Abstract
Antimicrobial resistance in Enterobacteriaceae, including resistance to carbapenems, is increasing worldwide. However, using U.S. Study for Monitoring Antimicrobial Resistance Trends (SMART) data for 2009 to 2013, no statistically significant decreasing susceptibility trends were found overall for Escherichia coli isolates from patients with intra-abdominal infections. In the subset of isolates from community-associated infections, susceptibility to levofloxacin decreased significantly and the increasing rate of multidrug-resistant E. coli approached statistical significance. In 2013, ertapenem, imipenem, and amikacin showed the highest susceptibility rates (≥99%) and fluoroquinolones the lowest (<70%). The 10 non-ertapenem-susceptible isolates (0.3% of all E. coli isolates) encoded one or more carbapenemases, extended-spectrum β-lactamases (ESBLs), AmpC β-lactamases, or non-ESBL β-lactamases.
TEXT
As Enterobacteriaceae producing extended-spectrum β-lactamases (ESBLs) spread, carbapenems are often considered the treatment of choice for intra-abdominal infections (IAIs) (1–3). However, reports of decreased susceptibility to carbapenems in Enterobacteriaceae, due to carbapenemases or porin deficiency combined with production of ESBLs or AmpC cephalosporinases, are mounting (4–7). Monitoring changes in the susceptibility of Escherichia coli, the most common IAI pathogen, is crucial for decision-making regarding empirical therapy, as well as for efforts to control the spread of ESBLs and carbapenemases. The Study for Monitoring Antimicrobial Resistance Trends (SMART) program has been monitoring IAIs for antimicrobial susceptibility, to assess worldwide trends, since 2002. This report examines trends in the activity of ertapenem and comparator agents against E. coli isolates collected over the past 5 years from patients with IAIs in the United States. Susceptibility is reported for agents recommended in the Surgical Infection Society and the Infectious Diseases Society of America guidelines for the diagnosis and management of complicated intra-abdominal infections (2).
(The results of this report were presented in part as an abstract at IDWeek 2014, Philadelphia, PA.)
Between 2009 and 2013, 29 hospitals in 17 states participated in the SMART program in the United States. A map of the participating states is presented in Fig. S1 in the supplemental material. Participating sites each collected up to 100 consecutive aerobic or facultatively anaerobic Gram-negative IAI pathogens per year. Only one isolate per species per patient was allowed. Of 7,907 IAI isolates, 2,897 (37%) were E. coli. Isolates were identified to the species level and were sent to a central laboratory (International Health Management Associates, Inc., Schaumburg, IL) for susceptibility testing and confirmation of identification. MICs and phenotypic ESBL status were determined by broth microdilution, following the Clinical and Laboratory Standards Institute (CLSI) guidelines, using custom dehydrated MicroScan panels (Siemens Medical Solutions Diagnostics, West Sacramento, CA) (8, 9). MIC interpretive criteria followed 2014 CLSI guidelines (9). As in other studies, multidrug resistance (MDR) was defined as resistance to three or more drug classes (in this study, aminoglycosides, β-lactam/β-lactamase inhibitors, cephems, carbapenems, and quinolones) (10). An IAI was defined as hospital associated or community associated if cultured ≥48 or <48 h postadmission, respectively.
All non-ertapenem-susceptible and >70% of phenotypically ESBL-positive E. coli isolates were molecularly characterized for β-lactamase genes. According to the SMART protocol, 50% of phenotypically ESBL-positive E. coli, Klebsiella pneumoniae, Klebsiella oxytoca, and Proteus mirabilis isolates from each site were to be randomly selected for molecular characterization. However, sometimes additional isolates were characterized for special analyses in support of publications, resulting in an overall final proportion of characterized ESBL-positive isolates that was greater than 50%. Genes encoding ESBLs (TEM, SHV, and CTX-M-type), carbapenemases (KPC, NDM, IMP, VIM, and OXA-48-type), and AmpC β-lactamases (CMY, DHA, FOX, MOX, ACC, MIR, and ACT) were detected using a combination of microarray (Check-MDR CT101; Check-Points B.V., Wageningen, the Netherlands), as described previously (11), and multiplex PCR assays, as described in the supplemental material. Detected genes were sequenced and compared to public databases available from the National Center for Biotechnology Information and the Lahey Clinic. Annual rates of genotypically ESBL-positive isolates were estimated by using as weights the yearly sampling fractions of phenotypically ESBL-positive isolates (i.e., the proportion of phenotypically ESBL-positive isolates that were molecularly characterized each year).
ESBL, MDR, and susceptibility rates were evaluated for linear trends with the Cochran-Armitage test, while trends in MICs were assessed using Pearson's correlations between logarithmically transformed MICs and calendar years. The main analyses included all 2,897 E. coli isolates from all 29 U.S. sites. Sensitivity analyses included only 1,856 isolates from the 12 sites in 9 states that participated in all 5 years. P values of <0.05 were considered statistically significant. Analyses were performed with XLSTAT v2011.1.05.
Of 2,897 E. coli isolates, 69% were from general hospital wards and 17% from intensive care units. Medical wards contributed 44% of isolates and surgical units 36%. Susceptibility, MIC, and prevalence results shown in the tables and figures are for the main analyses using all sites; the statistical test results of the sensitivity analyses are reported in the footnotes. Overall, activities were highest for amikacin, ertapenem, and imipenem, with susceptibility rates being consistently ≥98%; piperacillin-tazobactam and all cephalosporins except for cefotaxime and ceftriaxone demonstrated susceptibility rates of >90%, and the fluoroquinolones showed the lowest susceptibility rates, with rates falling below 70% in 2013 (Table 1). Susceptibility rates did not differ between hospital-associated and community-associated IAIs for amikacin, ertapenem, and imipenem, while small differences were observed for cephalosporins (on average, 1 to 3% lower for hospital-associated infections) and fluoroquinolones (on average, 4% lower). Susceptibility rates appeared fairly stable over the past 5 years, with no statistically significant trends in the main analysis except for a decreasing trend for levofloxacin susceptibility among isolates from community-associated IAIs (P = 0.04) (Table 1); however, this trend was not confirmed in the sensitivity analysis. Decreasing trends for ciprofloxacin and cefotaxime susceptibility among isolates from community-associated IAIs approached significance (P < 0.1) in the main analysis, and the latter was statistically significant in the sensitivity analysis (decreasing from 94% in 2009 to 87% in 2013; P = 0.02). Ertapenem activity also appeared remarkably stable when the MIC distribution was examined (Fig. 1), with no statistical evidence of a shift in MICs (P > 0.05). Furthermore, our data showed no increase in isolates with MICs of 0.25 or 0.5 μg/ml, which is important in light of reports of carbapenemases and other resistance mechanisms increasing carbapenem MICs in Enterobacteriaceae but leaving the isolates susceptible (ertapenem MICs of ≤0.5 μg/ml) (4).
TABLE 1.
Trends in susceptibility to ertapenem and comparators of E. coli isolates from IAIs in the United States in 2009 to 2013
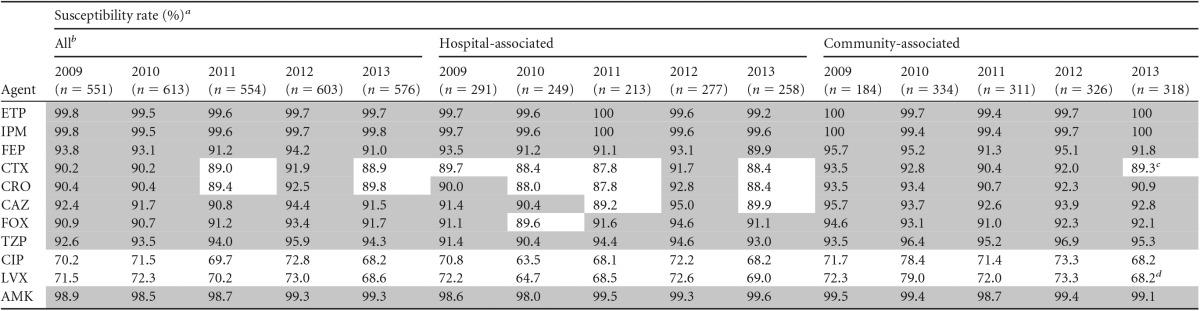
Susceptibility rates of ≥90% are shaded gray. ETP, ertapenem; IPM, imipenem; FEP, cefepime; CTX, cefotaxime; CRO, ceftriaxone; CAZ, ceftazidime; FOX, cefoxitin; TZP, piperacillin-tazobactam; CIP, ciprofloxacin; LVX, levofloxacin; AMK, amikacin.
Includes isolates from hospital-associated and community-associated IAIs, as well as isolates for which the time of collection postadmission was not reported.
Statistically significant decrease in the susceptible proportion in the sensitivity analysis of the 12 continuously participating sites (P < 0.05).
Statistically significant decrease in the susceptible proportion in the main analysis of all 29 sites (P < 0.05).
FIG 1.
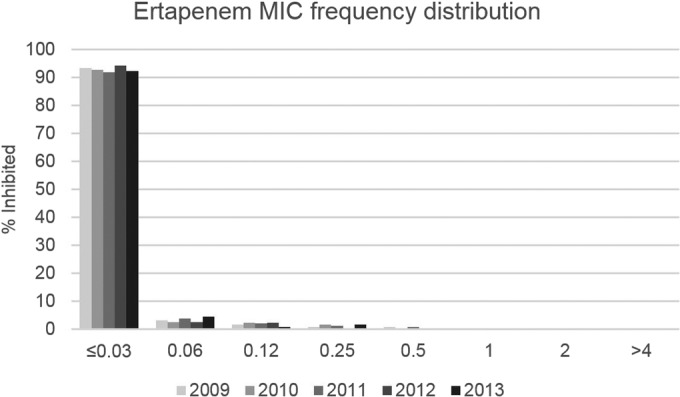
Frequency distribution of ertapenem MICs (in micrograms per milliliter) for E. coli isolates from IAIs in the United States in 2009 to 2013. No statistically significant trends in MICs were noted in the main or sensitivity analyses (P > 0.05). E. coli sample sizes were as follows: 2009, 551 isolates; 2010, 613 isolates; 2011, 554 isolates; 2012, 603 isolates; 2013, 576 isolates.
Between 2009 and 2013, nine isolates were resistant to ertapenem and one exhibited intermediate resistance (total of 0.3% non-ertapenem-susceptible isolates), without evidence of an increasing trend. Molecular characterization of these 10 isolates revealed four KPC carbapenemase producers from New York and Pennsylvania (Table 2); although KPC is usually associated with K. pneumoniae, KPC-producing E. coli isolates were recently noted in the mid-Atlantic region (12). Another four isolates encoded CMY-2 or CTX-M-15 enzymes, which have been reported to cause increased carbapenem MICs in E. coli when expressed at high levels in combination with porin deficiency (13–16). The isolate encoding only CTX-M-71 was carbapenemase negative by the CarbaNP test (17). CTX-M-71 was first observed in a K. pneumoniae isolate that was resistant to ertapenem and meropenem. The purified CTX-M-71 enzyme displayed only weak carbapenemase activity, suggesting that an additional nonenzymatic mechanism (e.g., porin deficiency) was required for the reduction in susceptibility (18). The last isolate encoded only non-ESBL TEM. That isolate displayed MICs of 2 μg/ml for ertapenem and 4 to 8 μg/ml for cefepime in multiple determinations, was susceptible to cefotaxime, ceftazidime, and imipenem, and was not susceptible to cefoxitin and piperacillin-tazobactam. This pattern is similar to that observed by Beceiro et al. for a non-ESBL TEM-1 expressed under the control of a promoter with elevated activity in a porin-deficient E. coli strain (19); however, in contrast to that case, the isolate in this study was not sensitive to the combination of cefepime and clavulanic acid (data not shown), suggesting that additional resistance mechanisms may be involved.
TABLE 2.
MICs and β-lactamases found in 10 non-ertapenem-susceptible isolates from IAIs in the United States in 2009 to 2013
| Year | State | Patient age (yr) | Typea | MIC (μg/ml)b |
Molecular characteristicsc |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ETP | IPM | FEP | CTX | CAZ | FOX | TZP | CIP | AMK | OSBL | ESBL | AmpC | Carbapenemase | ||||
| 2009 | Ohio | 61 | HA | >4 | 8 | 8 | 128 | 128 | >16 | 64 | ≤0.25 | ≤4 | CMY-2 | |||
| 2010 | California | 2 | CA | >4 | >8 | 8 | 128 | >128 | >16 | >64 | 0.5 | ≤4 | TEM | CMY-2 | ||
| 2010 | California | 46 | NA | 1 | 0.25 | >32 | >128 | >128 | >16 | >64 | >2 | 32 | CTX-M-15 | |||
| 2010 | Pennsylvania | 49 | HA | >4 | >8 | >32 | >128 | >128 | >16 | >64 | >2 | ≤4 | SHV-12 | KPC-2 | ||
| 2011 | Pennsylvania | 51 | CA | >4 | 8 | >32 | >128 | 128 | >16 | >64 | >2 | ≤4 | SHV-12 | KPC-2 | ||
| 2011 | New York | 56 | CA | >4 | 4 | >32 | >128 | >128 | >16 | >64 | >2 | >32 | TEM | SHV-5 | KPC-3 | |
| 2012 | California | 55 | HA | 2 | 0.25 | 4 | ≤0.5 | ≤0.5 | 16 | 64 | >2 | 16 | TEM | |||
| 2012 | Georgia | 86 | CA | >4 | 8 | 8 | 32 | 128 | >16 | 64 | >2 | ≤4 | TEM | CMY-2 | ||
| 2013 | Michigan | 67 | HA | 2 | 0.25 | 32 | 32 | 16 | 16 | 32 | >2 | 16 | CTX-M-71 | |||
| 2013 | New York | 80 | HA | >4 | 4 | >32 | >128 | 128 | >16 | >64 | >2 | ≤4 | TEM | KPC-3 | ||
HA, hospital-associated (hospital stay of ≥48 h at the time of specimen collection); CA, community-associated (hospital stay of <48 h); NA, not available.
ETP, ertapenem; IPM, imipenem; FEP, cefepime; CTX, cefotaxime; CAZ, ceftazidime; FOX, cefoxitin; TZP, piperacillin-tazobactam; CIP, ciprofloxacin; AMK, amikacin.
OSBL, original-spectrum β-lactamase; ESBL, extended-spectrum β-lactamase; AmpC, plasmid-encoded class C β-lactamase.
ESBL rates increased slightly from 6.5% in 2009 to 8.0% in 2013, with a faster increase among isolates from community-associated IAIs (from 4.3 to 7.3%), but none of these trends was statistically significant (Fig. 2). Multidrug-resistant (MDR) E. coli presented a similar pattern, with the rate more than doubling from 2009 to 2013 among community-associated infections (Fig. 3). This trend approached statistical significance in both the main analysis (P = 0.10) and the sensitivity analysis (P = 0.07). We cannot explain the decreases in the ESBL and MDR rates seen in 2012. Since these decreases were also found in the sensitivity analyses, they do not appear to be caused by sampling bias due to sites not participating every year. They are likely at least partly due to sampling variations common in surveillance studies, in which the characteristics of a population are estimated by examining a limited subset of that population. The finding underscores the importance of looking at longer-term trends, rather than placing undue significance on individual yearly estimates.
FIG 2.
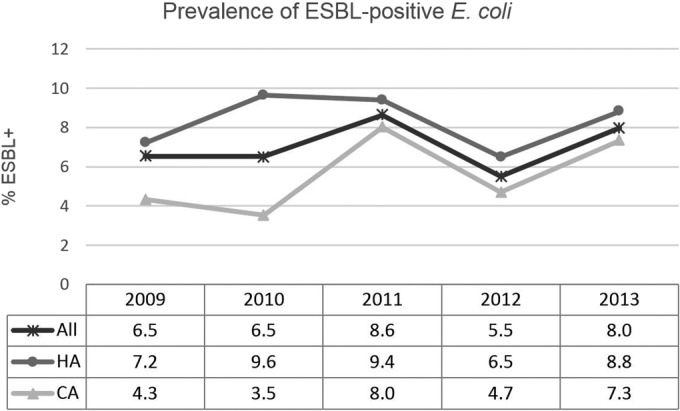
Trends in the prevalence of genotypically ESBL-positive isolates of E. coli from IAIs in the United States in 2009 to 2013. No statistically significant trends were noted in main (all 29 sites) or sensitivity (12 continuously participating sites) analyses (all P > 0.05). HA, hospital-associated; CA, community-associated. E. coli sample sizes (denominators) were as follows: all (including isolates from HA and CA IAIs, as well as isolates for which the time of collection postadmission was not reported), 2009, 551 isolates; 2010, 613 isolates; 2011, 554 isolates; 2012, 603 isolates; 2013, 576 isolates; HA, 2009, 291 isolates; 2010, 249 isolates; 2011, 213 isolates; 2012, 277 isolates; 2013, 258 isolates; CA, 2009, 184 isolates; 2010, 334 isolates; 2011, 311 isolates; 2012, 326 isolates; 2013, 318 isolates.
FIG 3.
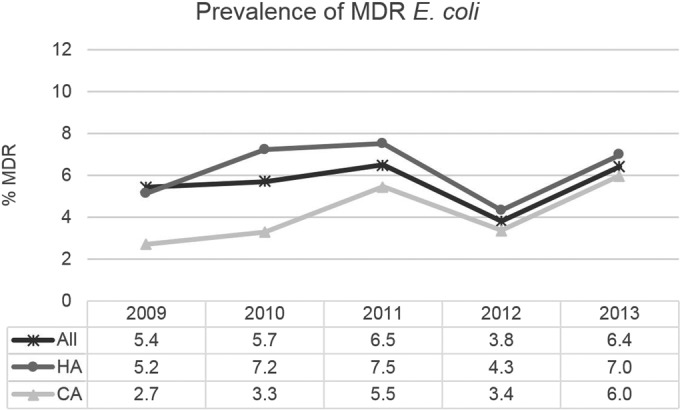
Trends in the prevalence of multidrug-resistant isolates of E. coli from IAIs in the United States in 2009 to 2013. No statistically significant trends were noted in main (all 29 sites) or sensitivity (12 continuously participating sites) analyses (all P > 0.05). MDR, multidrug-resistant; HA, hospital-associated; CA, community-associated. E. coli sample sizes (denominators) were as follows: all (including isolates from HA and CA IAIs, as well as isolates for which the time of collection postadmission was not reported), 2009, 551 isolates; 2010, 613 isolates; 2011, 554 isolates; 2012, 603 isolates; 2013, 576 isolates; HA, 2009, 291 isolates; 2010, 249 isolates; 2011, 213 isolates; 2012, 277 isolates; 2013, 258 isolates; CA, 2009, 184 isolates; 2010, 334 isolates; 2011, 311 isolates; 2012, 326 isolates; 2013, 318 isolates.
This study has limitations. The categorization of IAIs into hospital-associated and community-associated infections based on the length of time between hospital admission and specimen collection is imperfect, since patients may be transferred to a hospital from another health care facility. Nevertheless, publications on various infection types, regions, and time periods using this definition have consistently found higher susceptibility and lower ESBL rates for community-associated infections, thus helping to depict community trends (20–23). Another limitation common in longitudinal surveillance studies is that analyses are often negatively affected by changes in local sites from year to year. Therefore, sensitivity analyses were performed using only continuously participating sites. About one-third of the isolates had to be excluded, leading to smaller sample sizes and less power to find statistical significance. On the other hand, sensitivity analyses may make it easier to discern trends, as the “noise” and diluting effects of sites that enter and leave the study are reduced. The sensitivity analyses performed for this report corroborated the stability of susceptible rates found for most agents, as well as the stability of MICs for ertapenem, and they confirmed the weak statistical evidence of an increase in MDR rates in the community. The decreasing trends in susceptibility to levofloxacin and cefotaxime in the community were found only in either the main analysis or the sensitivity analysis. Both are plausible, considering the spread of E. coli ST131 (24) and the slight increase in ESBL rates, affecting cephalosporins and often being associated with coresistance to other drug classes.
Both globally and in North America, decreasing susceptibility to many drugs of E. coli isolates from IAIs was reported in several studies for the years leading up to 2009/2010 (25–27). In those studies, ertapenem, imipenem, and amikacin were the only tested agents without evidence of activity loss in North America. The current report found that, since 2009, resistance rates have stabilized for almost all drugs, and carbapenems have maintained their excellent activity, despite increasing reports of carbapenem resistance worldwide (4, 6). However, this report did find some evidence of increasing resistance among isolates from community-associated infections, including the decreasing trends in levofloxacin and cefotaxime susceptibility in the main and/or sensitivity analyses, which were statistically significant, the increasing trends in MDR rates, which approached significance, and the slight increase in ESBL-positive isolates, which, although not statistically significant, is worrisome considering other reports of the spread of ESBLs in the community (28, 29). Worldwide reports of increasing resistance and new resistance mechanisms, the mobility of plasmid-mediated resistance, and today's human mobility all combine to underscore the importance of remaining vigilant and continuing surveillance efforts. Surveillance is important at every level (global, national, and local), as resistance patterns and trends help inform empirical treatment decisions and support infection control efforts.
Supplementary Material
ACKNOWLEDGMENTS
This investigation was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. (Whitehouse Station, NJ).
The sponsor approved the overall study design. All investigative sites were recruited and study supplies were provided by International Health Management Associates, Inc. Analyses of the MIC and molecular data were independently performed by International Health Management Associates, Inc.
We thank all of the participants in the SMART program for their continuing contributions to its success.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.05186-14.
REFERENCES
- 1.Vardakas KZ, Tansarli GS, Petros I, Rafailidis PI, Falagas ME. 2012. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum β-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother 67:2793–2803. doi: 10.1093/jac/dks301. [DOI] [PubMed] [Google Scholar]
- 2.Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJC, Baron EJ, O'Neill PJ, Chow AW, Patchen Dellinger E, Eachempai SR, Gorbach S, Hilfiker M, May AK, Nathens AB, Sawyer RG, Bartlett JG. 2010. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect 11:79–109. doi: 10.1089/sur.2009.9930. [DOI] [PubMed] [Google Scholar]
- 3.Malloy AM, Campos JM. 2011. Extended-spectrum beta-lactamases: a brief clinical update. Pediatr Infect Dis J 30:1092–1093. doi: 10.1097/INF.0b013e31823c0e9d. [DOI] [PubMed] [Google Scholar]
- 4.Nordmann P, Naas T, Poirel L. 2011. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 17:1791–1798. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guh AY, Limbago BM, Kallen AJ. 2014. Epidemiology and prevention of carbapenem-resistant Enterobacteriaceae in the United States. Expert Rev Anti Infect Ther 12:565–580. doi: 10.1586/14787210.2014.902306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta N, Limbago BM, Patel JB, Kallen AJ. 2011. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis 53:60–67. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- 7.Jacoby GA, Mills DM, Chow N. 2004. Role of β-lactamases and porins in resistance to ertapenem and other β-lactams in Klebsiella pneumoniae. Antimicrob Agents Chemother 48:3203–3206. doi: 10.1128/AAC.48.8.3203-3206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically—9th ed Approved standard M7-A9 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2014. Performance standards for antimicrobial susceptibility testing; 24th informational supplement. CLSI document M100-S24 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 10.Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 11.Hoban DJ, Lascols C, Nicolle LE, Badal RE, Bouchillon S, Hackel M, Hawser S. 2012. Antimicrobial susceptibility of Enterobacteriaceae, including molecular characterization of extended-spectrum beta-lactamase-producing species, in urinary tract isolates from hospitalized patients in North America and Europe: results from the SMART study 2009–2010. Diagn Microbiol Infect Dis 74:62–67. doi: 10.1016/j.diagmicrobio.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 12.Castanheira M, Farrell SE, Krause KM, Jones RN, Sader HS. 2014. Contemporary diversity of β-lactamases among Enterobacteriaceae in the nine U.S. Census regions and ceftazidime-avibactam activity tested against isolates producing the most prevalent β-lactamase groups. Antimicrob Agents Chemother 58:833–838. doi: 10.1128/AAC.01896-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goessens WH, van der Bij AK, van Boxtel R, Pitout JD, van Ulsen P, Melles DC, Tommassen J. 2013. Antibiotic trapping by plasmid-encoded CMY-2 β-lactamase combined with reduced outer membrane permeability as a mechanism of carbapenem resistance in Escherichia coli. Antimicrob Agents Chemother 57:3941–3949. doi: 10.1128/AAC.02459-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chia JH, Siu LK, Su LH, Lin HS, Kuo AJ, Lee MH, Wu TL. 2009. Emergence of carbapenem-resistant Escherichia coli in Taiwan: resistance due to combined CMY-2 production and porin deficiency. J Chemother 21:621–626. doi: 10.1179/joc.2009.21.6.621. [DOI] [PubMed] [Google Scholar]
- 15.Mammeri H, Guillon H, Eb F, Nordmann P. 2010. Phenotypic and biochemical comparison of the carbapenem-hydrolyzing activities of five plasmid-borne AmpC β-lactamases. Antimicrob Agents Chemother 54:4556–4560. doi: 10.1128/AAC.01762-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adler M, Anjum M, Andersson DI, Sandegren L. 2013. Influence of acquired β-lactamases on the evolution of spontaneous carbapenem resistance in Escherichia coli. J Antimicrob Chemother 68:51–59. doi: 10.1093/jac/dks368. [DOI] [PubMed] [Google Scholar]
- 17.Nordmann P, Poirel L, Dortet L. 2012. Rapid detection of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis doi: 10.3201/eid1809.120355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider I, Queenan AM, Markovska R, Markova B, Keuleyan E, Bauernfeind A. 2009. New variant of CTX-M-type extended-spectrum β-lactamases, CTX-M-71, with a Gly238Cys substitution in a Klebsiella pneumoniae isolate from Bulgaria. Antimicrob Agents Chemother 53:4518–4521. doi: 10.1128/AAC.00461-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beceiro A, Maharjan S, Gaulton T, Doumith M, Soares NC, Dhanji H, Warner M, Doyle M, Hickey M, Downie G, Bou G, Livermore DM, Woodford N. 2011. False extended-spectrum β-lactamase phenotype in clinical isolates of Escherichia coli associated with increased expression of OXA-1 or TEM-1 penicillinases and loss of porins. J Antimicrob Chemother 66:2006–2010. doi: 10.1093/jac/dkr265. [DOI] [PubMed] [Google Scholar]
- 20.Badal R, Morrissey I, Lob S, Biedenbach D, Hackel M, Bouchillon S. 2014. Epidemiology and antimicrobial susceptibility of Gram-negative pathogens causing intra-abdominal infections (IAI) in pediatric patients in Europe— SMART 2010–2013, abstr 104. Abstr 32nd Annu Meet Eur Soc Paediatr Infect Dis. [Google Scholar]
- 21.Lob SH, Badal RE, Hoban DJ, Bouchillon SK, Hackel MA, Biedenbach DJ, Hawser SP, Morrissey I. 2013. Epidemiology and susceptibility of pathogens from hospital- and community-associated urinary tract infection in Latin America: SMART 2010–2012, abstr 1596. Abstr IDWeek 2013 https://idsa.confex.com/idsa/2013/webprogram/Paper41819.html. [Google Scholar]
- 22.Hawser SP, Bouchillon SK, Hoban DJ, Badal RE, Cantón R, Baquero F. 2010. Incidence and antimicrobial susceptibility of Escherichia coli and Klebsiella pneumoniae with extended-spectrum β-lactamases in community- and hospital-associated intra-abdominal infections in Europe: results of the 2008 Study for Monitoring Antimicrobial Resistance Trends (SMART). Antimicrob Agents Chemother 54:3043–3046. doi: 10.1128/AAC.00265-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawser SP, Bouchillon SK, Hoban DJ, Badal RE. 2010. Epidemiologic trends, occurrence of extended-spectrum beta-lactamase production, and performance of ertapenem and comparators in patients with intra-abdominal infections: analysis of global trend data from 2002–2007 from the SMART study. Surg Infect 11:371–378. doi: 10.1089/sur.2009.057. [DOI] [PubMed] [Google Scholar]
- 24.Johnson JR, Urban C, Weissman SJ, Jorgensen JH, Lewis JS II, Hansen G, Edelstein PH, Robicsek A, Cleary T, Adachi J, Paterson D, Quinn J, Hanson ND, Johnston BD, Clabots C, Kuskowski MA, AMERECUS Investigators . 2012. Molecular epidemiological analysis of Escherichia coli sequence type ST131 (O25:H4) and blaCTX-M-15 among extended-spectrum-β-lactamase-producing E. coli from the United States, 2000–2009. Antimicrob Agents Chemother 56:2364–2370. doi: 10.1128/AAC.05824-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hawser SP, Badal RE, Bouchillon SK, Hoban DJ, Biedenbach DJ, Cantón R, Paterson DL. 2013. Monitoring the global in vitro activity of ertapenem against Escherichia coli from intra-abdominal infections: SMART 2002–2010. Int J Antimicrob Agents 41:224–228. doi: 10.1016/j.ijantimicag.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Hawser SP, Badal RE, Bouchillon SK, Hoban DJ. 2011. Trending eight years of in vitro activity of ertapenem and comparators against Escherichia coli from intra-abdominal infections in North America: SMART 2002–2009. J Chemother 23:266–272. doi: 10.1179/joc.2011.23.5.266. [DOI] [PubMed] [Google Scholar]
- 27.Babinchak T, Badal R, Hoban D, Hackel M, Hawser S, Lob S, Bouchillon S. 2013. Trends in susceptibility of selected Gram-negative bacilli isolated from intra-abdominal infections in North America: SMART 2005–2010. Diagn Microbiol Infect Dis 76:379–381. doi: 10.1016/j.diagmicrobio.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 28.Pitout JD. 2013. Enterobacteriaceae that produce extended-spectrum β-lactamases and AmpC β-lactamases in the community: the tip of the iceberg? Curr Pharm Des 19:257–263. doi: 10.2174/138161213804070348. [DOI] [PubMed] [Google Scholar]
- 29.Pitout JD, Nordmann P, Laupland KB, Poirel L. 2005. Emergence of Enterobacteriaceae producing extended-spectrum β-lactamases (ESBLs) in the community. J Antimicrob Chemother 56:52–59. doi: 10.1093/jac/dki166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


