Abstract
In response to a call for the global eradication of malaria, drug discovery has recently been extended to identify compounds that prevent the onward transmission of the parasite, which is mediated by Plasmodium falciparum stage V gametocytes. Lately, metabolic activity has been used in vitro as a surrogate for gametocyte viability; however, as gametocytes remain relatively quiescent at this stage, their ability to undergo onward development (gamete formation) may be a better measure of their functional viability. During gamete formation, female gametocytes undergo profound morphological changes and express translationally repressed mRNA. By assessing female gamete cell surface expression of one such repressed protein, Pfs25, as the readout for female gametocyte functional viability, we developed an imaging-based high-throughput screening (HTS) assay to identify transmission-blocking compounds. This assay, designated the P. falciparum female gametocyte activation assay (FGAA), was scaled up to a high-throughput format (Z′ factor, 0.7 ± 0.1) and subsequently validated using a selection of 50 known antimalarials from diverse chemical families. Only a few of these agents showed submicromolar 50% inhibitory concentrations in the assay: thiostrepton, methylene blue, and some endoperoxides. To determine the best conditions for HTS, a robustness test was performed with a selection of the GlaxoSmithKline Tres Cantos Antimalarial Set (TCAMS) and the final screening conditions for this library were determined to be a 2 μM concentration and 48 h of incubation with gametocytes. The P. falciparum FGAA has been proven to be a robust HTS assay faithful to Plasmodium transmission-stage cell biology, and it is an innovative useful tool for antimalarial drug discovery which aims to identify new molecules with transmission-blocking potential.
INTRODUCTION
Despite the efforts made over decades of scientific research, malaria still remains a major health problem in tropical and subtropical areas, with more than 220 million cases and 600,000 deaths being registered per year (1). This parasitic disease is caused by Plasmodium infection through the bite of infected Anopheles female mosquitoes, with Plasmodium falciparum being responsible for the highest mortality rates (2).
Traditionally, pharmacological antimalarial treatments have targeted parasite asexual reproduction inside erythrocytes, which leads to the clinical symptoms of malaria. However, a small proportion of these asexual blood stages (0.2 to 1%) are committed to develop into sexual stages: male and female gametocytes (3, 4). Their differentiation process inside erythrocytes takes 8 to 12 days for P. falciparum, and inside erythrocytes they undergo a series of morphological and metabolic changes classically categorized into five stages of maturation (5, 6). While most schizonticides, such as chloroquine, affect young gametocytes (stages I, II, and III), gametocytes at late stages of maturation are not sensitive to them (7). These insensitive stage V gametocytes, which are responsible for malaria parasite transmission, remain quiescent but infectious in the peripheral bloodstream for approximately 22 days (8).
Currently administered pharmacological antimalarial treatments are highly effective against asexual blood stages, but only the 8-aminoquinoline (8-AQ) primaquine and methylene blue have been shown to be active against mature gametocytes in the field (9, 10). As these forms of the parasite are solely responsible for transmitting the disease, the discovery and development of new drugs targeting gametocytes will contribute to achieving the ultimate goal of malaria elimination. The challenge of global eradication was recently renewed by the malaria scientific community (11), highlighting that both asexual and sexual stages have to be targeted in order to control the disease and block its transmission. Therefore, dual-activity antimalarial drugs killing both asexual and sexual parasite stages in human blood are urgently required.
In the past few years, different groups have made a huge effort toward the development of bioassays to identify compounds with gametocytocidal activity. Diverse approaches have been followed to assess the viability of gametocytes, such as the development of green fluorescent protein and luciferase reporter lines controlled by gametocyte-specific promoters (12, 13) or the use of different indicators as a general measure of metabolic activity, such as ATP (14), alamarBlue (15), or parasite lactate dehydrogenase activity (16). Despite the number of assays developed, only a few compounds active against P. falciparum gametocytes have been identified so far; thus, further studies are still required.
With the aim of accelerating the discovery of new transmission-blocking drugs, it is essential to have the capacity to test several thousand compounds, and therefore, it is critical to develop fast, nonexpensive, and highly reproducible assays in a high-throughput screening (HTS) format. Although phenotypic screening has been widely used to identify compounds active against P. falciparum asexual stages (17–20), it is more laborious to scale up gametocyte-based assays because of the tedious, time-demanding, and expensive production of such stages. As of now, the production of gametocytes is one of the main impediments in the development of large-scale in vitro transmission-blocking assays. Furthermore, HTS of asexual stages has been based on asexual blood stage replication, but this approach is not translatable to sexual stage-based assays, as those stages do not divide during their maturation process. For all the reasons mentioned above, the development of gametocyte-based HTS assays constitutes a real challenge.
While the latest assays focused on early- and late-stage gametocytes as endpoints (12–14, 16), we have focused our attention on gamete formation as a key indicator of the functional viability of stage V mature female gametocytes after pharmacological treatment (21, 22). By using this readout, the biological content of the assay is increased to assess not only the effects of drugs on mature gametocytes (by targeting metabolic pathways) but also the possible inhibition of the gamete formation process (by gametocyte sterilization). Since both male and female gametes are required for zygote formation, the elimination of one of them would interrupt Plasmodium transmission. Here we present the P. falciparum female gametocyte activation assay (FGAA) scaled up for a high-throughput screening format, validated using 50 known antimalarial compounds, and currently being used for HTS campaigns.
MATERIALS AND METHODS
Asexual-stage culture.
P. falciparum 3D7 asexual-stage culture was performed as first described by Trager and Jensen (23). The culture medium composition was RPMI 1640 supplemented with 25 mM HEPES (Invitrogen), 50 μg/ml hypoxanthine (Gibco), 2 g/liter NaHCO3 (Sigma), 2 g/liter glucose (Sigma), and 10% pooled human male type A-positive (A+) serum (Interstate Blood Bank, TN). Asexual stages were maintained in culture at 0.5 to 5% parasitemia and 4% hematocrit, with a new vial of parasites being thawed every month. The human type A+ red blood cells used for culturing were obtained from the Spanish Red Cross. Parasites were constantly kept at 37°C in the presence of a fixed gas composition (3% CO2, 5% O2, 92% N2).
Gametocyte culture.
Gametocyte induction was performed as previously described (24). P. falciparum 3D7 asexual-stage cultures synchronized at the ring stage were used to start gametocyte cultures (day 0) at 1% parasitemia and 4% hematocrit in a 200-ml final volume. Complete culture medium (RPMI 1640 supplemented with 25 mM HEPES, 50 μg/ml hypoxanthine, 2 g/liter NaHCO3, and 10% pooled human male type A+ serum) was totally replaced daily for 14 days without fresh erythrocyte addition. To ensure maintenance of the temperature at 37°C, which is crucial for gametocyte production and maturation, prewarmed medium and a slide warmer (Premium) were used. Under these conditions, parasitemia reaches a peak of asexual stages on days 4 to 5, and the first gametocytes are differentiated in culture on day 6. Sexual-stage development was monitored microscopically by Giemsa-stained thin blood smears at day 7 (mainly asexual stages and stage I to III gametocytes) and day 14 (stage IV and V gametocytes).
Female gametocyte activation assay.
Cultures showing mainly stage V gametocytes were purified by differential sedimentation using Nycoprep 1.077 solution (Axis-Shield). After 20 min of centrifugation at 800 × g and 37°C, the collected interface band was washed in prewarmed RPMI 1640 (Sigma) and gametocytes were counted using a Neubauer chamber. The concentration was adjusted to plate 8,000 gametocytes per well (100 μl/well) in 384-well black plates with clear bottoms (CellCarrier; PerkinElmer). Gametocytes were then incubated with drugs (0.5 μl/well) for either 24 or 48 h at 37°C (3% O2, 5% CO2, 92% N2). Female gametocyte activation was then performed as previously described (21) by a temperature drop and addition of xanthurenic acid (Sigma). A volume of 10 μl/well of complete ookinete medium (RPMI 1640 with 25 mM HEPES, 50 μg/ml hypoxanthine, 2 g/liter NaHCO3, 100 μM xanthurenic acid, and 20% pooled human male type A+ serum) was added to each plate. For female gamete detection, the medium was supplemented with anti-Pfs25 antibody (final concentration, 0.5 μg/ml), produced from monoclonal hybridomas (25) and provided by the Malaria Research and Reference Reagent Resource Center (MR4), conjugated to the Cy3 fluorochrome (GE Healthcare). Activated cultures were then incubated at 26°C for 24 h (3% O2, 5% CO2, 92% N2) and protected from light. The plates were analyzed using an Opera high-content screening system (PerkinElmer).
Compounds and controls.
Compounds were dissolved in 100% dimethyl sulfoxide (DMSO), and 2-fold serial dilutions (starting at a 25 μM concentration) were used for the dose-response analysis. Each compound was tested in duplicate in at least 3 independent experiments. As a negative control (female gametocyte activation in the presence of the vehicle only), a minimum of 10 wells per plate containing 0.5% DMSO were analyzed. As a positive control (background, residual activation in the presence of a confirmed gametocytocidal drug), a minimum of 10 wells per plate containing the proteasome inhibitor thiostrepton at a 50 μM concentration were analyzed. The MMV50 set of 50 compounds tested for assay validation was kindly provided by the Medicines for Malaria Venture (MMV; Geneva, Switzerland).
Image acquisition and analysis.
Image acquisition and analysis were performed on the Opera high-content screening system (PerkinElmer). For statistical analysis purposes, five images per well were taken from the bottom of the PerkinElmer microplate using a 10× air objective. The intensity of the Cy3 fluorochrome was measured with a laser with excitation at 532 nm, and the distribution was determined with an exposure time of 80 ms. All images were analyzed with a Columbus image data storage and analysis system (PerkinElmer) using a script specifically designed for this assay. Once the number of activated female gametocytes per well was determined, positive and negative controls were used to define the percent inhibition and calculate the 50% inhibitory concentration (IC50) of each compound.
Data analysis.
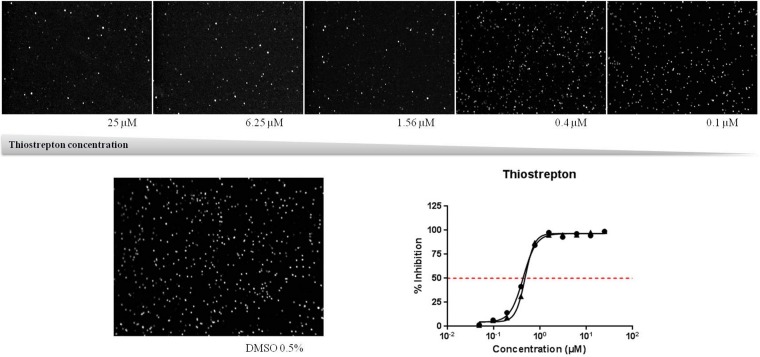
IC50 calculation was performed with GraphPad Prism software using a nonlinear regression analysis and a normalized 4-parameter log dose with variable slope curve. A graphical representation of the dose-response curve for the thiostrepton positive control is shown in Fig. 1. The signal-to-noise ratio (S/N), signal-to-background ratio (S/B), and Z′ factor were calculated as previously reported (26). All formulae contain the mean and standard deviation (SD) for the positive-control (C+; thiostrepton) and negative-control (C−; DMSO) signals. Values of the Z′ factor higher than 0.4 are required for HTS quality and robustness (27). The formulae are as follows: S/N = (mean for C− − mean for C+)/SD for C+, S/B = mean for C−/mean for C+, and Z′ factor = 1−(3 SDs for C− + 3 SDs for C+)/(mean for C− − mean for C+).
FIG 1.
HTS pharmacology validation. (Bottom right) Graphical representation of thiostrepton dose-response curve; (top and bottom left) Cy3 signal of activated female gametocytes after 48 h treatment with thiostrepton (top) or DMSO (bottom left) control. A 10× objective was used to obtain the images.
RESULTS
P. falciparum FGAA in high-throughput format.
The P. falciparum female gametocyte activation assay (FGAA) previously described by Delves et al. (21) was modified and scaled up to a 384-well format by achieving the large-scale production of gametocytes in culture and imaging detection in an automated Opera high-content screening system. Female gamete formation was used as the indicator of stage V gametocyte viability and functionality. Detection of female gametocyte activation was based on the specific expression of the Pfs25 protein at the surface of the female gametes that had egressed.
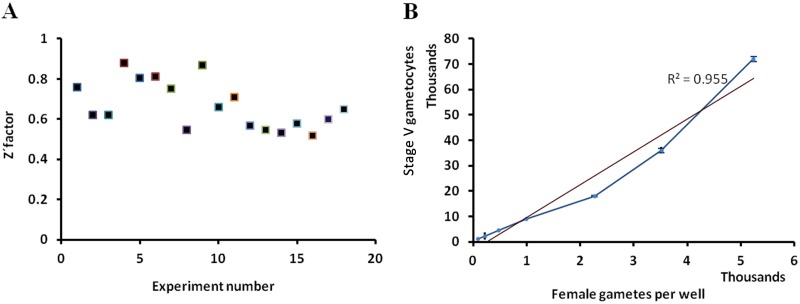
The large-scale production of viable gametocytes is the main bottleneck to developing in vitro high-throughput screenings for gametocytocidal compounds. This problem was addressed by performing gametocytogenesis in 200-ml bottles and including an enrichment step using Nycoprep. By this procedure, up to 2.5 × 107 stage V gametocytes per culture were obtained. Using 8,000 gametocytes per well, a 200-fold S/B ratio and 20-fold S/N ratio were obtained. The culture enrichment step was essential to achieving the optimal assay sensitivity and reproducibility in the 384-well format. Applying those conditions, more than 20,000 compounds could be tested per week. The average Z′ factor obtained in the P. falciparum FGAA high-throughput format was 0.7 ± 0.1 (SD) (Fig. 2).
FIG 2.
Quality and sensitivity of the P. falciparum FGAA for HTS. (A) Graphical representation of the Z′ factors obtained in 18 independent plates during assay validation for HTS. (B) Linearity of the number of stage V gametocytes plotted against the number of female gametes detected per well.
The process of gametogenesis, which naturally occurs inside the mosquito midgut shortly after an infected-blood meal, can be mimicked in vitro to obtain both male gamete exflagellation and female gametocyte activation. This process was triggered by a temperature drop of at least 5°C and supplementation of the medium with low concentrations of xanthurenic acid (28). To induce female gamete formation, medium supplemented with xanthurenic acid was added to stage V gametocyte cultures, followed by incubation at 26°C.
The expression of the Pfs25 protein on the plasma membrane surface has been demonstrated to be a suitable biomarker for female gamete formation (21, 29). A specific anti-Pfs25 antibody labeled with Cy3 enabled the detection of fluorescent round forms only 30 min after activation; however, the highest fluorescence signal was obtained at 24 h postactivation, because Pfs25 protein expression increases over time. Therefore, we decided to incubate the gametocytes for 48 h at 37°C with the corresponding compound and then trigger gamete formation, add Pfs25-Cy3 antibody, and incubate the mixture for 24 h at 26°C postactivation (Fig. 3).
FIG 3.
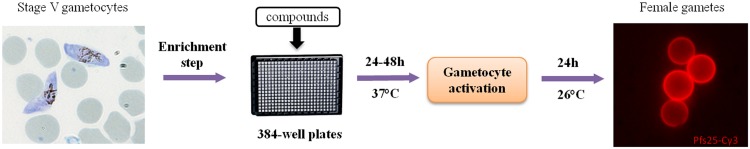
Diagram of P. falciparum female gametocyte activation assay protocol for HTS. The gametocyte culture was purified using a Nycoprep cushion, and stage V gametocytes (8,000 per well) were plated onto 384-well plates into which compounds had been predispensed. After 24 to 48 h of incubation at 37°C, the gametocytes were activated, followed by 24 h of incubation at 26°C with antibody against Pfs25 labeled with Cy3. Activated female gametocytes were detected by the Cy3 fluorescence signal in the Opera high-content screening system.
In the P. falciparum FGAA low-throughput format, the fluorescence signal from Pfs25-Cy3-positive cells was used as the final assay readout, based on a simple particle count ImageJ algorithm (21). For the high-throughput format, detection of viable activated female gametocytes was improved by using the automated Opera high-content screening system. Images were analyzed with an algorithm which calculates the number of events (activated female gametocytes) detected on the basis of the Pfs25-Cy3 antibody signal. In order to select the most appropriate discrimination parameters and discard any fluorescent background object or dead gametocyte/gamete, a cluster analysis using a K-means clustering algorithm (30) with morphology, texture, and fluorescence intensity properties was performed. As a result of this analysis, a reduced set of parameters, such as roundness, area, intensity, and second angular moment, was selected to be discriminant variables on the Columbus script in order to separate the spots associated with activated female gametocytes.
Comparison of P. falciparum FGAA high- and low-throughput formats.
A set of 20 known antimalarial drugs was tested by exposing stage V gametocytes to the drugs at a concentration of 10 μM for 24 h in order to compare the data obtained by the high-throughput format with those obtained by the previously described low-throughput format (Table 1). Four compounds, cycloheximide, mefloquine, pyronaridine, and thiostrepton, showed inhibition values higher than 50%. These results were comparable to those observed in the low-throughput format, confirming that few antimalarial drugs exerted activity against P. falciparum sexual stages. Methylene blue showed less potency in the high-throughput format than in the low-throughput format (42.0% and 95.82% inhibition, respectively). However, incubation of gametocytes for an additional 24 h increased the potency of methylene blue, with the inhibition value reaching 90%. Dose-response assays were performed for those compounds with an inhibitory effect greater than 50% after 24 h of exposure to mature gametocytes (Table 2). Among all the compounds tested, thiostrepton was the most potent against female gamete formation, showing submicromolar IC50s (0.27 μM) (Table 2).
TABLE 1.
Activity of a set of 20 antimalarial drugs in the P. falciparum FGAA for comparison of high- and low-throughput formats
| Drug | % inhibition of female gametocyte activationa |
|||
|---|---|---|---|---|
| High-throughput format |
Low-throughput formatb |
|||
| Mean | SD | Mean | SEM | |
| Amodiaquine | 34.6 | 5.3 | 27.25 | 4.5 |
| Artemether | 28.2 | 8.7 | 9.97 | 11.48 |
| Artemisinin | 27.9 | 13.7 | 11.44 | 8.23 |
| Artesunate | 38.9 | 14.7 | 10.3 | 2.3 |
| Azithromycin | 19.4 | 5.2 | 12.95 | 6.92 |
| Chloroquine | 13.7 | 10.3 | NDc | ND |
| Cycloguanil | 11.9 | 9.1 | 17.63 | 5.27 |
| Cycloheximide | 86.9 | 11.5 | ND | ND |
| Dihydroartemisinin | 43.3 | 15.1 | 9.36 | 4.26 |
| Doxycycline | 9.0 | 10.4 | 45.41 | 3.18 |
| Halofantrine | 15.6 | 10.9 | −0.89 | 9.63 |
| Lumefantrine | 14.1 | 11.2 | ND | ND |
| Mefloquine | 64.3 | 12.0 | 34.2 | 9.21 |
| Methylene blue | 42.0 | 17.6 | 95.82 | 1.99 |
| Primaquine | 21.7 | 13.2 | 17.77 | 11.22 |
| Pyrimethamine | 16.9 | 12.2 | 7.43 | 6.52 |
| Pyronaridine | 100.0 | 2.7 | 95.56 | 1.17 |
| Quinine | 11.2 | 8.8 | ND | ND |
| Sulfadoxine | 10.4 | 11.7 | ND | ND |
| Thiostrepton | 97.4 | 1.2 | 97.85 | 0.46 |
Data are mean percent inhibition values with respect to the level of inhibition for the DMSO carrier controls (n = 6) ± SD when each of the drugs was used at a concentration of 10 μM.
Data are from Delves et al. (21).
ND, not determined.
TABLE 2.
Dose-response evaluation of selected compounds with inhibition potential in the P. falciparum FGAA
| Drug | IC50a (μM) |
|
|---|---|---|
| Mean | SD | |
| Thiostrepton | 0.27 | 0.08 |
| Methylene blue | 11.9 | 2.24 |
| Pyronaridine | 2.42 | 0.72 |
| Cycloheximide | 1 | 0.34 |
| Mefloquine | 4.51 | 3.85 |
Data are the mean IC50s after 24 h of incubation with respect to the level of inhibition for the DMSO carrier controls (n = 4) ± SD.
Validation of the P. falciparum FGAA in high-throughput format using a test set of 50 antimalarial agents.
The new assay was further validated using a panel of 50 antimalarial compounds from diverse chemical families (the MMV50 set), which included those previously tested in the 20 antimalarials. Given the results obtained with methylene blue and in order to identify slow-acting molecules, the activities of these compounds were evaluated after 48 h of exposure in a dose-response manner; IC50s are summarized in Table 3. Similar to the data obtained previously with the set of 20 compounds, the MMV50 set contained only a few drugs with submicromolar IC50s against female gametocyte activation: endoperoxides (dihydroartemisinin [IC50, 0.99 μM], artesunate [IC50, 0.64 μM], artemisone [IC50, 0.94 μM]), thiostrepton (IC50, 0.12 μM), and methylene blue (IC50, 0.92 μM) were the most potent compounds.
TABLE 3.
Gametocytocidal activity of the MMV50 set of antimalarials in the high-throughput format of the P. falciparum FGAA
| Drug | IC50a (μM) |
|
|---|---|---|
| Avg | SD | |
| Endoperoxides | ||
| Artemether | 9.96 | 2.06 |
| Dihydroartemisinin | 0.99 | 0.74 |
| Artesunate | 0.64 | 0.47 |
| Artemisinin | 7.59 | 5.96 |
| OZ277 (RBX-11160) | 3.59 | 0.81 |
| Artemisone | 0.94 | 0.25 |
| 8-AQs | ||
| Pamaquine | 2.37 | 1.02 |
| Primaquine | 11.04 | 2.94 |
| Tafenoquine | 7.60 | 0.80 |
| NPC-1161B | 6.07 | 2.54 |
| Antifolates | ||
| Pyrimethamine | >25 | |
| Chlorproguanil | 4.68 | 0.28 |
| Proguanil | 11.94 | 1.32 |
| P218 | >25 | |
| Antibiotics | ||
| Azithromycin | 13.02 | 0.00 |
| Dapsone | >25 | |
| Doxycycline | >25 | |
| Trimethoprim | >25 | |
| Thiostrepton | 0.12 | 0.01 |
| Clindamycin | >25 | |
| cis-Myrincamycin | 16.69 | 1.94 |
| trans-Myrincamycin | 17.45 | 1.85 |
| Fosmidomycin | >25 | |
| Pentamidine | 7.41 | 1.40 |
| Tetracycline | >25 | |
| 4-AQs | ||
| Amodiaquine | 9.01 | 0.86 |
| AQ-13 | 15.89 | 1.33 |
| Chloroquine | >25 | |
| Hydroxychloroquine | >25 | |
| Naphthoquine | 1.98 | 1.12 |
| Piperaquine | >25 | |
| Pyronaridine | 1.57 | 0.10 |
| Amino alcohols | ||
| Halofantrine | >25 | |
| Lumefantrine | >25 | |
| Mefloquine (racemic) | 7.48 | 1.41 |
| Mefloquine (+RS) | 4.87 | 0.99 |
| Quinine sulfate dihydrate | 9.80 | 8.28 |
| Sulfonamides | ||
| Sulfadiazine | 16.54 | 0.00 |
| Sulfamethoxazole | >25 | |
| Sulfadoxine | >25 | |
| Miscellaneous | ||
| Dehydroepiandrosterone | >25 | |
| Cycloheximide | 5.36 | 0.55 |
| Riboflavin | >25 | |
| N-Acetyl-d-penicillamine | >25 | |
| Deferoxamine mesylate | >25 | |
| Atovaquone | 5.31 | 2.74 |
| Methylene blue | 0.92 | 0.24 |
| Quinidine | >25 | |
Data are the IC50s with respect to the level of inhibition for the DMSO carrier controls (n = 3) ± SD.
Establishment of the best conditions for HTS.
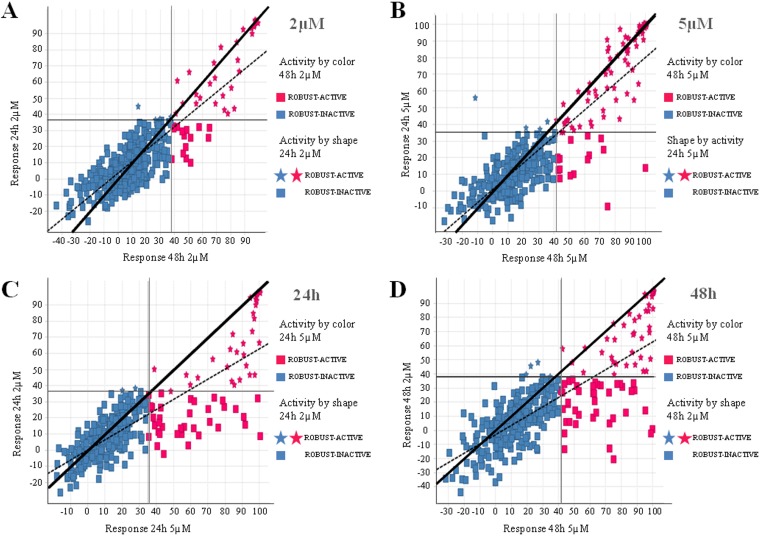
Once the assay was validated, the next step was to investigate the optimum screening conditions for the P. falciparum FGAA. The GlaxoSmithKline (GSK) Tres Cantos Antimalarial Set (TCAMS) is a collection of 13,533 compounds with activities against P. falciparum asexual stages at concentrations lower than 2 μM (17). A randomly selected subset of 356 compounds was used to perform a robustness test in order to determine the optimum assay concentration and incubation time. This test was performed with two different concentrations (2 and 5 μM) as well as two distinct incubation times (24 and 48 h). The definition of a hit was established on the basis of the mean cutoff, which was defined as the mean inhibition value for all tested compounds plus 3 times the standard deviation. We performed a pairwise comparison of compound activities obtained under each condition of concentration and incubation time (Fig. 4).
FIG 4.
Determination of the best screening conditions: graphical representations of the robustness of test results. Pairwise comparisons of incubation times (24 and 48 h) and compound concentrations (2 and 5 µM) are provided. (A and B) Response of increasing incubation time; (C and D) response of increasing concentration. Blue squares, compounds inactive under both conditions; pink stars, compounds active under both conditions; pink squares and blue stars, compounds active when the incubation time or concentration was increased.
As expected, more compounds were active when both concentration and incubation time were increased: 33 and 44 hits at 24 and 48 h, respectively, at 2 μM and 69 and 81 hits at 24 and 48 h, respectively, at 5 μM. Hit rates obtained at 2 μM were considered acceptable for HTS after either 24 or 48 h of incubation: 9.27% and 12.36%, respectively. These hit rates are higher than expected in a regular screening campaign (1 to 2%) because these compounds were preselected against asexual stages. In order to include the possibility of detecting slow-acting compounds, it was decided to use the longer drug exposure time. Final screening conditions were then defined to be a 48-h incubation time and a 2 μM concentration.
DISCUSSION
Malaria control and eradication are essential to reduce the disease burden in countries where malaria is endemic. This goal is strongly supported by the World Health Organization and institutions supporting research on diseases of the developing world (1, 11). Once clinical symptoms are controlled by pharmacological elimination of circulating asexual stages, gametocyte clearance from infected carriers is crucial to reduce the spread of malaria in affected areas. In this context, here we describe a robust imaging-based HTS assay using female gamete formation as a reporter of gametocyte viability. This new method enables the identification of molecules with gametocytocidal activity and dual-acting agents (drugs with both gametocytocidal and blood schizonticidal activities) capable of simultaneously relieving clinical symptoms and blocking parasite transmission.
Gametocyte-based assays determine gametocyte death or metabolic inhibition (13–16), but they lack the ability to measure the effects of those drugs that provoke delayed death or inhibition of onward development. Since gamete formation requires the activation of many transcriptional pathways initiated in the gametocyte, these could also be considered possible druggable targets to block malaria parasite transmission. Using the gamete formation as endpoint, the number of targets is therefore increased. Gametocytes are sexually dimorphic, and both male and female are required for parasite transmission. Detection of fluorescent female gametes was considered to be more suitable for HTS than detection of exflagellation, as they can be detected over a longer time window. Interestingly, it has recently been reported that exflagellation can be quantified in 96- and 384-well formats (22).
The P. falciparum FGAA in the high-throughput format was validated with 50 known antimalarial drugs, and we have established the optimum conditions for the testing of large libraries of compounds. On the basis of the Z′ factor, linearity, signal-to-noise ratio, and signal-to-background ratio obtained (Fig. 4), the reproducibility and reliability of this assay for HTS are ensured. Moreover, the test performed with a selection of TCAM set compounds provided valuable information about the P. falciparum FGAA robustness and demonstrated that this assay is reliable for the screening of large libraries of compounds. On the basis of data obtained from this robustness test, the hit rate expected in the screening of the TCAM set will be higher than that obtained in a regular screening campaign (9% versus 1%). This is a consequence of screening of a collection of compounds with activity against P. falciparum asexual stages.
The evaluation of the MMV50 set identified five compounds with activities against P. falciparum female gamete formation at submicromolar concentrations, which highlights the infrequent activity of most current antimalarial agents against sexual stages. Compounds with activity at submicromolar concentrations in the P. falciparum FGAA were the antibiotic thiostrepton, the 4-aminoquinoline (4-AQ) pyronaridine, some endoperoxides, and methylene blue. The ribosome-targeting antibiotic thiostrepton has been described to be a potent inhibitor of apicoplast protein synthesis and parasite proteasome activity (31). Although its activity against asexual blood stages is not remarkable (IC50 = 8.9 μM) (31), it has been reported to be highly active against mature gametocytes (IC50 = 0.56 μM) (13), inhibiting both male gamete exflagellation (32) and female gamete formation (IC50 = 0.12 μM). The recently developed 4-AQ pyronaridine is an inhibitor of P. falciparum topoisomerase II, which is in phase II-III clinical trials in a combined treatment with artesunate (33, 34). The in vitro gametocytocidal activity of pyronaridine has been previously reported (35), although recently developed assays revealed that it has high IC50s against late-stage gametocytes: 4.26 μM (13) and 3.25 μM (14). Interestingly, pyronaridine inhibits P. falciparum exflagellation as well as oocyst production (36). These data correlate well with our results for pyronaridine in the P. falciparum female gamete formation assay (IC50 = 1.57 μM). Methylene blue has been shown to interfere with the parasite heme detoxification process and both human and Plasmodium disulfide reductases (37). Many studies have characterized methylene blue to be a potent inhibitor of young and mature gametocytes (12), but its IC50 is variable, probably because of the different assay conditions used for testing (different culture treatments, times of exposure, and assay sensitivities or readouts). This difference in potency observed between the P. falciparum FGAA low- and high-throughput formats may be explained by the methylene blue redox mode of action and how it is modulated depending on the erythrocyte density in the assay.
Artemisinin combination therapies (ACTs) constitute the first line of treatment for P. falciparum malaria (38). Endoperoxides, such as artemisinin and its derivatives, have demonstrated potent antimalarial activity against asexual blood stages (39) and young gametocytes (40) but exert little or no effect against stage IV and V gametocytes in vitro (14, 41). However, endoperoxides have been shown to be effective antigamete drugs, causing exflagellation inhibition (80% at 1 μM) (21) and interrupting female gamete formation at submicromolar concentrations (Table 2). Furthermore, the transmission-blocking activity of ACTs in the field is still controversial, as only a few clinical trials showed moderate activity in the standard membrane feeding assay (SMFA) (42–44). The 8-AQ primaquine is the only drug with activity against mature gametocytes used in the field (9). However, it has been described that the efficacy of primaquine is mainly due to its metabolites, since there is no significant evidence of its in vitro activity (IC50 in the P. falciparum FGAA, 11.04 μM).
The P. falciparum FGAA provides highly valuable information on potential transmission-blocking compounds. However, the measure of female gametocyte activation has not been proven to be a direct measure of mosquito infectivity, as no female-specific compounds have been described so far. Thus, being conscious of this limitation and the fact that this assay may miss compounds that specifically exert activity against the male gametocyte, work is under way to develop dual male-female gametocyte HTS assays (22). An assay that could assess the effects of drugs on both male and female gamete formation would be the closest in vitro approach to the “gold standard” SMFA.
During the past decade, HTS assays have been successfully applied to P. falciparum asexual stages to screen millions of compounds (17, 19, 20). These phenotypic screenings have identified new active compounds, and some of them (KAE609 [45], OZ439 [46]) are currently in clinical development. To achieve the goal of malaria eradication proposed by the scientific community, the same approach should be applied to parasite stages, responsible for transmission.
Here we validated a new HTS assay based on imaging technologies to screen large libraries and select thousands of molecules with activity against stage V gametocytes. Our current strategy aims to test collections with known activity against asexual stages to discover molecules with dual activity targeting both asexual stages of the parasite and mature gametocytes. Compounds identified during the validation of the P. falciparum FGAA, such as methylene blue and thiostrepton, will be used as positive controls. This extensive screening will enable not only the discovery of new antimalarials for malaria elimination and eradication but also the identification of tool compounds to describe new molecular targets involved in malaria transmission.
ACKNOWLEDGMENTS
The following reagent was obtained through MR4 as part of the BEI Resources Repository, NIAID, NIH: Plasmodium falciparum anti-Pfs25 monoclonal antibody 4B7, MRA-28, deposited by D. C. Kaslow.
This work was supported by Bill and Melinda Gates Foundation grant OPP1043501.
We thank Richard Priest from Biological Sciences at GSK, Stevenage, United Kingdom, for the production and conjugation of anti-Pfs25 antibody. We also thank the GSK Sample Management Technologies facilities for the preparation of 384-well plates into which compounds had been predispensed.
REFERENCES
- 1.World Health Organization. 2013. World malaria report 2013. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/world_malaria_report_2013/report/en/. [Google Scholar]
- 2.Flannery EL, Chatterjee AK, Winzeler EA. 2013. Antimalarial drug discovery—approaches and progress towards new medicines. Nat Rev Microbiol 11:849–862. doi: 10.1038/nrmicro3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinden RE. 1983. Sexual development of malarial parasites. Adv Parasitol 22:153–216. doi: 10.1016/S0065-308X(08)60462-5. [DOI] [PubMed] [Google Scholar]
- 4.Kafsack BF, Rovira-Graells N, Clark TG, Bancells C, Crowley VM, Campino SG, Williams AE, Drought LG, Kwiatkowski DP, Baker DA, Cortes A, Llinas M. 2014. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature 507:248–252. doi: 10.1038/nature12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawking F, Wilson ME, Gammage K. 1971. Evidence for cyclic development and short-lived maturity in the gametocytes of Plasmodium falciparum. Trans R Soc Trop Med Hyg 65:549–559. doi: 10.1016/0035-9203(71)90036-8. [DOI] [PubMed] [Google Scholar]
- 6.Baker DA. 2010. Malaria gametocytogenesis. Mol Biochem Parasitol 172:57–65. doi: 10.1016/j.molbiopara.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smalley ME. 1977. Plasmodium falciparum gametocytes: the effect of chloroquine on their development. Trans R Soc Trop Med Hyg 71:526–529. doi: 10.1016/0035-9203(77)90149-3. [DOI] [PubMed] [Google Scholar]
- 8.Sinden RE, Smalley ME. 1979. Gametocytogenesis of Plasmodium falciparum in vitro: the cell-cycle. Parasitology 79:277–296. doi: 10.1017/S003118200005335X. [DOI] [PubMed] [Google Scholar]
- 9.White NJ. 2013. Primaquine to prevent transmission of falciparum malaria. Lancet Infect Dis 13:175–181. doi: 10.1016/S1473-3099(12)70198-6. [DOI] [PubMed] [Google Scholar]
- 10.Coulibaly B, Zoungrana A, Mockenhaupt FP, Schirmer RH, Klose C, Mansmann U, Meissner PE, Muller O. 2009. Strong gametocytocidal effect of methylene blue-based combination therapy against falciparum malaria: a randomised controlled trial. PLoS One 4:e5318. doi: 10.1371/journal.pone.0005318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinden RE, Carter R, Drakeley C, Leroy D. 2012. The biology of sexual development of Plasmodium: the design and implementation of transmission-blocking strategies. Malar J 11:70. doi: 10.1186/1475-2875-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adjalley SH, Johnston GL, Li T, Eastman RT, Ekland EH, Eappen AG, Richman A, Sim BK, Lee MC, Hoffman SL, Fidock DA. 2011. Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. Proc Natl Acad Sci U S A 108:E1214–E1223. doi: 10.1073/pnas.1112037108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duffy S, Avery VM. 2013. Identification of inhibitors of Plasmodium falciparum gametocyte development. Malar J 12:408. doi: 10.1186/1475-2875-12-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lelievre J, Almela MJ, Lozano S, Miguel C, Franco V, Leroy D, Herreros E. 2012. Activity of clinically relevant antimalarial drugs on Plasmodium falciparum mature gametocytes in an ATP bioluminescence “transmission blocking” assay. PLoS One 7:e35019. doi: 10.1371/journal.pone.0035019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka TQ, Dehdashti SJ, Nguyen DT, McKew JC, Zheng W, Williamson KC. 2013. A quantitative high throughput assay for identifying gametocytocidal compounds. Mol Biochem Parasitol 188:20–25. doi: 10.1016/j.molbiopara.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Alessandro S, Silvestrini F, Dechering K, Corbett Y, Parapini S, Timmerman M, Galastri L, Basilico N, Sauerwein R, Alano P, Taramelli D. 2013. A Plasmodium falciparum screening assay for anti-gametocyte drugs based on parasite lactate dehydrogenase detection. J Antimicrob Chemother 68:2048–2058. doi: 10.1093/jac/dkt165. [DOI] [PubMed] [Google Scholar]
- 17.Gamo FJ, Sanz LM, Vidal J, de Cozar C, Alvarez E, Lavandera JL, Vanderwall DE, Green DV, Kumar V, Hasan S, Brown JR, Peishoff CE, Cardon LR, Garcia-Bustos JF. 2010. Thousands of chemical starting points for antimalarial lead identification. Nature 465:305–310. doi: 10.1038/nature09107. [DOI] [PubMed] [Google Scholar]
- 18.Avery VM, Bashyam S, Burrows JN, Duffy S, Papadatos G, Puthukkuti S, Sambandan Y, Singh S, Spangenberg T, Waterson D, Willis P. 2014. Screening and hit evaluation of a chemical library against blood-stage Plasmodium falciparum. Malar J 13:190. doi: 10.1186/1475-2875-13-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guiguemde WA, Shelat AA, Bouck D, Duffy S, Crowther GJ, Davis PH, Smithson DC, Connelly M, Clark J, Zhu F, Jimenez-Diaz MB, Martinez MS, Wilson EB, Tripathi AK, Gut J, Sharlow ER, Bathurst I, El Mazouni F, Fowble JW, Forquer I, McGinley PL, Castro S, Angulo-Barturen I, Ferrer S, Rosenthal PJ, Derisi JL, Sullivan DJ, Lazo JS, Roos DS, Riscoe MK, Phillips MA, Rathod PK, Van Voorhis WC, Avery VM, Guy RK. 2010. Chemical genetics of Plasmodium falciparum. Nature 465:311–315. doi: 10.1038/nature09099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plouffe D, Brinker A, McNamara C, Henson K, Kato N, Kuhen K, Nagle A, Adrian F, Matzen JT, Anderson P, Nam TG, Gray NS, Chatterjee A, Janes J, Yan SF, Trager R, Caldwell JS, Schultz PG, Zhou Y, Winzeler EA. 2008. In silico activity profiling reveals the mechanism of action of antimalarials discovered in a high-throughput screen. Proc Natl Acad Sci U S A 105:9059–9064. doi: 10.1073/pnas.0802982105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delves MJ, Ruecker A, Straschil U, Lelievre J, Marques S, Lopez-Barragan MJ, Herreros E, Sinden RE. 2013. Male and female Plasmodium falciparum mature gametocytes show different responses to antimalarial drugs. Antimicrob Agents Chemother 57:3268–3274. doi: 10.1128/AAC.00325-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruecker A, Mathias DK, Straschil U, Churcher TS, Dinglasan RR, Leroy D, Sinden RE, Delves MJ. 2014. A male and female gametocyte functional viability assay to identify biologically relevant malaria transmission-blocking drugs. Antimicrob Agents Chemother 58:7292–7302. doi: 10.1128/AAC.03666-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trager W, Jensen JB. 1976. Human malaria parasites in continuous culture. Science 193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 24.Kaushal DC, Carter R, Miller LH, Krishna G. 1980. Gametocytogenesis by malaria parasites in continuous culture. Nature 286:490–492. doi: 10.1038/286490a0. [DOI] [PubMed] [Google Scholar]
- 25.Barr PJ, Green KM, Gibson HL, Bathurst IC, Quakyi IA, Kaslow DC. 1991. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J Exp Med 174:1203–1208. doi: 10.1084/jem.174.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang JH, Chung TD, Oldenburg KR. 1999. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 27.Coma I, Herranz J, Martin J. 2009. Statistics and decision making in high-throughput screening. Methods Mol Biol 565:69–106. doi: 10.1007/978-1-60327-258-2_4. [DOI] [PubMed] [Google Scholar]
- 28.Billker O, Lindo V, Panico M, Etienne AE, Paxton T, Dell A, Rogers M, Sinden RE, Morris HR. 1998. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature 392:289–292. doi: 10.1038/32667. [DOI] [PubMed] [Google Scholar]
- 29.Kaslow DC, Quakyi IA, Syin C, Raum MG, Keister DB, Coligan JE, McCutchan TF, Miller LH. 1988. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature 333:74–76. doi: 10.1038/333074a0. [DOI] [PubMed] [Google Scholar]
- 30.Hartigan JA, Wong MJ. 1979. Algorithm AS 136: a K-means clustering algorithm. Appl Stat 28:100–108. doi: 10.2307/2346830. [DOI] [Google Scholar]
- 31.Aminake MN, Schoof S, Sologub L, Leubner M, Kirschner M, Arndt HD, Pradel G. 2011. Thiostrepton and derivatives exhibit antimalarial and gametocytocidal activity by dually targeting parasite proteasome and apicoplast. Antimicrob Agents Chemother 55:1338–1348. doi: 10.1128/AAC.01096-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delves MJ, Ramakrishnan C, Blagborough AM, Leroy D, Wells TN, Sinden RE. 2012. A high-throughput assay for the identification of malarial transmission-blocking drugs and vaccines. Int J Parasitol 42:999–1006. doi: 10.1016/j.ijpara.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Vivas L, Rattray L, Stewart L, Bongard E, Robinson BL, Peters W, Croft SL. 2008. Anti-malarial efficacy of pyronaridine and artesunate in combination in vitro and in vivo. Acta Trop 105:222–228. doi: 10.1016/j.actatropica.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Duparc S, Borghini-Fuhrer I, Craft CJ, Arbe-Barnes S, Miller RM, Shin CS, Fleckenstein L. 2013. Safety and efficacy of pyronaridine-artesunate in uncomplicated acute malaria: an integrated analysis of individual patient data from six randomized clinical trials. Malar J 12:70. doi: 10.1186/1475-2875-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chavalitshewinkoon-Petmitr P, Pongvilairat G, Auparakkitanon S, Wilairat P. 2000. Gametocytocidal activity of pyronaridine and DNA topoisomerase II inhibitors against multidrug-resistant Plasmodium falciparum in vitro. Parasitol Int 48:275–280. doi: 10.1016/S1383-5769(99)00028-8. [DOI] [PubMed] [Google Scholar]
- 36.Delves M, Plouffe D, Scheurer C, Meister S, Wittlin S, Winzeler EA, Sinden RE, Leroy D. 2012. The activities of current antimalarial drugs on the life cycle stages of Plasmodium: a comparative study with human and rodent parasites. PLoS Med 9:e1001169. doi: 10.1371/journal.pmed.1001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buchholz K, Schirmer RH, Eubel JK, Akoachere MB, Dandekar T, Becker K, Gromer S. 2008. Interactions of methylene blue with human disulfide reductases and their orthologues from Plasmodium falciparum. Antimicrob Agents Chemother 52:183–191. doi: 10.1128/AAC.00773-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anthony MP, Burrows JN, Duparc S, Moehrle JJ, Wells TN. 2012. The global pipeline of new medicines for the control and elimination of malaria. Malar J 11:316. doi: 10.1186/1475-2875-11-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dechy-Cabaret O, Benoit-Vical F. 2012. Effects of antimalarial molecules on the gametocyte stage of Plasmodium falciparum: the debate. J Med Chem 55:10328–10344. doi: 10.1021/jm3005898. [DOI] [PubMed] [Google Scholar]
- 40.Chotivanich K, Sattabongkot J, Udomsangpetch R, Looareesuwan S, Day NP, Coleman RE, White NJ. 2006. Transmission-blocking activities of quinine, primaquine, and artesunate. Antimicrob Agents Chemother 50:1927–1930. doi: 10.1128/AAC.01472-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peatey CL, Leroy D, Gardiner DL, Trenholme KR. 2012. Anti-malarial drugs: how effective are they against Plasmodium falciparum gametocytes? Malar J 11:34. doi: 10.1186/1475-2875-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price RN, Nosten F, Luxemburger C, ter Kuile FO, Paiphun L, Chongsuphajaisiddhi T, White NJ. 1996. Effects of artemisinin derivatives on malaria transmissibility. Lancet 347:1654–1658. doi: 10.1016/S0140-6736(96)91488-9. [DOI] [PubMed] [Google Scholar]
- 43.Price RN. 2013. Potential of artemisinin-based combination therapies to block malaria transmission. J Infect Dis 207:1627–1629. doi: 10.1093/infdis/jit079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sutherland CJ, Ord R, Dunyo S, Jawara M, Drakeley CJ, Alexander N, Coleman R, Pinder M, Walraven G, Targett GA. 2005. Reduction of malaria transmission to Anopheles mosquitoes with a six-dose regimen of co-artemether. PLoS Med 2:e92. doi: 10.1371/journal.pmed.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leong FJ, Li R, Jain JP, Lefevre G, Magnusson B, Diagana TT, Pertel P. 2014. A first-in-human randomized, double-blind, placebo-controlled, single- and multiple-ascending oral dose study of novel antimalarial spiroindolone KAE609 (cipargamin) to assess its safety, tolerability, and pharmacokinetics in healthy adult volunteers. Antimicrob Agents Chemother 58:6209–6214. doi: 10.1128/AAC.03393-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Dong Y, Wittlin S, Charman SA, Chiu FC, Chollet J, Katneni K, Mannila J, Morizzi J, Ryan E, Scheurer C, Steuten J, Santo Tomas J, Snyder C, Vennerstrom JL. 2013. Comparative antimalarial activities and ADME profiles of ozonides (1,2,4-trioxolanes) OZ277, OZ439, and their 1,2-dioxolane, 1,2,4-trioxane, and 1,2,4,5-tetraoxane isosteres. J Med Chem 56:2547–2555. doi: 10.1021/jm400004u. [DOI] [PubMed] [Google Scholar]