.86 | 2.20 | 0.32 | 0.34 |
| 32 | 21.64 | 6.42 | 0.30 | 0.31 | |
| Tazobactam | 8 | 2.05 | 0.91 | 0.45 | 0.60 |
| 32 | 7.25 | 4.51 | 0.62 | 0.83 |
Free-drug ELF/free-drug plasma.
DISCUSSION
In this study, we found that the pharmacokinetics of both tazobactam and ceftolozane were linear over a range of doses from 4 to 64 mg/kg. The half-life of tazobactam was approximately 10 min. We found no earlier reference to tazobactam pharmacokinetics in mice, except for the studies by Fournier et al. (14) and Bulik et al. (8). However, in the study by Fournier et al., no specific pharmacokinetic data are provided other than a concentration-time plot; in the study by Bulik et al., the authors simulated human pharmacokinetics in their specific model, so the results cannot be directly compared. In the current study, i.p. injections were utilized, while most previous in vivo studies used subcutaneous doses. Of note, we did not find any pharmacokinetic interaction between tazobactam and ceftolozane, as was alluded to in the preliminary study by Bulik et al. (8). One possible reason might be that the doses used in our study were low enough not to show any; in the study by Bulik et al., the dosing regimens were adjusted by lowering the dose but giving doses more often to account for drug interactions. In humans, such a drug interaction is not observed (9).
The pharmacokinetics of ceftolozane were in agreement with that found by Craig and Andes (15) using a biological assay (single doses of 25, 100, and 400 mg/kg), although the half-life we found was slightly longer (17 min versus 12 to 14 min). In their discussion, the authors note that the half-life they found in their studies may have been too short due to the use of a bioassay, which thereby explained the relatively short percent free time above the MIC found for stasis in their mouse model. Our results show a slightly longer half-life, but this does not fully explain that result. The protein binding values were also comparable. Overall, the two studies provide a good framework for ceftolozane plasma pharmacokinetics in mice.
Both ceftolozane and tazobactam were found to penetrate well in the ELF. Although we used a thigh infection model, we think that a lung infection will not alter ELF penetrations, since these were independent to the infection model for ceftazidime and avibactam in another study by our group (13). Recently, ceftolozane penetration was described in human ELF as being 48% (16) for the total drug and 59% for the unbound fraction. The authors assumed a protein binding of 20% for ceftolozane in humans, which is higher than in mice. The penetration we found for ceftolozane in mice was lower, but it was slightly higher than the penetration of ceftazidime found in the same model (13). We do not have a clear explanation for the slightly higher penetration of ceftolozane compared to ceftazidime. Tazobactam penetration in the ELF was significantly higher than that of ceftolozane; after correction for protein binding, penetration exceeded 70% on average, which is a promising result to further investigate the pharmacodynamics of tazobactam combined with a beta-lactam compound. It was also much higher than the avibactam penetration in the ELF of 27% found in one of our other studies, using the same murine models (13). The penetration ratio of tazobactam in humans was described as 0.49 for the total drug and, when corrected for protein binding, was comparable to our results. Fournier et al. (14) looked at tazobactam concentrations in lung homogenates and concluded that tazobactam penetrated well and appeared to be dose dependent. However, interpretations of such studies should be viewed with great caution (17), because, by grinding up tissue consisting of distinct compartments in which the drug is not necessarily distributed in an homogeneous fashion or available for activity, this method may result in underestimation or overestimation of the drug levels on the side infection.
We conclude that both ceftolozane and tazobactam display linear pharmacokinetics in mice and show a good penetration in ELF. Additionally, similar to that seen in humans, we did not detect an interaction between ceftolozane and tazobactam in our murine model.
ACKNOWLEDGMENT
This study was performed with an unrestricted grant from Cubist Pharmaceuticals USA.
REFERENCES
- 1.Sader HS, Farrell DJ, Castanheira M, Flamm RK, Jones RN. 2014. Antimicrobial activity of ceftolozane-tazobactam tested against Pseudomonas aeruginosa and Enterobacteriaceae with various resistance patterns isolated in European hospitals (2011 to 2012). J Antimicrob Chemother 69:2713–2722. doi: 10.1093/jac/dku184. [DOI] [PubMed] [Google Scholar]
- 2.Sader HS, Farrell DJ, Flamm RK, Jones RN. 2014. Ceftolozane/tazobactam activity tested against aerobic Gram-negative organisms isolated from intraabdominal and urinary tract infections in European and United States hospitals (2012). J Infect 69:266–277. doi: 10.1016/j.jinf.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Farrell DJ, Flamm RK, Sader HS, Jones RN. 2013. Antimicrobial activity of ceftolozane-tazobactam tested against Enterobacteriaceae and Pseudomonas aeruginosa with various resistance patterns isolated in U.S. hospitals (2011 to 2012). Antimicrob Agents Chemother 57:6305–6310. doi: 10.1128/AAC.01802-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farrell DJ, Sader HS, Flamm RK, Jones RN. 2014. Ceftolozane/tazobactam activity tested against Gram-negative bacterial isolates from hospitalised patients with pneumonia in US and European medical centres (2012). Int J Antimicrob Agents 43:533–539. doi: 10.1016/j.ijantimicag.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 5.Snydman DR, McDermott LA, Jacobus NV. 2014. Activity of ceftolozane-tazobactam against a broad spectrum of recent clinical anaerobic isolates. Antimicrob Agents Chemother 58:1218–1223. doi: 10.1128/AAC.02253-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bretonnière C, Boutoille D, Caillon J, Desessard C, Guitton C, Potel G, Jacqueline C. 2014. In vivo efficacy of ceftolozane against Pseudomonas aeruginosa in a rabbit experimental model of pneumonia: comparison with ceftazidime, piperacillin/tazobactam and imipenem. Int J Antimicrob Agents 44:218–221. doi: 10.1016/j.ijantimicag.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Jacqueline C, Roquilly A, Desessard C, Boutoille D, Broquet A, Le Mabecque V, Amador G, Potel G, Caillon J, Asehnoune K. 2013. Efficacy of ceftolozane in a murine model of Pseudomonas aeruginosa acute pneumonia: in vivo antimicrobial activity and impact on host inflammatory response. J Antimicrob Chemother 68:177–183. doi: 10.1093/jac/dks343. [DOI] [PubMed] [Google Scholar]
- 8.Bulik CC, Tessier PR, Keel RA, Sutherland CA, Nicolau DP. 2012. In vivo comparison of CXA-101 (FR264205) with and without tazobactam versus piperacillin-tazobactam using human simulated exposures against phenotypically diverse gram-negative organisms. Antimicrob Agents Chemother 56:544–549. doi: 10.1128/AAC.01752-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller B, Hershberger E, Benziger D, Trinh M, Friedland I. 2012. Pharmacokinetics and safety of intravenous ceftolozane-tazobactam in healthy adult subjects following single and multiple ascending doses. Antimicrob Agents Chemother 56:3086–3091. doi: 10.1128/AAC.06349-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuluaga AF, Salazar BE, Rodriguez CA, Zapata AX, Agudelo M, Vesga O. 2006. Neutropenia induced in outbred mice by a simplified low-dose cyclophosphamide regimen: characterization and applicability to diverse experimental models of infectious diseases. BMC Infect Dis 6:55. doi: 10.1186/1471-2334-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louie A, Castanheira M, Liu W, Grasso C, Jones RN, Williams G, Critchley I, Thye D, Brown D, Vanscoy B, Kulawy R, Drusano GL. 2012. Pharmacodynamics of beta-lactamase inhibition by NXL104 in combination with ceftaroline: examining organisms with multiple types of beta-lactamases. Antimicrob Agents Chemother 56:258–270. doi: 10.1128/AAC.05005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seyedmousavi S, Bruggemann RJ, Melchers WJ, Verweij PE, Mouton JW. 2014. Intrapulmonary posaconazole penetration at the infection site in an immunosuppressed murine model of invasive pulmonary aspergillosis receiving oral prophylactic regimens. Antimicrob Agents Chemother 58:2964–2967. doi: 10.1128/AAC.00053-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berkhout J, Melchers MJ, van Mil AC, Seyedmousavi S, Lagarde CM, Nichols WW, Mouton JW. 2015. Pharmacokinetics and penetration of ceftazidime and avibactam into epithelial lining fluid in thigh- and lung-infected mice. Antimicrob Agents Chemother 59:2299–2304. doi: 10.1128/AAC.04627-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fournier JL, Ramisse F, Jacolot AC, Szatanik M, Petitjean OJ, Alonso JM, Scavizzi MR. 1996. Assessment of two penicillins plus beta-lactamase inhibitors versus cefotaxime in treatment of murine Klebsiella pneumoniae infections. Antimicrob Agents Chemother 40:325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Craig WA, Andes DR. 2013. In vivo activities of ceftolozane, a new cephalosporin, with and without tazobactam against Pseudomonas aeruginosa and Enterobacteriaceae, including strains with extended-spectrum beta-lactamases, in the thighs of neutropenic mice. Antimicrob Agents Chemother 57:1577–1582. doi: 10.1128/AAC.01590-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandorkar G, Huntington JA, Gotfried MH, Rodvold KA, Umeh O. 2012. Intrapulmonary penetration of ceftolozane/tazobactam and piperacillin/tazobactam in healthy adult subjects. J Antimicrob Chemother 67:2463–2469. doi: 10.1093/jac/dks246. [DOI] [PubMed] [Google Scholar]
- 17.Mouton JW, Theuretzbacher U, Craig WA, Tulkens PM, Derendorf H, Cars O. 2008. Tissue concentrations: do we ever learn? J Antimicrob Chemother 61:235–237. [DOI] [PubMed] [Google Scholar]