Abstract
Exposure to biocides may result in cross-resistance to other antimicrobials. Changes in biocide and antibiotic susceptibilities, metabolism, and fitness costs were studied here in biocide-selected Escherichia coli and Klebsiella pneumoniae mutants. E. coli and K. pneumoniae mutants with various degrees of triclosan susceptibility were obtained after exposure to triclosan (TRI), benzalkonium chloride (BKC), chlorhexidine (CHX) or sodium hypochlorite (SHC), and ampicillin or ciprofloxacin. Alterations in antimicrobial susceptibility and metabolism in mutants were tested using Phenotype MicroArrays. The expression of AcrAB pump and global regulators (SoxR, MarA, and RamA) was measured by quantitative reverse transcription-PCR (qRT-PCR), and the central part of the fabI gene was sequenced. The fitness costs of resistance were assessed by a comparison of relative growth rates. Triclosan-resistant (TRIr) and triclosan-hypersusceptible (TRIhs) mutants of E. coli and K. pneumoniae were obtained after selection with biocides and/or antibiotics. E. coli TRIr mutants, including those with mutations in the fabI gene or in the expression of acrB, acrF, and marA, exhibited changes in susceptibility to TRI, CHX, and antibiotics. TRIr mutants for which the TRI MIC was high presented improved metabolism of carboxylic acids, amino acids, and carbohydrates. In TRIr mutants, resistance to one antimicrobial provoked hypersusceptibility to another one(s). TRIr mutants had fitness costs, particularly marA-overexpressing (E. coli) or ramA-overexpressing (K. pneumoniae) mutants. TRI, BKC, and CIP exposure frequently yielded TRIr mutants exhibiting alterations in AraC-like global regulators (MarA, SoxR, and RamA), AcrAB-TolC, and/or FabI, and influencing antimicrobial susceptibility, fitness, and metabolism. These various phenotypes suggest a trade-off of different selective processes shaping the evolution toward antibiotic/biocide resistance and influencing other adaptive traits.
INTRODUCTION
Biocides are commonly used in hospitals, farms, agriculture, a wide variety of industries, and in households for inhibiting or killing potentially pathogenic bacteria (1). Exposure to biocides can cause a decrease in bacterial susceptibility to different antimicrobial compounds due to the adaptive expression of different mechanisms influencing efflux pump activity, membrane permeability, lipid and cell wall synthesis, and cellular respiration (2, 3). Some of these metabolic routes are also involved in stress response, virulence, and even invasiveness (4–11). Frequently, antibiotics and biocides are simultaneously used at homes, hospitals, and farms, which may increase the risk of cross-selection of resistant variants (1, 12–14). Multidrug-resistant (MDR) Enterobacteriaceae, mainly Escherichia coli and Klebsiella pneumoniae, are currently considered a serious public health threat by the Centers for Disease Control and Prevention (CDC) (http://www.cdc.gov/drugresistance/threat-report-2013/), and the use of biocides, such as chlorhexidine (CHX), is now recommended for decontaminating areas of high risk of MDR Enterobacteriaceae (13–15).
The overproduction of efflux pumps is the most commonly described mechanism of resistance to biocides and is associated with low-level resistance to both biocides and antibiotics (β-lactams, chloramphenicol, fluoroquinolones, tetracyclines, and macrolides) (6, 16, 17). AcrAB-like efflux pumps are intrinsically expressed in different species of Enterobacteriaceae; the overexpression of acrAB or acrEF is controlled by global transcriptional regulators, such as MarAB, RamA, and SoxR. Besides controlling the expression of efflux pumps, these regulators also control the levels of porins when they are induced by oxidative stress (18). Besides classical Qac efflux proteins, some SMR-type efflux pumps, such as KpnEF and SugE, have also recently been involved in antibiotic and biocide resistance (19). Furthermore, alterations in FabI, an enoyl-acyl carrier protein reductase that acts on fatty acid synthesis, constitute the main mechanism of resistance to triclosan (TRI) in E. coli; however, cross-resistance to antibiotics has not been proven (20).
The effects of biocides on antibiotic susceptibility, the expression of different pumps, or growth kinetics have been reported mainly in Salmonella enterica strain SL1344 (2, 3, 21). However, only a few studies with limited data have been performed with either E. coli or K. pneumoniae, and less is known in the case of clinical isolates (19, 22–27). The aim of this study was to determine the effect of preexposure to the more commonly used biocides in hospitals and domestic settings (TRI, CHX, benzalkonium chloride [BKC], and sodium hypochlorite [SHC]), or to antibiotics of wide consumption (ampicillin [AMP] and ciprofloxacin [CIP]) on the selection of biocide-resistant variants and to explore the associated changes in metabolism and bacterial growth rate. For this purpose, instead of using classic strains from laboratory collections, which are already “domesticated” and may not have all the capabilities of natural isolates to cope with the exposure to antimicrobials, we used clinical E. coli and K. pneumoniae strains belonging to relevant phylogroups to achieve observations closer to those in the clinical setting.
MATERIALS AND METHODS
Bacterial strains.
The clinical E. coli HEC30 strain belonging to the B23 phylogenetic group, recovered at Ramón y Cajal University Hospital from a blood culture in 2010, and the K. pneumoniae 39.11 strain, belonging to phylogroup KpI, recovered from a urinary tract infection in an outpatient in 2011, were used for the experimental selection of biocide resistance. Phylogroup B2 E. coli comprises human-adapted strains associated with extraintestinal diseases and containing a high number of adaptive traits enhancing its ability to colonize and, often, to infect (28). The K. pneumoniae phylogroup KpI is the most frequent K. pneumoniae phylogenetic group detected in clinical isolates (29). Both isolates investigated in this work were susceptible to both first- and last-line antibiotics, including third-generation cephalosporins, carbapenems, fluoroquinolones, aminoglycosides, and tetracyclines, and also to chloramphenicol. Both isolates were also susceptible to biocides, according to suggested epidemiological cutoff (ECOFF) values (30). The E. coli isolate was resistant to ampicillin, streptomycin, and sulfonamides, while the K. pneumoniae isolate was resistant to ampicillin and fosfomycin, according to EUCAST breakpoints (http://mic.eucast.org/Eucast2/).
Selection of mutants.
As biocides have different degrees of affinity toward multiple bacterial targets, we facilitated the isolation of a variety of diverse mutants by using preconditioning broth cultures with different antimicrobials at subinhibitory concentrations. These cultures were seeded and incubated overnight in rich medium (Columbia blood agar) plates. A single colony from each plate was inoculated into plain Luria-Bertani broth (LB) and into LB supplemented with subinhibitory concentrations (0.5× the MIC) of biocides (TRI, CHX, BKC, and SHC, with one biocide per tube) (Sigma-Aldrich, Inc., St. Louis, MO) or antibiotics (AMP and CIP) and further incubated overnight at 37°C with shaking at 150 rpm. Aliquots of 100 μl were plated onto LB plates, each containing biocide or antibiotic at concentrations between 2.5× and 33× MIC and incubated at 30°C. The plates were examined for growth for 7 days. Three colonies were randomly picked from each plate and stored for subsequent analyses. Colonies capable of growing in plates containing inhibitory concentrations of biocides (TRI, CHX, and BKC) and antibiotics (AMP and CIP) were submitted to subcultures in medium without selection (3 daily passages) to determine whether the observed phenotype was stable or was just due to transient resistance induction after challenge.
As biocides are rarely used for treating infections, there is not a clear definition of resistance based on breakpoints for these compounds. For this reason, we define as resistant any mutant able to grow in plates with a higher biocide concentration than that of the wild-type parental strain for a given biocide (31). Mutants were designed by the abbreviation of the compound used for exposure before plating, followed by the name and concentration of the compound used in the selective plates from which it had been retrieved. Mutants obtained without antimicrobial preexposure were named nonexposed (NE). The morphologies of mutant colonies and their corresponding parental strains were compared in LB and Columbia blood agar plates to establish the colony morphotype. The genomic digestion profiles of diverse mutants and parent strains were also assessed by pulsed-field gel electrophoresis (PFGE) of XbaI-digested genomic DNA (see Fig. S1 and S2 in the supplemental material).
Antimicrobial susceptibility testing.
The susceptibilities of parental and mutant strains against biocides (TRI, CHX, BKC, and SHC) and antibiotics (AMP, CIP, ceftazidime [CAZ], erythromycin [ERY], gentamicin [GEN], chloramphenicol [CLO], and tetracycline [TET]) (bioMérieux, Marcy l'Etoile, France) were determined by both broth microdilution and agar diffusion using Etest strip methods, respectively, according to CLSI guidelines (60). E. coli ATCC 10536 and S. aureus ATCC 6538 were used as control strains (3). Susceptibility to biocides was assessed in at least two independent experiments, and only consistent variations in two or more double dilutions were considered significant for a definition of biocide variants with altered susceptibility.
Susceptibility to 240 cell growth-inhibiting chemical compounds (using the Phenotype MicroArray; Biolog, Hayward, CA, USA) was performed using tryptic soy broth (TSB) and tetrazolium dye at a final concentration of 1%, according to the manufacturer's instructions for Gram-negative bacteria (32). Mutant growth at sub-MICs was performed in 96-well microtiter plates sealed with gas-permeable sealing membranes (Breathe-Easy; Diversified Biotech, Boston, MA) and incubated at 37°C for 24 h in a thermostatic kinetic microplate reader; the data were recorded using an OmniLog instrument (VersaMax; Molecular Devices, Sunnyvale, CA). The optical density at 590 nm (OD590) was measured every 10 min. The plates were gently shaken for 2 s prior to each reading. An increase in the kinetic curve area of 33% was taken as a parameter for the identification of significant phenotypic differences.
Metabolic profile assay.
An analysis of growth using different carbon and nitrogen sources was performed using Biolog Phenotype MicroArray panels PM1 and PM2A for carbon sources and PM3B for nitrogen sources (Biolog, Inc., Hayward, CA, USA). Carbon and nitrogen utilization pathways were tested for a variety of E. coli HEC30 and K. pneumoniae 39.11 mutants and the respective wild-type strains, according to the manufacturer's instructions. The goal was to assess changes in the physiological response associated with biocide resistance in E. coli and K. pneumoniae.
Growth kinetics.
The growth kinetics of 14 mutants with different phenotypes against biocides (5 from E. coli strain HEC30 and 9 from K. pneumoniae strain 39.11) and their corresponding parental strains were measured by inoculating 400 μl of a 1:1,000 dilution of an overnight culture in fresh LB broth (approximately 104 CFU/ml). The samples were analyzed in triplicate in a microtiter plate, and the optical density at 600 nm was measured every 10 min for 24 h in Bioscreen C (Labsystems, Helsinki, Finland) at 37°C, adapting the method described by Foucault et al. (33). The data were averaged for each strain, and the fitness cost (FC) was determined by calculating the relative growth rates.
RNA isolation and qRT-PCR.
The expression of components of the AcrAB, AcrCD, and AcrEF efflux pumps (encoded by the acrB, acrD, and acrF genes, respectively), related to resistance to both biocides and antibiotics, and the expression of global transcriptional factors that regulate the expression of efflux pumps and other pathways (marA, ramA, and soxS) were evaluated by reverse transcription-quantitative PCR (qRT-PCR), using gapA as the reference gene.
Three independent cultures of wild-type strains and their corresponding mutants were grown in 25 ml of plain LB at exponential phase and cooled rapidly on ice before centrifugation. All cultures were analyzed in duplicate. Five milliliters from each duplicate inoculum was mixed and centrifuged at 8,000 rpm and 4°C for 20 min. RNA was isolated by means of the RNeasy commercial kit (Qiagen, GmbH, Hilden, Germany), according to the manufacturer's instructions, treated with Turbo DNase (Ambion) to remove genomic DNA contamination, and cleaned using the Qiagen kit. The quality and concentration of RNA were assessed using a NanoDrop (ND-1000; Labtech). cDNA was synthesized using the High-Capacity cDNA reverse transcriptase kit (Applied Biosystems, Foster City, CA, USA), according to the manufacturer's instructions. The qRT-PCR experiments were carried out in the 7500 and 7300 Fast real-time PCR devices (Applied Biosystems) using Power SYBR green master mix (Applied Biosystems) on 50 ng of initially isolated RNA with 100 nM primers. The primers used are listed in Table 1. The quantities of initial cDNA in each sample were inferred from cycle threshold (CT) values. The data were normalized against both the value of the corresponding reference gene (gapA) and the value of the analyzed gene in the parent strain. The amount of RNA was calculated according to the 2−ΔΔCT method (34).
TABLE 1.
Oligonucleotides used for the study of gene expression in this study
| Species | Gene | Primer directiona | Primer sequence (5′ to 3′) | Amplicon size (bp) | Melting temp (°C) | Annealing temp (°C) |
|---|---|---|---|---|---|---|
| E. coli and K. pneumoniae | acrB | F | TTTGCGTGGGTGATCGC | 192 | 54 | 50 |
| R | GTTATCGATACCGTTCATA | 52 | ||||
| E. coli | acrF | F | TGGCCATTATYCTGATGA | 179 | 50 | 48 |
| R | GTTATCGATACCGTTCATA | 52 | ||||
| K. pneumoniae | F | TTCATCGACCGGTCTTCG | 208 | 56 | 52 | |
| R | AGATTGTCGATGCCGTTC | 54 | ||||
| E. coli and K. pneumoniae | acrD | F | GTGCTGGCAATCCTGTT | 181 | 52 | 50 |
| R | TATCGAGGCCGGTCATAT | 54 | ||||
| E. coli | marA | F | TCATAGCATTTTGGACTGG | 172 | 54 | 52 |
| R | TTGCGCGATTTCCGTCAT | 54 | ||||
| K. pneumoniae | F | AGCGCTCCGGTTACTCTAA | 171 | 58 | 56 | |
| R | GCTGCGACTCGAAACCATA | 58 | ||||
| E. coli | soxS | F | ATTGACCAGCCGCTTAACAT | 197 | 58 | 54 |
| R | ACATAACCCAGGTCCATTG | 56 | ||||
| K. pneumoniae | F | TAGTCGCCAGAAAGTCAGG | 190 | 58 | ||
| R | GAGAAGGTTTGCTGCGAGA | 56 | ||||
| K. pneumoniae | ramA | F | CTGCAACGGCTGTTTTTACA | 180 | 58 | 56 |
| R | ATTGAAGGTCCGGGTGAAG | 58 | ||||
| E. coli | gapA | F | GCTAACCTGAAATGGGACG | 189 | 58 | 56 |
| R | GTCCTGGCCAGCATATTTG | 58 | ||||
| K. pneumoniae | gapA | F | GAAAGGCGTTCTGGGTTAC | 184 | 58 | |
| R | GATGTGGGCAATCAGATCC | 58 |
F, forward; R, reverse.
Analysis of FabI amino acid changes.
Amplified DNA from both parental strains and mutants showing different MICs against TRI were analyzed for mutations in the fabI gene, which codes for the enoyl-acyl carrier protein reductase within bacterial fatty acid synthesis. Primers FabIF (5′-ATGGGTTTTCTTTCCGGTAAG-3′) and FabIR (5′-AATGCTGAANCCGCCGTCAA-3′) were designed using consensus sequences from fabI of E. coli and K. pneumoniae strain MGH 78578. The fabI amplicons (759 bp) were sequenced by the Sanger method (Macrogen Korea, Seoul, Republic of Korea), and both nucleotides and amino acid sequences of the parental strains and their corresponding mutants were aligned by Clustal W for comparison.
RESULTS
Selection of biocide mutants presenting different phenotypes.
Considering only variants with susceptibility changes involving two or more double dilutions, the only biotypes consistently obtained were triclosan resistance or triclosan hypersusceptibility (Table 2). In E. coli, triclosan-resistant (TRIr) variants were recovered from plates that contained TRI, BKC, or CIP after preconditioning broth exposure with TRI, BKC, or CIP and without preexposure to antimicrobials. Similarly, in K. pneumoniae, TRIr variants were obtained not only from plates containing TRI, BKC, or CIP, after being preexposed to subinhibitory concentrations of TRI, BKC, or CIP, but also to SHC, CHX, and AMP. In K. pneumoniae, TRI-hypersusceptible (TRIhs) variants were recovered from plates containing CHX as a selective agent. Of note, half of the TRIr E. coli variants showed slight increases (less than two double dilutions) in susceptibility to CHX (hypersusceptibility to CHX [CHXhs]), which suggests negative epistasis between the mechanisms of resistance to these two compounds. In K. pneumoniae, TRIr variants appeared to be slightly more resistant to both BKC and CHX than E. coli mutants, but the difference with the wild-type strain MIC was not sufficient to ascertain the robustness of these trends under our experimental conditions.
TABLE 2.
Diversity of mutants and their biocide and antibiotic susceptibility
| Species and biocide phenotypea | Preconditioning agentb | Biocide MIC (mg/liter) |
Antibiotic MIC (mg/liter)c |
Mutant designationd | No. of CFU by platee | No. of mutants | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TRI | BKC | CHX | AMP | CAZ | CIP | ERY | GEN | CLO | TET | |||||
| E. coli | ||||||||||||||
| Parental strain HEC30 | 0.06 | 16 | 8 | ≥256 | 0.25 | 0.023 | 16 | 1–1.5 | 6 | 3 | ||||
| TRIr | TRI | 4 | 16 | 2 | ≥256 | 0.13 | 0.032 | 16 | 0.75 | 3 | 3 | Ec_TRI/TRI2 | 2 | 3 |
| CIP | 1 | 32 | 8 | ≥256 | 0.75 | 0.75 | 16 | 1 | 12 | 0.75 | Ec_CIP/CIP | 7 | ||
| BKC | 0.25 | 16 | 8 | ≥256 | 0.5 | 0.032 | 16 | 1.5 | 4–12 | 3 | Ec_BKC/BKC2 | 1 | ||
| TRIr CHXs | NE | 1 | 16 | 4 | ≥256 | 0.38 | 0.023 | 16 | 1–1.5 | 6 | 2 | Ec_NE/TRI1 | UC | 1 |
| K. pneumoniae | ||||||||||||||
| Parental strain 39.11 | 0.5 | 16 | 32 | 32 | 0.38 | 0.047 | 16 | 1 | 2 | 3 | ||||
| TRIr | AMP | 2 | 32 | 64 | 64 | 0.75 | 0.094 | 16 | 1 | 128 | 4 | Kpn_AMP/TRI2 | 7 | 16 |
| NE | 2 | 32 | 64 | 128 | 0.5 | 0.5 | 16 | 1 | >256 | 12 | Kpn_NE/CIP | 126 | ||
| TRI | 2 | 32 | 64 | ≥256 | 0.5 | 0.5 | 16 | 1.5 | >256 | 8 | Kpn_TRI/CIP | 177 | ||
| CHX | 2 | 32 | 64 | ≥256 | 0.38 | 0.38 | 32 | 0.75 | >256 | 8 | Kpn_CHX/CIP | 59 | ||
| BKC | 2 | 32 | 64 | ≥256 | 1 | 0.38 | 96 | 0.75 | 64 | >128 | Kpn_BKC/CIP | 53 | ||
| SHC | 2 | 32 | 64 | 128–192 | 0.75 | 0.5 | 32–48 | 1.5 | >256 | 16–24 | Kpn_SHC/CIP | 31 | ||
| NE | 2 | 32 | 32 | 32 | 0.5 | 0.38 | 16 | 1 | >256 | 4 | Kpn_NE/TRI1 | UC | ||
| TRI | 2 | 32 | 32 | 64 | 0.5 | 0.38 | 12 | 1 | 128 | 6 | Kpn_TRI/TRI2 | 272 | ||
| BKC | 2 | 32 | 32 | 48 | 0.5 | 0.38 | 12 | 1 | 128 | 4 | Kpn_BKC/TRI2 | 12 | ||
| CHX | 2 | 32 | 32 | 32 | 0.5 | 0.5 | 16 | 1 | >256 | 4 | Kpn_CHX/TRI2 | 6 | ||
| CIP | 2 | 32 | 32 | 32 | 0.38 | 0.5 | 32 | 1 | >256 | 24–32 | Kpn_CIP/TRI2 | 20 | ||
| TRI | 2 | 32 | 32 | ≥256 | 0.38 | 0.5 | 16 | 0.5 | >256 | 16–24 | Kpn_TRI/BKC1 | UC | ||
| BKC | 2 | 32 | 32 | ≥256 | 1.5 | 0.38 | 16 | 0.5 | 192 | 32 | Kpn_BKC/BKC1 | UC | ||
| CIP | 2 | 32 | 32 | ≥256 | 1.5 | 0.75 | >256 | 0.19 | 96 | 12 | Kpn_CIP/BKC2 | 11 | ||
| AMP | 2 | 32 | 32 | ≥256 | 0.5 | 0.5 | 16 | 0.5 | >256 | 8 | Kpn_AMP/CIP | 152 | ||
| CIP | 2 | 32 | 32 | 192 | 0.5 | 0.38 | 16 | 1 | >256 | 12 | Kpn_NE/CIP | 126 | ||
| TRIhs | NE | 0.12 | 8 | 32 | 48 | 0.25 | 0.094 | 16 | 1.5 | 3 | 2 | Kpn_NE/CHX2 | 3 | 2 |
| CIP | 0.12 | 16 | 16 | 32 | 0.25–0.38 | 0.094 | 16 | 1 | 4 | 1.5 | Kpn_CIP/CHX2 | UC | ||
TRI, triclosan; CHX, chlorhexidine.
CIP, ciprofloxacin; BKC, benzalkonium chloride; NE, no exposure; AMP, ampicillin; SHC, sodium hypochlorite.
CAZ, ceftazidime; ERY, erythromycin; GEN, gentamicin; CLO, chloramphenicol; TET, tetracycline.
When the selecting agent was TRI, the numbers in the nomenclature of mutants refer to the concentrations of the compounds in the plates as follows: 1 = 1 mg/liter and 2 = 1.5 mg/liter. When the selecting agent was CHX, the concentration in the plate was as follows: 1 = 64 mg/liter or 2 = 128 mg/liter. In the case of BKC, the numbers 1 to 3 refer to the concentrations in plates as follows: 1 = 32 mg/liter, 2 = 64 mg/liter, and 3 = 128 mg/liter. The concentration of CIP was 0.12 mg/liter in all plates.
UC, uncountable.
A large number of colonies of E. coli and K. pneumoniae mutants was obtained in plates supplemented with CIP (either the culture was previously exposed to antibiotics or not), in cases when the same compound was applied during preexposure and plate selection, and when low concentrations of TRI or BKC were used (Table 2).
Antibiotic susceptibility of triclosan mutants.
As described above, TRI resistance was achieved after selection with TRI but also under selection in plates containing BKC or CIP. Table 2 shows the antibiotic susceptibility profile of the TRIr mutants obtained. It is noteworthy that one of the E. coli TRIr variants showing hypersusceptibility to CHX (CHXhs) also presented slightly increased susceptibility to CAZ, GEN, and CLO (Table 2). Some K. pneumoniae TRIr variants showed a remarkable decrease in susceptibility to AMP (1.5- to ≥8-fold MIC increases), CIP (2- to 16-fold), CLO (32- to ≥128-fold), TET (2.6- to ≥16-fold), and CAZ (1.3- to 4-fold). One of them (Kpn_CIP/BKC2) also presented a loss of susceptibility to ERY (≥16-fold) but increased GEN susceptibility (5-fold).
An extended analysis of susceptibility to a wide variety of antimicrobial substances using Phenotype MicroArrays (Biolog, Inc.) was performed not only for TRIr mutants (4 E. coli and 2 K. pneumoniae) but also for mutants for which the MIC of TRI (MICTRI) showed either a slight increase (only a one-dilution difference) or a slight decrease in comparison with that of the wild parental strain (2 E. coli and 3 K. pneumoniae strains, respectively). The parental strains Ec_HEC30 and Kpn_39.11 were also included. The results are shown in Tables S1 and S2 in the supplemental material. Some common patterns were observed, even though the phenotypes of susceptibility were diverse in the different mutants.
All six E. coli TRIr mutants analyzed were more resistant to cefoxitin, cefuroxime, tetracycline, and dodine (a fungicide) but showed enhanced susceptibility to highly diverse antimicrobials, remarkably, those that increase membrane permeability (such as poly-l-lysine, polymyxin B, and metaborate, a herbicide), and to many others. The five K. pneumoniae mutants analyzed (2 TRIr and 3 triclosan susceptible [TRIs]) were more tolerant to penicillin, the tetracycline derivative penimepicycline, and novobiocin and more susceptible to compounds acting on membrane permeability, such as colistin (a polypeptide polycationic antibiotic), methyl viologen or paraquat (oxidant herbicide), and cobalt (a toxic cation). The two TRIr K. pneumoniae mutants (Kpn_CIP/BKC2 and Kpn_TRI/TRI2) exhibited decreased antimicrobial susceptibility to a wide panel of agents (to 57 and 58 chemicals, respectively, with 52 compounds being commonly affected), which comprised several antibiotic classes (see Table S2 in the supplemental material). On the contrary, TRIhs K. pneumoniae variants were mainly susceptible to several more antimicrobials than their parental strain. These results indicate that the acquisition of resistance to biocides can either decrease or increase susceptibility to a wide variety of different cell growth-inhibiting agents.
Acquisition of resistance to biocides alters the physiology of E. coli and K. pneumoniae.
One interesting issue derived from testing some of the analyzed mutants was the possible alteration of their normal physiology. Alterations in colony morphology were observed for some of the TRIr mutants (Ec_CIP/CIP, Kpn_CIP/BKC2, Kpn_AMP/TRI2, and Kpn_TRI/CIP), as reflected in the smaller size exhibited by their colonies in comparison with that of the corresponding parental strains, whereas other TRIr and TRIhs mutants (Kpn_AMP/Tri2, Kpn_BKC/BKC1, and Kpn_SHC/CHX2) formed mucous colonies (see Fig. S1 in the supplemental material) with enhanced biofilm formation (not shown). All of them exhibited the same XbaI-digested genomic DNA profile as their respective parental ancestor, suggesting that the alterations in physiological behavior are not due to large genomic reorganizations (see Fig. S2 in the supplemental material).
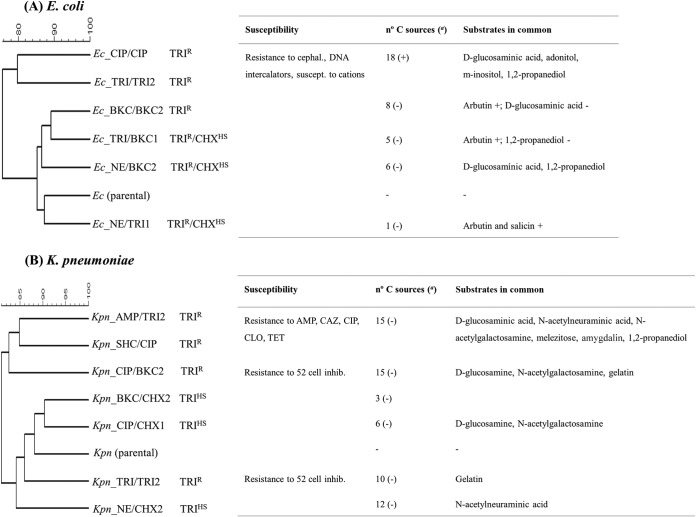
To further understand the effect of acquiring biocide resistance on the physiology of K. pneumoniae and E. coli, the use of carbon sources in each mutant was compared to that in the parental strains. The clustering of metabolic profiles of both the mutants and their respective wild-type strains is shown in Fig. 1 and in Tables S3 and S4 in the supplemental material. Among E. coli TRIr mutants, the most remarkable finding was the enhanced use of d-malic acid by all strains analyzed and the more efficient growth of most mutants in arbutin (β-d-glucopyranoside). Some TRIr mutants (Ec_TRI/TRI2 and Ec_CIP/CIP), characterized by a similar antibiotic susceptibility profile, were able to use some amino acids (hydroxyl-l-proline), carboxylic acids, carbohydrates, and alcohols more efficiently than the parental strain. However, they differ in their ability to metabolize different substrates (adonitol, d-glucosaminic acid, m-inositol, d-psicose, l-lyxose, d-allose, salicin, α-hydroxyglutaric acid γ-lactone, and dihydroxyacetone).
FIG 1.
Cluster of metabolic profiles of biocide mutants and respective wild-type strains of E. coli (A) and K. pneumoniae (B). Clusters of mutants according to their ability to metabolize carbon and nitrogen substrates by the unweighted-pair group method using average linkages (UPGMA) are shown. In the number of carbon (C) sources column, (+) indicates carbon substrates that were used more efficiently by mutants than by parental strains, and (−) indicates carbon substrates that were used less efficiently by mutants than by parental strains. cephal., cephalosporins; suscept., susceptible; inhib., inhibitors.
All K. pneumoniae mutants studied were unable to use Tween 80, γ-cyclodextrin, and 3-methylglucuronic acid compared to the wild-type strain, but almost all metabolized some amides (d-alaninamide), amino acids (l-ornithine and d-aspartic acid), sugars (d-fructose, d-arabinose, N-acetylneuraminic acid, and glycogen), and carboxylic acids (propionic acid) (Fig. 1; see also Table S4 in the supplemental material) better than the parental strain. Two clusters of K. pneumoniae mutants were inferred. One grouped TRIr mutants (Kpn_AMP/TRI2, Kpn_SHC/CIP, and Kpn_CIP/BKC2), which used some sugars (d-melezitose and d-amygdalin), carboxylic acids (citramalic, oxalic, and oxomalic), amino sugar components of peptidoglycan (d-glucosaminic acid and N-acetylneuraminic acid), and alcohols (1,2-propanediol) less efficiently than their parental strain. The second group comprises both TRIr (Kpn_CIP/BKC2) and TRIhs mutants with disparate antibiotic susceptibility but a similar metabolic profile consisting of enhanced use (for ornithine) or diminished use (for β-d-allose, d-melezitose, amygdalin, d-arabinose, and sedoheptulosan) in comparison with that of the wild-type strain. The TRIhs CHXhs mutants metabolized some peptidoglycan amino sugars (d-glucosaminic acid/N-acetyl-d-glucosamine, and N-acetylneuraminic acid) less efficiently than the parental strain. Mutants with remarkable and distinct antibiotic susceptibility changes (Kpn_CIP/BKC2 and Kpn_SHC/CIP) only showed impaired use of l-glucose.
Fitness costs associated with the acquisition of biocide resistance in E. coli and K. pneumoniae.
Various FC values were observed for TRIr mutants of both E. coli (1 to 75%) and K. pneumoniae (4 to 66%) (Table 3). For E. coli, in the TRIr mutants with the highest FC values (26 and 75%, respectively) and increased lag phases, notable changes were shown in the susceptibility to TRI or CIP (10-fold increase in MIC), as was an increased metabolism of some carbon sources (Ec_TRI/TRI2 and Ec_CIP/CIP).
TABLE 3.
Possible mechanisms of resistance and phenotype alterations to biocides
| Species and biocide phenotypea | Colony morphology | Fitness cost (%) | FabI mutation | Differential gene expression | Mutant designationb |
|---|---|---|---|---|---|
| E. coli | |||||
| TRIr | Changed | 75 | NDc | acrF (1.99 ± 0.81) | Ec_CIP/CIP |
| marA (4.31 ± 0.71) | |||||
| Not changed | 26 | 1 nt, Gly93→Val | Not changed | Ec_TRI/TRI2 | |
| Not changed | 6 | ND | acrB (0.26 ± 0.17) | Ec_BKC/BKC2 | |
| acrF (0.18 ± 0.15) | |||||
| marA (0.32 ± 0.18) | |||||
| soxS (0.21 ± 0.18) | |||||
| TRIr CHXhs | Not changed | 1 | 1 nt, Leu94→Phe (E. coli K-12 F)d | Not changed | Ec_NE/TRI1 |
| K. pneumoniae | |||||
| TRIr | Changed | 11 | 0 | ND | Kpn_AMP/TRI2 |
| Not changed | 9 | ND | ND | Kpn_NE/CIP | |
| Changed | 8 | 0 | ND | Kpn_TRI/CIP | |
| Not changed | ND | 0 | ND | Kpn_CHX/CIP | |
| Not changed | ND | ND | ND | Kpn_BKC/CIP | |
| Not changed | 15 | ND | acrF (357.44 ± 246.36) | Kpn_SHC/CIP | |
| Not changed | ND | ND | ND | Kpn_NE/TRI1 | |
| Not changed | 4 | 0 | acrB (62.62 ± 40.01) | Kpn_TRI/TRI2 | |
| acrF (2,923.37 ± 1,101.81) | |||||
| marA (1.64 ± 0.26) | |||||
| Not changed | ND | ND | ND | Kpn_BKC/TRI2 | |
| Not changed | ND | ND | ND | Kpn_CHX/TRI2 | |
| Not changed | ND | ND | ND | Kpn_SHC/TRI2 | |
| Not changed | ND | ND | ND | Kpn_CIP/TRI2 | |
| Not changed | ND | ND | ND | Kpn_TRI/BKC1 | |
| Not changed | ND | ND | ND | Kpn_BKC/BKC1 | |
| Changed | 66 | 0 | ramA (45.01 ± 40.81) | Kpn_CIP/BKC2 | |
| Not changed | ND | ND | ND | Kpn_AMP/CIP | |
| Not changed | 9 | ND | ND | Kpn_NE/CIP | |
| TRIhs | Not changed | NCe | 0 | acrB (0.29 ± 0.11) | Kpn_NE/CHX2 |
| acrF (0.01 ± 0.00) | |||||
| marA (2.21 ± 0.18) | |||||
| Not changed | NC | ND | acrB (35.50 ± 13.52) | Kpn_CIP/CHX2 | |
| acrD (3.02 ± 0.83) | |||||
| marA (0.28 ± 0.13) |
TRI, triclosan; CHX, chlorhexidine.
CIP, ciprofloxacin; BKC, benzalkonium chloride; NE, no exposure; AMP, ampicillin; SHC, sodium hypochlorite.
ND, not determined.
This amino acid change was identified here for the first time.
NC, no cost.
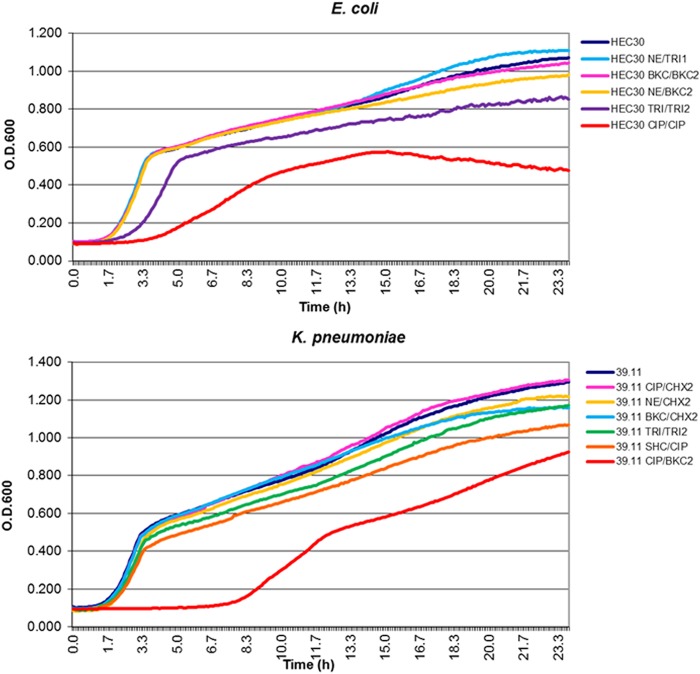
Seven out of 9 TRIr K. pneumoniae mutants showed variable FC (4 to 66%). The Kpn_CIP/BKC2 mutant with striking reduced susceptibility to several antibiotics (CAZ, AMP, CIP, ERY, CLO, and TET) showed the highest FC value and the longest lag phase (Fig. 2). This variant was unique in presenting such an increase in the susceptibility to GEN. Together with other two mutants that demonstrated appreciable FCs (Kpn_AMP/TRI2 and Kpn_SHC/CIP, with FC values of 11 and 15%, respectively), an impaired ability to metabolize a high number of carboxylic acids, amino acids, sugars, and amino sugars involved in peptidoglycan synthesis was observed in comparison with parental strains. Finally, K. pneumoniae TRIhs variants (Kpn_NE/CHX2 and Kpn_CIP/CHX2), with phenotypes of increased susceptibility, showed low FC.
FIG 2.
Growth kinetics of E. coli and K. pneumoniae mutants and their parental strains. The parental strains are HEC30 (E. coli) and 39.11 (K. pneumoniae). The average data are represented in the graphics. Growth rates were determined in plain LB broth at 37°C for 24 h. O.D.600, optical density at 600 nm.
A comparison of the mutants reveals that decreased susceptibility to TRI accompanied by a remarkable decreased susceptibility to CIP or ERY is associated with higher physiological costs in both E. coli and K. pneumoniae. The finding that mutants with high FC show increased metabolism of some carbon sources, in the case of E. coli, or decreased metabolic ability for other substrates, in the case of K. pneumoniae, suggests that FC may depend on the environment and available nutrients.
Gene expression of acrAB and marA, and soxS and ramA.
Gene expression of acrAB, acrEF, and acrCD and marA, soxS, and ramA was evaluated in different TRIr and TRIhs mutants exhibiting different FC values (Table 3). Increased expression of both acrF and marA was detected for one E. coli mutant (Ec_CIP/CIP), with notable changes in the susceptibility to TRI or CIP (10-fold increase in MIC) and an increased metabolism of some carbon sources, as well as a high FC value (26%). The mutant Ec_TRI/TRI2 presented the highest FC and increased metabolism of certain carbon sources, although the expression of the tested genes was not modified. Conversely, decreased expression of acrB, acrF, marA, and soxS (fold changes and standard deviations: 0.26 ± 0.17, 0.18 ± 0.15, 0.32 ± 0.18, and 0.21 ± 0.18, respectively) was observed in a TRIr mutant (4-fold increase in MIC for Ec_BKC/BKC) that showed 6% FC and a 2-fold increase in the MIC of CAZ (MICCAZ).
Mutants with a low fitness cost, corresponding to phenotypes of decreased (4-fold increase in MICTRI for Kpn_TRI/TRI2) and increased susceptibility to TRI (4-fold decrease in MICTRI for Kpn_NE/CHX2 and Kpn_CIP/CHX2), showed increased expression of acrB and acrF and either down- or overexpression of acrB and marA, respectively. The mutant Kpn_SHC/CIP, with a 15% FC, showed increased acrF expression. However, the variant with the highest FC had enhanced expression of the transcriptional regulator ramA (fold change, 45.01 ± 40.81). Overexpression of ramA was previously involved in ciprofloxacin resistance of S. enterica obtained after increasing passages in CIP (35).
Analysis of FabI amino acid changes.
Different amino acid changes were identified in TRIr E. coli mutants with MICs of 4 (Gly93Val in Ec_TRI/TRI2 mutant) and 1 mg/liter (Leu94Phe in Ec_NE/TRI1 mutant), both located in the binding site of FabI and the second reported here for the first time. Another isolate showing up to five mutations did not show any TRI resistance phenotype. Mutations in the fabI gene were not observed in K. pneumoniae mutants.
DISCUSSION
The effect of biocides in antibiotic resistance has been explored in different studies specifically focused on particular situations of cross-resistance or induction of resistance (2, 23, 36–39). In this study, we went one step further and comprehensively analyzed the high diversity of effects exerted by several biocides and antibiotics on the most relevant enterobacterial pathogenic species, E. coli and K. pneumoniae. For this purpose, we studied not only cross-resistance to a limited set of drugs but also other aspects, such as fitness costs and the effect of the selected mutations on susceptibility to a large number of compounds and on bacterial metabolism; these are aspects that were not usually taken into consideration in previous studies in this field. Further, in order to obtain more applicable results, we used clinical isolates instead of the domesticated collection strains regularly used in previous studies on this topic. The biocides tested in this study (the bisphenol TRI, the biguanide CHX, the quaternary ammonium BKC, and the oxidizer SHC) are representatives of the four main classes of biocides commonly used in the food industry, hospitals, farms, and households (39). To the best of our knowledge, this is the first study including preconditioning antimicrobial agents at subinhibitory concentrations in the selection of mutants of E. coli and K. pneumoniae resistant to several biocides and antibiotics (40).
As in other works, TRI was the biocide that led more consistently to the selection of resistant mutants. Also, in accordance with what was already reported, TRI resistance is frequently associated with reduced susceptibility to different antimicrobials (4, 41). The finding that TRI resistance could be selected by low concentrations of either BKC or CIP in addition to TRI is disturbing, due to the extensive use of several of these agents in different settings. Nevertheless, our results show that the acquisition of resistance to this biocide may increase susceptibility to other antimicrobial compounds, which may reflect negative epistasis between the involved mechanisms of resistance. This is also suggested by the fact that TRIr entails more susceptibility to CHX, and TRIhs mutants were selected with CHX, a phenomenon reported for TRIr S. enterica mutants (42). Our results support the presence of different mechanisms simultaneously conferring resistance phenotypes to a diverse number of compounds and enhanced susceptibility to other antimicrobials. Whereas the risks of using biocides for the development of resistance have been discussed in several studies, the possibility that biocides may enhance the activity of some antibiotics has never been explored and is a topic that may require more attention.
This work reports that changes in AraC-like global regulators (MarA, SoxR, and RamA), AcrAB-TolC, and FabI are involved in biocide/antibiotic alterations in susceptibility and result in variations in the metabolism and bacterial fitness of E. coli and K. pneumoniae. Overall, our data indicate that selection by biocides results in the emergence of a complex variety of mutants affecting not only susceptibility to TRI but also to other antimicrobials. Previous studies have documented the involvement of efflux pumps and AraC global regulators on resistance and virulence (43) and the frequent contribution of different mechanisms of resistance to both biocides (such as TRI, BKC, and CHX) and antibiotics (44), some of them associated with metabolic changes (45). In contrast to previous works that are focused on the effect of specific antimicrobials on laboratory strains (46–49), our work is based on the analysis of mutants derived from clinical isolates belonging to predominant E. coli and K. pneumoniae infective clones. A subset of AraC regulators belonging to the AraC/XylS family, including MarA, SoxS, and RarA, confers an MDR phenotype in K. pneumoniae through the upregulation of the AcrAB, AcrCD, and AcrEF efflux pumps and downregulation of the OmpF porin (50). We identified only induced expression of the transcriptional activator ramA, which controls a wealth of genes associated with cellular metabolism and virulence in different species of Enterobacteriaceae (46–49, 51, 52). While MarA, RamA, and SoxS can be simultaneously expressed (down- and/or upregulated) in response to different signals, as seems to have happened in our study, RarA seems to be associated with the increased expression of both the AcrAB and OqxAB efflux pumps in Klebsiella and is seemingly independent of other AraC-type regulators (24). To explain the lack of altered expression of AcrAB-like efflux pumps in some mutants, we cannot discard the possible late expression of these during stationary phase, its expression in the presence of antimicrobials, or even a more remarkable effect on acrA or tolC genes not tested here. Mutants with the highest FC showed increased expression of marA (E. coli) or ramA transcriptional regulators (K. pneumoniae). They correspond to mutants with remarkable changes in their antibiotic susceptibility and metabolic profiles.
Known mechanisms of TRI resistance, such as modification of FabI or modified expression of AcrAB-TolC under the control of specific regulators, such as AcrR, or global regulators, such as MarA and SoxA, have been identified for different E. coli mutants (53, 54). However, mutations in FabI do not completely justify TRIr in E. coli or in K. pneumoniae mutants. Enhanced expression of the acrF gene was observed for TRIr E. coli and K. pneumoniae mutants selected in TRI or CIP showing remarkable changes in susceptibility to a broad range of antimicrobials, which suggests that AcrEF also had a role here, as already reported for Salmonella TRIr mutants (2, 42). While enzymatic modification of FabI alters the synthesis of lipids and membrane permeability, the effects of increased expression of efflux pumps and their regulators may result in an alteration of metabolic pathways linked to enhanced stress response, persistence phenotypes, and eventually, pathogenicity (11, 55, 56). Despite most mutants showing fitness costs when growing under laboratory conditions, some of them have improved capabilities for degrading some compounds, including amino acids and glycosidic carbohydrates, which are present in the intestinal mucus layer. This improved metabolic capability may enhance the ability of such mutants to colonize and eventually infect the host (57). This might be also the case of mutants able to improve the metabolism of 1,2-propanediol, adonitol, d-glucosaminic acid, d-psicose, arbutin, m-inositol, or N-glucosaminic acid (Table 3). 1,2-Propanediol is a three-carbon glycol that is produced during the catabolism of rhamnose and fucose, which are abundant sugars in mammalian glycoconjugates. In this respect, it is worth mentioning that operons of genes for 1,2-propanediol utilization have been identified in Salmonella and Listeria monocytogenes and are suggested to be virulence mechanisms (58). Along the same line, the m-inositol-positive phenotype seems to be highly related to enhanced pathogenicity, as the ability to use some inositol derivatives is associated with an inflammatory host response or fluid secretion (59).
In summary, this work reports the effects of exposure to biocides and antibiotics in selected mutants showing polymorphic changes, including resistance to antimicrobial compounds, differential fitness costs, and changes in the metabolic profiles in E. coli and K. pneumoniae. The complex variety of antimicrobial mechanisms of action for biocides affecting different basic networks of bacterial physiology is consequently mirrored by the complex variety of resistance mechanisms, determining secondary effects on susceptibility to other compounds, metabolism, and ultimately environment-dependent influences on fitness and colonization/virulence.
Supplementary Material
ACKNOWLEDGMENTS
During the execution of this study, T.C. was a recipient of a fellow research contract (grant FI09/00901) from the Instituto Salud Carlos III, Spanish Ministry of Science and Innovation. The research in the authors' laboratories is funded by the European Commission (grant KBBE-2008-2B-227258 to J.L.M. and T.M.C., grant EVOTAR HEALTH-F3-2011-282004 to J.L.M., T.M.C., and F.B.), the Plan Nacional de I+D+i 2008-2011, the Ministry of Economy and Competitiveness (grants BIO2011-25255 and JPIW2013-089-C02-01 to J.L.M.), Instituto de Salud Carlos III (grants PI12-01581 to T.M.C. and PI10-02588 to F.B.), the Spanish Network for Research on Infectious Diseases (grant REIPI RD12/0015 to J.L.M.), the CIBERESP Network for Biomedical Research in Epidemiology and Public Health (grant CIBERESP_CB06/02/0053 to T.C., T.M.C., and F.B.), and the Regional Government of Madrid in Spain (grant PROMPT-S2010/BMD2414 to J.L.M. and F.B.) cofinanced by the European Regional Development Fund [ERDF] “A Way to Achieve Europe.”
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00187-15.
REFERENCES
- 1.McDonnell G, Russell AD. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 12:147–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitehead RN, Overton TW, Kemp CL, Webber MA. 2011. Exposure of Salmonella enterica serovar Typhimurium to high level biocide challenge can select multidrug resistant mutants in a single step. PLoS One 6:e22833. doi: 10.1371/journal.pone.0022833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Randall LP, Cooles SW, Coldham NG, Penuela EG, Mott AC, Woodward MJ, Piddock LJV, Webber MA. 2007. Commonly used farm disinfectants can select for mutant Salmonella enterica serovar Typhimurium with decreased susceptibility to biocides and antibiotics without compromising virulence. J Antimicrob Chemother 60:1273–1280. doi: 10.1093/jac/dkm359. [DOI] [PubMed] [Google Scholar]
- 4.Karatzas KAG, Webber MA, Jorgensen F, Woodward MJ, Piddock LJV, Humphrey TJ. 2007. Prolonged treatment of Salmonella enterica serovar Typhimurium with commercial disinfectants selects for multiple antibiotic resistance, increased efflux and reduced invasiveness. J Antimicrob Chemother 60:947–955. doi: 10.1093/jac/dkm314. [DOI] [PubMed] [Google Scholar]
- 5.Cheung H, Wong MM, Cheung S, Liang LY, Lam Y, Chiu S. 2012. Differential actions of chlorhexidine on the cell wall of Bacillus subtilis and Escherichia coli. PLoS One 7:e36659. doi: 10.1371/journal.pone.0036659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piddock LJV. 2006. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin Microbiol Rev 19:382–402. doi: 10.1128/CMR.19.2.382-402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey AM, Ivens A, Kingsley R, Cottell JL, Wain J, Piddock LJV. 2010. RamA, a member of the AraC/XylS family, influences both virulence and efflux in Salmonella enterica serovar Typhimurium. J Bacteriol 192:1607–1616. doi: 10.1128/JB.01517-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webber MA, Bailey AM, Blair JMA, Morgan E, Stevens MP, Hinton JCD, Ivens A, Wain J, Piddock LJV. 2009. The global consequence of disruption of the AcrAB-TolC efflux pump in Salmonella enterica includes reduced expression of SPI-1 and other attributes required to infect the host. J Bacteriol 191:4276–4285. doi: 10.1128/JB.00363-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heath RJ, Rubin JR, Holland DR, Zhang E, Snow ME, Rock CO. 1999. Mechanism of triclosan inhibition of bacterial fatty acid synthesis. J Biol Chem 274:11110–11114. doi: 10.1074/jbc.274.16.11110. [DOI] [PubMed] [Google Scholar]
- 10.Heath RJ, Yu YT, Shapiro MA, Olson E, Rock CO. 1998. Broad spectrum antimicrobial biocides target the FabI component of fatty acid synthesis. J Biol Chem 273:30316–30320. doi: 10.1074/jbc.273.46.30316. [DOI] [PubMed] [Google Scholar]
- 11.Pérez A, Poza M, Fernández A, Fernández Mdel C, Mallo S, Merino M, Rumbo-Feal S, Cabral MP, Bou G. 2012. Involvement of the AcrAB-TolC efflux pump in the resistance, fitness, and virulence of Enterobacter cloacae. Antimicrob Agents Chemother 56:2084–2090. doi: 10.1128/AAC.05509-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR). 2009. Assessment of the antibiotic resistance effects of biocides. European Commission, Directorate-General for Health & Consumers, Brussels, Belgium: http://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_021.pdf. [Google Scholar]
- 13.Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR). 2010. Research strategy to address the knowledge gaps on the antimicrobial resistance effects of biocides. European Commission, Directorate-General for Health & Consumers, Brussels, Belgium: http://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_028.pdf. [Google Scholar]
- 14.Parliamentary Office of Science & Technology. 2013. Antibiotic resistance in the environment, POST Note no. 446. Parliamentary Office of Science & Technology, Houses of Parliament, London, United Kingdom: http://www.parliament.uk/business/publications/research/briefing-papers/POST-PN-446/antibiotic-resistance-in-the-environment. [Google Scholar]
- 15.Naparstek L, Carmeli Y, Chmelnitsky I, Banin E, Navon-Venezia S. 2012. Reduced susceptibility to chlorhexidine among extremely-drug-resistant strains of Klebsiella pneumoniae. J Hosp Infect 81:15–19. doi: 10.1016/j.jhin.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez-Ortega C, Olivares J, Martínez JL. 2013. RND multidrug efflux pumps: what are they good for? Front Microbiol 4:7. doi: 10.3389/fmicb.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bialek-Davenet S, Marcon E, Leflon-Guibout V, Lavigne J-P, Bert F, Moreau R, Nicolas-Chanoine M-H. 2011. In vitro selection of ramR and soxR mutants overexpressing efflux systems by fluoroquinolones as well as cefoxitin in Klebsiella pneumoniae. Antimicrob Agents Chemother 55:2795–2802. doi: 10.1128/AAC.00156-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viveiros M, Dupont M, Rodrigues L, Couto I, Davin-Regli A, Martins M, Pagès J-M, Amaral L. 2007. Antibiotic stress, genetic response and altered permeability of E. coli. PLoS One 2:e365. doi: 10.1371/journal.pone.0000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srinivasan VB, Rajamohan G. 2013. KpnEF, a new member of the Klebsiella pneumoniae cell envelope stress response regulon, is an SMR-type efflux pump involved in broad-spectrum antimicrobial resistance. Antimicrob Agents Chemother 57:4449–4462. doi: 10.1128/AAC.02284-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMurry LM, Oethinger M, Levy SB. 1998. Triclosan targets lipid synthesis. Nature 394:531–532. doi: 10.1038/28970. [DOI] [PubMed] [Google Scholar]
- 21.Webber MA, Randall LP, Cooles S, Woodward MJ, Piddock LJV. 2008. Triclosan resistance in Salmonella enterica serovar Typhimurium. J Antimicrob Chemother 62:83–91. doi: 10.1093/jac/dkn137. [DOI] [PubMed] [Google Scholar]
- 22.Bailey AM, Constantinidou C, Ivens A, Garvey MI, Webber MA, Coldham N, Hobman JL, Wain J, Woodward MJ, Piddock LJV. 2009. Exposure of Escherichia coli and Salmonella enterica serovar Typhimurium to triclosan induces a species-specific response, including drug detoxification. J Antimicrob Chemother 64:973–985. doi: 10.1093/jac/dkp320. [DOI] [PubMed] [Google Scholar]
- 23.Abuzaid A, Hamouda A, Amyes SGB. 2012. Klebsiella pneumoniae susceptibility to biocides and its association with cepA, qacΔE and qacE efflux pump genes and antibiotic resistance. J Hosp Infect 81:87–91. doi: 10.1016/j.jhin.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 24.De Majumdar S, Veleba M, Finn S, Fanning S, Schneiders T. 2013. Elucidating the regulon of the multidrug resistance regulator RarA in Klebsiella pneumoniae. Antimicrob Agents Chemother 57:1603–1609. doi: 10.1128/AAC.01998-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srinivasan VB, Venkataramaiah M, Mondal A, Vaidyanathan V, Govil T, Rajamohan G. 2012. Functional characterization of a novel outer membrane porin KpnO, regulated by PhoBR two-component system in Klebsiella pneumoniae NTUH-K2044. PLoS One 7:e41505. doi: 10.1371/journal.pone.0041505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grare M, Dibama HM, Lafosse S, Ribon A, Mourer M, Regnouf-de-Vains J-B, Finance C, Duval RE. 2010. Cationic compounds with activity against multidrug-resistant bacteria: interest of a new compound compared with two older antiseptics, hexamidine and chlorhexidine. Clin Microbiol Infect 16:432–438. doi: 10.1111/j.1469-0691.2009.02837.x. [DOI] [PubMed] [Google Scholar]
- 27.Warnke PH, Lott AJS, Sherry E, Wiltfang J, Podschun R. 2013. The ongoing battle against multi-resistant strains: in-vitro inhibition of hospital-acquired MRSA, VRE, Pseudomonas, ESBL E. coli and Klebsiella species in the presence of plant-derived antiseptic oils. J Craniomaxillofac Surg 41:321–326. doi: 10.1016/j.jcms.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Clermont O, Bonacorsi S, Bingen E. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol 66:4555–4558. doi: 10.1128/AEM.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brisse S, Verhoef J. 2001. Phylogenetic diversity of Klebsiella pneumoniae and Klebsiella oxytoca clinical isolates revealed by randomly amplified polymorphic DNA, gyrA and parC genes sequencing and automated ribotyping. Int J Syst Evol Microbiol 51(Pt 3):915–924. doi: 10.1099/00207713-51-3-915. [DOI] [PubMed] [Google Scholar]
- 30.Morrisey I, Oggioni MR, Knight D, Curiao T, Coque TM, Kalkanci A, Martinez JL, BIOHYPO Consortium . 2013. Evaluation of epidemiological cut-off values indicates that biocide resistant subpopulations are uncommon in natural isolates of clinically-relevant microorganisms. PLoS One 9:e86669. doi: 10.1371/journal.pone.086669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martínez JL, Coque TM, Baquero F. 2014. What is a resistance gene? Ranking risk in resistomes. Nat Rev Microbiol 13:116–123. doi: 10.1038/nrmicro3399. [DOI] [PubMed] [Google Scholar]
- 32.Shea A, Wolcott M, Daefler S, Rozak DA. 2012. Biolog phenotype microarrays. Methods Mol Biol 881:331–373. doi: 10.1007/978-1-61779-827-6_12. [DOI] [PubMed] [Google Scholar]
- 33.Foucault M-L, Depardieu F, Courvalin P, Grillot-Courvalin C. 2010. Inducible expression eliminates the fitness cost of vancomycin resistance in enterococci. Proc Natl Acad Sci U S A 107:16964–16969. doi: 10.1073/pnas.1006855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Sun Y, Dai M, Hao H, Wang Y, Huang L, Almofti YA, Liu Z, Yuan Z. 2011. The role of RamA on the development of ciprofloxacin resistance in Salmonella enterica serovar Typhimurium. PLoS One 6:e23471. doi: 10.1371/journal.pone.0023471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCay PH, Ocampo-Sosa AA, Fleming GTA. 2010. Effect of subinhibitory concentrations of benzalkonium chloride on the competitiveness of Pseudomonas aeruginosa grown in continuous culture. Microbiology 156:30–38. doi: 10.1099/mic.0.029751-0. [DOI] [PubMed] [Google Scholar]
- 37.Karatzas KAG, Randall LP, Webber M, Piddock LJV, Humphrey TJ, Woodward MJ, Coldham NG. 2008. Phenotypic and proteomic characterization of multiply antibiotic-resistant variants of Salmonella enterica serovar Typhimurium selected following exposure to disinfectants. Appl Environ Microbiol 74:1508–1516. doi: 10.1128/AEM.01931-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moen B, Rudi K, Bore E, Langsrud S. 2012. Subminimal inhibitory concentrations of the disinfectant benzalkonium chloride select for a tolerant subpopulation of Escherichia coli with inheritable characteristics. Int J Mol Sci 13:4101–4123. doi: 10.3390/ijms13044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Condell O, Iversen C, Cooney S, Power KA, Walsh C, Burgess C, Fanning S. 2012. Efficacy of biocides used in the modern food industry to control Salmonella enterica, and links between biocide tolerance and resistance to clinically relevant antimicrobial compounds. Appl Environ Microbiol 3087–3097. 78:3087–3097. doi: 10.1128/AEM.07534-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanchez P, Moreno E, Martinez JL. 2005. The biocide triclosan selects Stenotrophomonas maltophilia mutants that overproduce the SmeDEF multidrug efflux pump. Antimicrob Agents Chemother 49:781–782. doi: 10.1128/AAC.49.2.781-782.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rensch U, Klein G, Schwarz S, Kaspar H, de Jong A, Kehrenberg C. 2013. Comparative analysis of the susceptibility to triclosan and three other biocides of avian Salmonella enterica isolates collected 1979 through 1994 and 2004 through 2010. J Food Prot 76:653–656. doi: 10.4315/0362-028X.JFP-12-420. [DOI] [PubMed] [Google Scholar]
- 42.Rensch U, Klein G, Kehrenberg C. 2013. Analysis of triclosan-selected Salmonella enterica mutants of eight serovars revealed increased aminoglycoside susceptibility and reduced growth rates. PLoS One 8:e78310. doi: 10.1371/journal.pone.0078310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pérez A, Poza M, Aranda J, Latasa C, Medrano FJ, Tomás M, Romero A, Lasa I, Bou G. 2012. Effect of transcriptional activators SoxS, RobA, and RamA on expression of multidrug efflux pump AcrAB-TolC in Enterobacter cloacae. Antimicrob Agents Chemother 56:6256–6266. doi: 10.1128/AAC.01085-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu BJ, Kim JA, Ju HM, Choi S-K, Hwang SJ, Park S, Kim E, Pan J-G. 2012. Genome-wide enrichment screening reveals multiple targets and resistance genes for triclosan in Escherichia coli. J Microbiol 50:785–791. doi: 10.1007/s12275-012-2439-0. [DOI] [PubMed] [Google Scholar]
- 45.Giraud E, Baucheron S, Virlogeux-Payant I, Nishino K, Cloeckaert A. 2013. Effects of natural mutations in the ramRA locus on invasiveness of epidemic fluoroquinolone-resistant Salmonella enterica serovar Typhimurium isolates. J Infect Dis 207:794–802. doi: 10.1093/infdis/jis755. [DOI] [PubMed] [Google Scholar]
- 46.Bratu S, Landman D, George A, Salvani J, Quale J. 2009. Correlation of the expression of acrB and the regulatory genes marA, soxS and ramA with antimicrobial resistance in clinical isolates of Klebsiella pneumoniae endemic to New York City. J Antimicrob Chemother 64:278–283. doi: 10.1093/jac/dkp186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenblum R, Khan E, Gonzalez G, Hasan R, Schneiders T. 2011. Genetic regulation of the ramA locus and its expression in clinical isolates of Klebsiella pneumoniae. Int J Antimicrob Agents 38:39–45. doi: 10.1016/j.ijantimicag.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruzin A, Visalli MA, Keeney D, Bradford PA. 2005. Influence of transcriptional activator RamA on expression of multidrug efflux pump AcrAB and tigecycline susceptibility in Klebsiella pneumoniae. Antimicrob Agents Chemother 49:1017–1022. doi: 10.1128/AAC.49.3.1017-1022.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Regan E, Quinn T, Pagès J-M, McCusker M, Piddock L, Fanning S. 2009. Multiple regulatory pathways associated with high-level ciprofloxacin and multidrug resistance in Salmonella enterica serovar Enteritidis: involvement of RamA and other global regulators. Antimicrob Agents Chemother 53:1080–1087. doi: 10.1128/AAC.01005-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Veleba M, Higgins PG, Gonzalez G, Seifert H, Schneiders T. 2012. Characterization of RarA, a novel AraC family multidrug resistance regulator in Klebsiella pneumoniae. Antimicrob Agents Chemother 56:4450–4458. doi: 10.1128/AAC.00456-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chollet R, Chevalier J, Bollet C, Pages J-M, Davin-Regli A. 2004. RamA is an alternate activator of the multidrug resistance cascade in Enterobacter aerogenes. Antimicrob Agents Chemother 48:2518–2523. doi: 10.1128/AAC.48.7.2518-2523.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kehrenberg C, Cloeckaert A, Klein G, Schwarz S. 2009. Decreased fluoroquinolone susceptibility in mutants of Salmonella serovars other than Typhimurium: detection of novel mutations involved in modulated expression of ramA and soxS. J Antimicrob Chemother 64:1175–1180. doi: 10.1093/jac/dkp347. [DOI] [PubMed] [Google Scholar]
- 53.McMurry LM, Oethinger MLS, Levy SB. 1998. Triclosan targets lipid synthesis. Nature 94:531–532. [DOI] [PubMed] [Google Scholar]
- 54.McMurry LM, Oethinger MLS, Levy SB. 1998. Overexpression of marA, soxS, or acrAB produces resistance to triclosan in laboratory and clinical strains of Escherichia coli. FEMS Microbiol Lett 166:305–309. doi: 10.1111/j.1574-6968.1998.tb13905.x. [DOI] [PubMed] [Google Scholar]
- 55.Padilla E, Llobet E, Domenech-Sanchez A, Martínez-Martínez L, Bengoechea JA, Alberti S. 2010. Klebsiella pneumoniae AcrAB efflux pump contributes to antimicrobial resistance and virulence. Antimicrob Agents Chemother 54:177–183. doi: 10.1128/AAC.00715-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Casaz P, Garrity-Ryan LK, McKenney D, Jackson C, Levy SB, Tanaka SK, Alekshun MN. 2006. MarA, SoxS and Rob function as virulence factors in an Escherichia coli murine model of ascending pyelonephritis. Microbiology 152:3643–3650. doi: 10.1099/mic.0.2006/000604-0. [DOI] [PubMed] [Google Scholar]
- 57.Chaudhuri RR, Sebaihia M, Hobman JL, Webber MA, Leyton DL, Goldberg MD, Cunningham AF, Scott-Tucker A, Ferguson PR, Thomas CM, Frankel G, Tang CM, Dudley EG, Roberts IS, Rasko DA, Pallen MJ, Parkhill J, Nataro JP, Thomson NR, Henderson IR. 2010. Complete genome sequence and comparative metabolic profiling of the prototypical enteroaggregative Escherichia coli strain 042. PLoS One 5:e8801. doi: 10.1371/journal.pone.0008801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xue J, Murrieta CM, Rule DC, Miller KW. 2008. Exogenous or l-rhamnose-derived 1,2-propanediol is metabolized via a pduD-dependent pathway in Listeria innocua. Appl Environ Microbiol 74:7073–7079. doi: 10.1128/AEM.01074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Norris FA, Wilson MP, Wallis TS, Galyov EE, Majerus PW. 1998. SopB, a protein required for virulence of Salmonella dublin, is an inositol phosphate phosphatase. Proc Natl Acad Sci U S A 95:14057–14059. doi: 10.1073/pnas.95.24.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.CLSI. 2011. Performance standards for antimicrobial susceptibility testing; 21st informational supplement. CLSI document M100-S21 CLSI, Wayne, PA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.