Abstract
Tenofovir disoproxil fumarate (TDF) and entecavir (ETV) are effective antivirals recommended as first-line monotherapies for treatment of chronic hepatitis B (CHB) infection. This study aimed to compare the short-term efficacies of TDF and ETV in the treatment of CHB with severe acute exacerbation. From 2008 to 2013, 189 consecutive treatment-naive CHB patients receiving TDF (n = 41) or ETV (n = 148) for severe acute exacerbation were enrolled. The primary endpoint was overall mortality or receipt of liver transplantation by week 24. The baseline characteristics were comparable between these two groups. By week 24, 8 (19% [95% confidence interval {CI}, 7% to 32%]) patients in the TDF group and 26 (18% [95% CI, 11 to 24%]) patients in the ETV group died (n = 30) or received liver transplantation (n = 4) (P = 0.749). The two groups of patients developed similar rates of liver-related complications and achieved comparable biochemical and virological responses at week 24. Cox regression analysis showed that baseline viral DNA level (P = 0.002), hypertension (P = 0.002), model for end-stage liver disease (MELD) score (P = 0.01), platelet count (P = 0.005), early presence (within 4 weeks) of ascites (P = 0.005), hepatic encephalopathy (P = 0.002), and hepatorenal syndrome (P < 0.001) were independent factors for mortality or liver transplantation. Among the patients who survived by week 24, there was no difference between the two groups in the percentage of patients who had a serum creatinine increase of ≥0.5 mg/dl from baseline (6.7% [95% CI, 0% to 16%] versus 2.0% [95% CI, 0% to 4.8%] in the TDF and ETV groups, respectively; P = 0.231), whereas a significant reduction in the estimated glomerular filtration rate (eGFR) was found in the two groups (P = 0.001 for both). In conclusion, TDF and ETV produce a similar treatment response and clinical outcome in patients with severe acute exacerbation of CHB.
INTRODUCTION
Chronic hepatitis B virus (HBV) infection is a major global health issue, affecting around 370 million people worldwide (1). Patients with chronic hepatitis B (CHB) are at a significantly increased risk for the development of liver failure, cirrhosis, and hepatocellular carcinoma (HCC) (2). In its natural course, up to 30% of CHB patients experience a spontaneous reactivation of hepatitis every year (3). Severe acute exacerbation of CHB characterized by high serum alanine aminotransferase (ALT) level, jaundice, and hepatic decompensation leads to a high mortality rate, ranging from 30 to 70% (4, 5). Most guidelines recommend treatment with oral nucleos(t)ide analogues (NUCs) for CHB patients with severe acute exacerbation as soon as possible (6–8). Liver transplantation is the salvage treatment if medical therapy fails; however, this is neither readily available nor feasible in many parts of the world where HBV is highly endemic (5, 9).
Lamivudine (LAM) was the first effective oral HBV replication-suppressive agent and has been widely used in patients with severe acute exacerbation of CHB (4, 10). However, this therapy is limited by the high risks of virological breakthrough and drug resistance (11). Entecavir (ETV) is a newer potent NUC against HBV, with rare resistance in NUC-naive patients (12). Although the clinical data are inconsistent with regard to the efficacy and safety of ETV in CHB patients with severe acute exacerbation (13–17), recent studies have shown similar rates of short-term mortality between LAM and ETV treatment in such patients (15–17). In particular, our previous study based on a large cohort demonstrated that the choice between ETV and LAM was not an independent factor for mortality in CHB patients with acute exacerbation (17).
Tenofovir disoproxil fumarate (TDF), which has been available since 2008, is another rapidly acting oral NUC that has been shown to be highly effective in suppressing HBV replication (18). TDF has shown excellent activity against HBV in both LAM-naive and LAM-resistant patients (19). In a small randomized controlled study, TDF was shown to significantly reduce HBV DNA levels, improve Child-Turcotte-Pugh (CTP) and model for end-stage liver disease (MELD) scores, and reduce mortality in patients with severe spontaneous reactivation of CHB compared to those factors in the placebo group (20). However, its safety and effectiveness should be further evaluated in more patients.
In this study, we compared the short-term efficacy, safety, and clinical outcomes of severe acute exacerbation in CHB patients treated with TDF or ETV, which have been recommended oral first-line therapies for CHB (6–8).
MATERIALS AND METHODS
Patients.
From January 2008 to December 2013, consecutive CHB patients treated with TDF or ETV who fit the definition of severe acute exacerbation of CHB in single medical center were recruited in this study. Severe acute exacerbation of CHB was defined as an elevation of serum ALT level to ≥5 times the upper limit of normal (ULN) (7), accompanied by a raised serum bilirubin level of ≥3 mg/ml, prolonged prothrombin time of ≥3 s, and/or occurrence of complications, such as ascites or hepatic encephalopathy (4, 17). All patients were positive for hepatitis B surface antigen (HBsAg) for >6 months. Patients who had coinfection with human immunodeficiency virus (HIV), hepatitis A virus, hepatitis C virus (HCV), hepatitis D virus (HDV), or hepatitis E virus by serological assays or had HCC or biliary obstruction by imaging studies at the start of treatment were excluded. Patients who had evidence of drug-induced injury or who received immunosuppressants or systemic corticosteroids were also excluded. Cirrhosis was diagnosed by ultrasound findings as coarse liver parenchyma with nodularity and small liver size, as well as the presence of features of portal hypertension (21).
Treatment and follow-up.
The patients were antiviral treatment naive and received 300 mg of TDF or 0.5 mg of ETV daily. All patients were followed up at weeks 1, 2, 4, 8, and 12 and then every 12 weeks after. The follow-up studies included clinical assessment and conventional biochemical and blood tests. HBV DNA levels were checked at baseline, week 12, and week 24.
The primary endpoint was overall mortality or liver transplantation by week 24. The secondary endpoints included liver-related complications (ascites, variceal bleeding, hepatic encephalopathy, and hepatorenal syndrome). The biochemical response (normalization of ALT and total bilirubin level) and virological response at week 24 were compared between these two groups. Informed consent was obtained from each patient. This study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethics committees of Chang Gung Memorial Hospital, Kaohsiung, Taiwan.
Laboratory assays.
The presence of hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg), anti-HCV antibodies, and anti-HDV antibodies was assessed using commercially available kits (HBsAg enzyme immunoassay [EIA], HBeAg EIA, anti-HCV EIA 3.0, and anti-HDV radioimmunoassay, respectively; all from Abbott, North Chicago, IL). Serum HBV DNA levels were analyzed using the Cobas AmpliPrep-Cobas TaqMan HBV test (CAP-CTM) (Roche Molecular Systems, Inc., Branchburg, NJ, USA), with a lower detection limit of 70 copies/ml. The HBV genotypes were determined using restriction fragment length polymorphism on the surface gene sequence and amplified by PCR with nested primers, as described previously (22).
Statistical analysis.
The continuous data were expressed as the mean ± standard deviation, and the categorical data were expressed as the number (percentage). Comparisons of differences in the categorical data between the groups were performed using the chi-square test or Fisher's exact test. The distributions of the continuous variables were analyzed by the Student t test or Mann-Whitney U test, where appropriate. A paired t test was performed to compare the variables, such as ALT, bilirubin, and HBV DNA levels, and estimated glomerular filtration rate (GFR) in serial measurements. The cumulative incidences of mortality or emergent liver transplantation were analyzed by the Kaplan-Meier method with a log rank test. Univariate and multivariate analyses were carried out to identify independent factors using the Cox proportional hazards regression models. All statistical tests were 2-tailed, and a P value of <0.05 was considered statistically significant.
RESULTS
Baseline characteristics.
Forty-one and 148 patients who fulfilled the inclusion criteria received TDF and ETV treatment, respectively. The baseline characteristics of the study population are shown in Table 1. There were no significant differences regarding the demographic, virological, and laboratory characteristics between these two groups.
TABLE 1.
Comparisons of baseline characteristics between patients treated with tenofovir or entecavir
| Characteristica | Data (mean ± SD or no. [%]) for patients treated with: |
P | |
|---|---|---|---|
| Tenofovir (n = 41) | Entecavir (n = 148) | ||
| Age (yr) | 49.8 ± 13.1 | 50.6 ± 14.7 | 0.758 |
| Male gender | 30 (73) | 106 (72) | 1.000 |
| Body mass index (kg/m2) | 24.1 ± 3.5 | 24.6 ± 4.4 | 0.518 |
| Diabetes mellitus | 7 (17) | 26 (18) | 1.000 |
| Hypertension | 9 (22) | 39 (26) | 0.686 |
| Cirrhosis | 8 (20) | 50 (34) | 0.088 |
| HBV DNA (log10 copies/ml) | 7.0 ± 1.9 | 6.5 ± 1.9 | 0.076 |
| HBeAg positive | 14 (34) | 42 (28) | 0.562 |
| AST (U/liter) | 880 ± 837 | 857 ± 706 | 0.855 |
| ALT (U/liter) | 1,104 ± 918 | 1,084 ± 830 | 0.890 |
| Total bilirubin (mg/dl) | 8.8 ± 7.3 | 10.6 ± 7.7 | 0.172 |
| Albumin (g/dl) | 3.5 ± 0.7 | 3.3 ± 0.6 | 0.060 |
| Creatinine (mg/dl) | 1.0 ± 1.0 | 1.1 ± 1.4 | 0.837 |
| Estimated GFR (MDRD) | 102 ± 48 | 92 ± 33 | 0.118 |
| INR | 1.7 ± 0.7 | 1.7 ± 0.9 | 0.738 |
| Platelet (103/μl) | 139 ± 65 | 144 ± 69 | 0.658 |
| MELD score | 20.0 ± 6.6 | 20.6 ± 6.7 | 0.586 |
HBV, hepatitis B virus; HBeAg, hepatitis B e antigen; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GFR, glomerular filtration rate; MDRD, modification of diet in renal disease (in ml/min/1.73 m2); INR, international normalized ratio of prothrombin time; MELD, model for end-stage liver disease.
Overall mortality or liver transplantation and liver-related complications by week 24.
By week 24, 7 (17% [95% confidence interval {CI}, 5% to 29%]) patients in the TDF group and 23 (16% [95% CI, 10% to 21%]) patients in the ETV group died (Fig. 1A, P = 0.797). Of these, 60% (n = 18) of the deaths occurred in the first month (Table 2). All of the deaths were liver related. In addition, 1 patient (2% [95% CI, 0% to 7%]) in the TDF group and 3 patients (2% [95% CI, 0% to 4%]) in the ETV group received living-donor liver transplantation because of progressive liver failure. The reasons for mortality without transplantation were no available living donor or refusal in 13 patients, old age (>70 years) in 9 patients, history of malignancy in 4 patients, refractory sepsis in 3 patients, and schizophrenia in 1 patient. The cumulative rates of overall mortality or liver transplantation were similar between the TDF and ETV groups (P = 0.749) (Fig. 1B).
FIG 1.
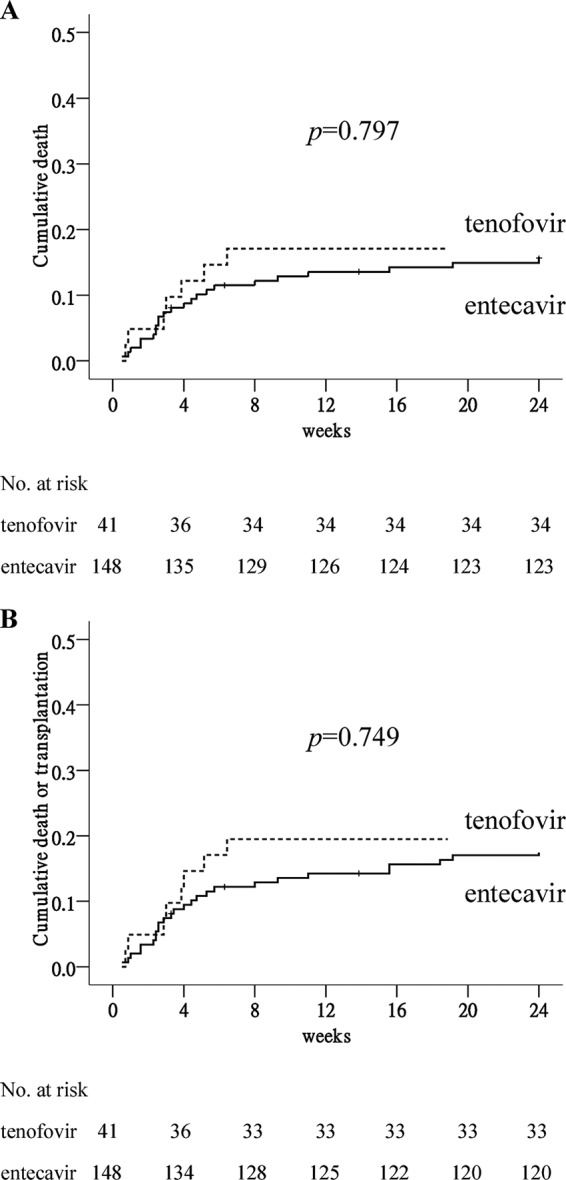
Cumulative rate of overall mortality or liver transplantation by week 24 in patients treated with tenofovir and entecavir. (A) Overall mortality. (B) Overall mortality or liver transplantation.
TABLE 2.
Clinical outcomes of patients with severe acute exacerbation of chronic hepatitis B treated with tenofovir or entecavir
| Outcome | No. (%) in patients treated with: |
P | |
|---|---|---|---|
| Tenofovir (n = 41) | Entecavir (n = 148) | ||
| Liver-related complications | |||
| Ascites | 14 (34) | 49 (33) | 1.000 |
| Within 4 wk | 11 (27) | 40 (27) | |
| Between 4 and 24 wk | 3 (7) | 9 (6) | |
| Hepatic encephalopathy | 7 (17) | 27 (18) | 1.000 |
| Within 4 wk | 5 (12) | 16 (11) | |
| Between 4 and 24 wk | 2 (5) | 11 (7) | |
| Hepatorenal syndrome | 3 (7) | 10 (7) | 1.000 |
| Within 4 wk | 2 (5) | 8 (5) | |
| Between 4 and 24 wk | 1 (2) | 2 (1) | |
| Variceal bleeding | 0 (0) | 2 (1) | 1.000 |
| Death | 7 (17) | 23 (16) | 0.811 |
| Within 4 wk | 5 (12) | 13 (9) | |
| Between 4 and 24 wk | 2 (5) | 10 (7) | |
| Liver transplantation | 1 (2) | 3 (2) | 1.000 |
| Within 4 wk | 1 (2) | 1 (1) | |
| Between 4 and 24 wk | 0 (0) | 2 (1) | |
As shown in Table 2, the patients in the two groups had comparable rates of liver-related complications, including ascites, hepatic encephalopathy, hepatorenal syndrome, and variceal bleeding.
Factors associated with overall mortality or liver transplantation by week 24.
Comparisons between the patients with and without mortality or liver transplantation by week 24 of treatment are shown in Table 3. Old age, diabetes mellitus (DM), hypertension, cirrhosis, higher levels of HBV DNA, bilirubin levels, international normalized ratio (INR) of prothrombin time, and MELD scores, lower levels of albumin, estimated GFR, platelet count, early (within 4 weeks) presence of ascites, hepatic encephalopathy, and hepatorenal syndrome were associated with mortality or liver transplantation. By a Cox proportional hazard model, hypertension (hazard ratio [HR], 3.49; P = 0.002), higher HBV DNA levels (HR, 1.51; P = 0.002), higher MELD scores (HR, 1.10; P = 0.01), lower platelet count (HR, 0.99; P = 0.005), early presence of ascites (HR, 3.35; P = 0.005), hepatic encephalopathy (HR, 4.36; P = 0.002), and hepatorenal syndrome (HR, 7.34; P < 0.001) were independent factors associated with overall mortality or liver transplantation (Table 4).
TABLE 3.
Comparisons of clinical features between patients with and those without mortality or liver transplantation by week 24 of treatment
| Featurea | Data (mean ± SD or no. [%]) for patients with: |
P | |
|---|---|---|---|
| No mortality or transplantation (n = 155) | Mortality or transplantation (n = 34) | ||
| Age (yr) | 47.6 ± 13.6 | 61.9 ± 11.5 | <0.001 |
| Male gender | 114 (74) | 22 (65) | 0.300 |
| Body mass index (kg/m2) | 24.4 ± 3.8 | 25.0 ± 5.9 | 0.474 |
| Diabetes mellitus | 22 (14) | 11 (32) | 0.022 |
| Hypertension | 32 (21) | 16 (47) | 0.002 |
| Cirrhosis | 37 (24) | 21 (62) | <0.001 |
| TDF/ETV | 33/122 | 8/26 | 0.819 |
| HBV DNA (log10 copies/ml) | 6.4 ± 1.9 | 7.3 ± 1.7 | 0.021 |
| HBeAg positive | 50 (32) | 6 (18) | 0.101 |
| HBV genotype B/Cb | 53/17 | 8/0 | 0.189 |
| AST (U/liter) | 858 ± 711 | 879 ± 840 | 0.884 |
| ALT (U/liter) | 1,126 ± 839 | 917 ± 878 | 0.194 |
| Total bilirubin (mg/dl) | 9.5 ± 6.9 | 13.7 ± 9.9 | 0.003 |
| Albumin (g/dl) | 3.4 ± 0.6 | 3.0 ± 0.6 | 0.001 |
| Creatinine (mg/dl) | 1.0 ± 1.1 | 1.5 ± 2.0 | 0.059 |
| Estimated GFR (MDRD) | 98 ± 35 | 78 ± 43 | 0.004 |
| INR | 1.6 ± 0.8 | 2.3 ± 0.9 | <0.001 |
| Platelet (103/μl) | 151 ± 70 | 107 ± 46 | 0.001 |
| MELD score | 19.3 ± 5.3 | 25.9 ± 9.2 | <0.001 |
| Ascitesc | 29 (19) | 22 (65) | <0.001 |
| Hepatic encephalopathyc | 7 (5) | 14 (41) | <0.001 |
| Hepatorenal syndromec | 0 (0) | 10 (29) | <0.001 |
TDF, tenofovir; ETV, entecavir; HBV, hepatitis B virus; HBeAg, hepatitis B e antigen; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GFR, glomerular filtration rate; MDRD, modification of diet in renal disease (in ml/min/1.73 m2); INR, international normalized ratio of prothrombin time; MELD, model for end-stage liver disease.
Available in 78 patients.
Developed within 4 weeks.
TABLE 4.
Univariate and multivariate analyses of factors associated with mortality or liver transplantation by week 24 of treatment
| Factor comparisona | Univariate analyses |
Stepwise multivariate analyses |
||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| Age, per 1 year increase | 1.07 (1.04–1.10) | <0.001 | ||
| Gender, male vs female | 0.711 (0.35–1.44) | 0.342 | ||
| BMI, per 1 kg/m2 increase | 1.03 (0.95–1.11) | 0.509 | ||
| DM, yes vs no | 2.43 (1.18–4.98) | 0.016 | ||
| Hypertension, yes vs no | 2.96 (1.51–5.81) | 0.002 | 3.49 (1.57–7.76) | 0.002 |
| Cirrhosis, yes vs no | 4.15 (2.08–8.30) | <0.001 | ||
| Antiviral drug, TDF vs ETV | 1.14 (0.52–2.51) | 0.750 | ||
| HBV DNA, per log10 copies/ml increase | 1.30 (1.04–1.61) | 0.021 | 1.51 (1.17–1.96) | 0.002 |
| HBeAg positive, yes vs no | 0.47 (0.19–1.12) | 0.089 | ||
| HBV genotype, B vs C | 0.033 (0–38.77) | 0.345 | ||
| ALT, per 1 U/L increase | 1.00 (1.00–1.00) | 0.198 | ||
| Total bilirubin, per 1 mg/dl increase | 1.07 (1.03–1.11) | 0.001 | ||
| Albumin, per 1 g/dl increase | 0.40 (0.23–0.68) | 0.001 | ||
| Creatinine, per 1 mg/dl increase | 1.17 (1.02–1.35) | 0.024 | ||
| INR, increase in ratio | 1.32 (1.13–1.54) | <0.001 | ||
| Platelet, per 103/μl increase | 0.99 (0.98–0.99) | 0.001 | 0.99 (0.98–0.99) | 0.005 |
| MELD, per score | 1.14 (1.09–1.19) | 0.001 | 1.10 (1.02–1.18) | 0.01 |
| Ascites,b yes vs no | 6.06 (3.00–12.27) | <0.001 | 3.35 (1.44–7.81) | 0.005 |
| Hepatic encephalopathy,b yes vs no | 9.16 (4.59–18.27) | <0.001 | 4.36 (1.71–11.09) | 0.002 |
| Hepatorenal syndrome,b yes vs no | 24.06 (10.47–55.30) | <0.001 | 7.34 (2.52–21.43) | <0.001 |
CI, confidence interval; TDF, tenofovir; ETV, entecavir; HBV, hepatitis B virus; HBeAg, hepatitis B e antigen; ALT, alanine aminotransferase; INR, international normalized ratio of prothrombin time; MELD, model for end-stage liver disease.
Developed within 4 weeks.
Biochemical and virological response.
As shown in Fig. 2A, the rates of decline in serum ALT levels were very similar between the TDF and ETV groups. Although bilirubin increased and peaked at week 2 in the TDF group, there was no significant difference in the serum bilirubin levels at each point between these two groups (Fig. 2B). Among the patients who survived by week 24, 26 of 31 (84% [95% CI, 70% to 98%]) in the TDF group had ALT level normalization compared to 93 of 114 (82% [95% CI, 74% to 89%]) patients in the ETV group (P = 1.0). Also, the normalization rate of the serum bilirubin level was not different between these two groups.
FIG 2.
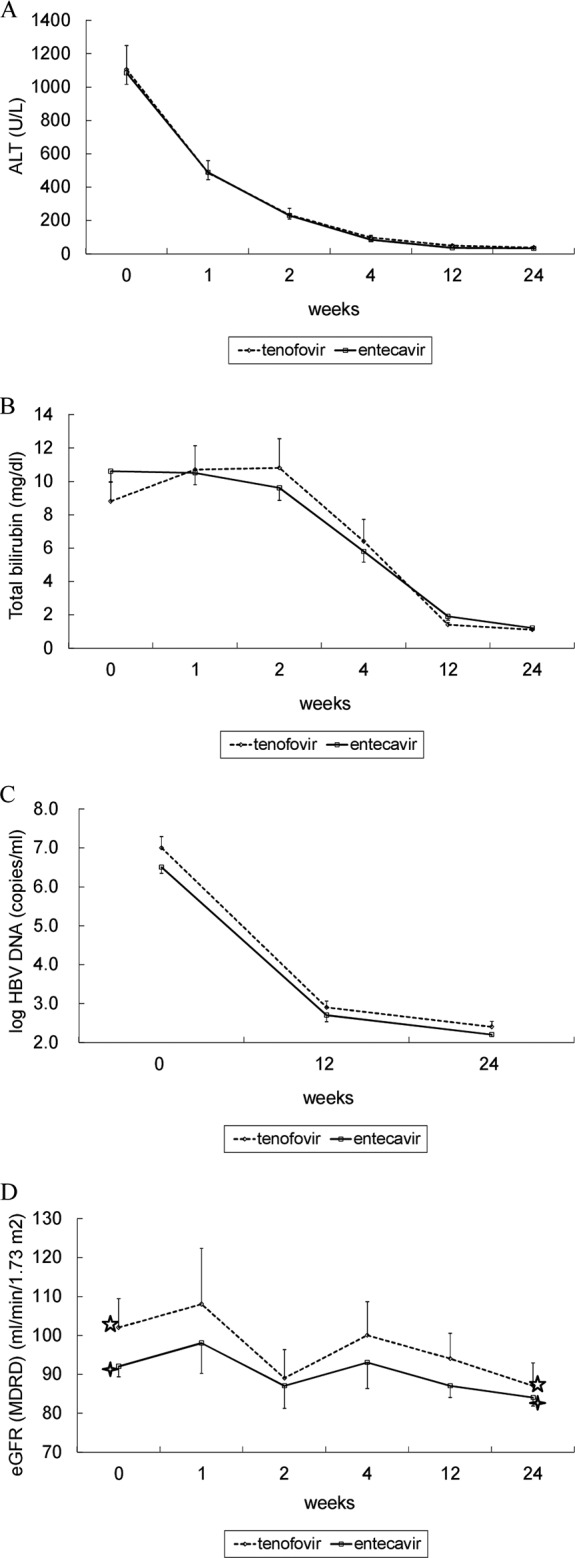
Serial mean ALT (A), total bilirubin (B), HBV DNA (C), and estimated GFR (eGFR) (D) levels by week 24 in patients treated with tenofovir and entecavir. (D) ✰, P < 0.05; ✧, P < 0.05 by paired t test. The data are presented as mean ± standard error of the mean ([SEM]). MDRD, modification of diet in renal disease.
The mean ± standard deviation HBV DNA levels in the TDF group and the ETV group were 2.9 ± 0.8 and 2.7 ± 1.3 log copies/ml at week 12 (P = 0.535) and 2.4 ± 0.7 and 2.2 ± 0.5 log copies/ml at week 24 (P = 0.154), respectively (Fig. 2C). The numbers of patients in the TDF and ETV groups with undetectable HBV DNA levels were 10 of 23 (43% [95% CI, 22% to 65%]) and 54 of 102 (53% [95% CI, 43% to 63%]), respectively, at week 24 (P = 0.491).
Overall renal safety.
There were no patients who discontinued antivirals early due to drug side effects. Among the patients who survived by week 24, 2 of 30 (6.7% [95% CI, 0% to 16%]) patients in the TDF group and 2 of 99 (2.0% [95% CI, 0% to 4.8%]) patients in the ETV group had a confirmed change in serum creatinine from baseline of 0.5 mg/dl at week 24 (P = 0.231). The significant factors associated with serum creatinine increase of ≥0.5 mg/dl from baseline were old age (P = 0.035), hypertension (P = 0.039), and low baseline estimated GFR (P = 0.035).
There was no significant difference in the estimated GFRs between the TDF and the ETV groups at baseline, weeks 1, 2, 4, 12, and 24 (Fig. 2D). However, a significant reduction in the estimated GFR was found at week 24 in both groups (108 to 87 ml/min/1.73 m2, P = 0.001 in the TDF group and 92 to 84 ml/min/1.73 m2, P = 0.001 in the ETV group, respectively) (Fig. 2D).
DISCUSSION
There is growing evidence to suggest that treatment for CHB with severe acute exacerbation or decompensated liver disease should use the most effective NUCs where available (9, 23). Despite the absence of randomized controlled trials with NUCs, they appear to improve survival (mean survival, almost 80%) in CHB patients with severe acute exacerbation compared to that without antiviral therapy (mortality or transplantation rate, nearly 50%) (24). The survival benefit is more evident if therapy starts early enough (before serum bilirubin level rise of >20 mg/dl or a MELD score of ≤30) (4, 25). In addition, a rapid decline in viral load has been considered a predictor of good outcome (25).
TDF and ETV are both effective antiviral agents and have been reported to be well tolerated in patients with decompensated liver disease. However, there are no head-to-head comparisons of TDF and ETV for the treatment of CHB with severe acute exacerbation. Furthermore, little information is available about the clinical efficacy and safety of TDF in such patients (20). In this study, we compared the short-term efficacy, safety, and clinical outcomes of severe acute exacerbation in naive CHB patients treated with TDF or ETV. Our data showed that patients receiving TDF or ETV developed the similar rates of liver-related complications, including ascites, hepatic encephalopathy, hepatorenal syndrome, and variceal bleeding. In addition, the cumulative rates of mortality or liver transplantation by week 24 were comparable between these two groups and were similar to those previously reported in patients treated with ETV (13, 17). These results demonstrated that the efficacies of TDF and ETV were similar in the treatment of naive patients with severe acute exacerbation of CHB. However, in treatment-experienced patients, further studies are necessary to confirm these findings. Although this study was not randomly controlled, our analysis with two groups with very similar baseline characteristics might provide important data for use in clinical practice.
There are some observational studies comparing TDF and ETV in terms of their antiviral responses to CHB (26, 27). These studies reported that the decline in serum HBV DNA levels and HBV DNA negativity rates were not different between TDF and ETV treatments for CHB (26, 27). A recent meta-analysis also confirmed that no differences were observed in the ALT normalization rates and HBeAg seroconversion rates after 24 weeks and 48 weeks of TDF or ETV therapy (28). In our study, we found that TDF and ETV achieved comparable biochemical and virological responses at week 24 in CHB with severe acute exacerbation. Nevertheless, the long-term responses with TDF and ETV should be monitored in prolonged therapy.
Previous studies have identified several important indicators of poor prognosis in CHB with severe exacerbation. These included the presence of cirrhosis, high bilirubin level and INR, high CTP score, high MELD score, low albumin level, and low platelet count (4, 5, 16). Consistent with these studies (4, 5, 16), our patients with mortality or liver transplantation had significantly high baseline HBV DNA level and MELD score, low platelet count, early presence of ascites, hepatic encephalopathy, and hepatorenal syndrome. In particular, metabolic factors, including DM and hypertension, were also significantly associated with higher rates of primary adverse outcomes, although DM was not shown to be an independent variable on multivariate analysis. Currently, there are limited data regarding the relationship between chronic HBV infection and metabolic factors (29, 30). Wong et al. (29) demonstrated that coincidental metabolic syndrome in CHB patients increased the risk of liver fibrosis progression, independent of viral load and hepatitis activity. Another study showed that metabolic factor-related hepatic steatosis was significantly associated with antiviral treatment failure in CHB patients (30). In our study, we provide the first evidence that hypertension is one of the significant factors of poor prognosis in patients with severe acute exacerbation of CHB, and further studies are warranted to explore the possible mechanism of this factor.
Nephrotoxicity may be a concern with TDF, based on evidence from the postmarketing surveillance of patients receiving TDF for HIV infection (31), but so far, this problem seems to be less evident in CHB patients. In clinical trials, creatinine clearance rates remained stable over 4 years, with <1% of CHB patients having confirmed increases of 0.5 mg/dl in serum creatinine levels (32). In comparison, the degree of serum creatinine increase of ≥0.5 mg/dl from baseline appeared to be higher in our patients, with a correspondingly significant decrease in the estimated GFR at week 24. These results might be attributed to a number of pathogenic mechanisms, such as renal hypoperfusion, drug-induced nephrotoxicity, or systemic inflammatory response during severe acute exacerbation of CHB (33). However, there was no significant difference in the estimated GFRs between the TDF and the ETV groups over the treatment course, suggesting that the renal safety of TDF was the same as that of ETV for treating patients with severe acute exacerbation of CHB. Instead, old age, hypertension, and low baseline estimated GFR significantly correlated with a serum creatinine increase of ≥0.5 mg/dl from baseline.
Our study has some limitations, the most important being that the treatment assignment was not done by randomization. Nevertheless, we believed that the bias was small, since the two groups of patients had very similar baseline characteristics. Ideally, a randomized controlled trial to compare the efficacy between entecavir and tenofovir is needed, but such a trial appears to be very difficult to perform, considering that these cases do not occur frequently; therefore, it is almost impossible to have two arms that are adequately numerous and homogenous for statistical evaluation. Second, there was no untreated arm for use as a control in our study. This might be because NUCs can be provided by the National Health Insurance for severe acute exacerbation of CHB in Taiwan; thus, most such patients were already under NUC treatment. Although the number of patients on TDF was relatively small, this study has been the largest cohort with this condition to date.
In conclusion, our data indicate that TDF and ETV produce a similar treatment response and clinical outcome in patients with severe acute exacerbation of CHB. There was no significant difference in terms of renal safety between these two groups. Baseline HBV DNA level, hypertension, MELD score, platelet count, early presence of ascites, hepatic encephalopathy, and hepatorenal syndrome were independent factors affecting primary adverse outcomes by week 24.
ACKNOWLEDGMENT
This study was funded in part by contract grant CMRPG8C0961 from Chang Gung Memorial Hospital, Taiwan.
REFERENCES
- 1.Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, Guan R, Lau GK, Locarnini S, Chronic Hepatitis B Guideline Working Party of the Asian-Pacific Association for the Study of the Liver . 2008. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int 2:263–283. doi: 10.1007/s12072-008-9080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee WM. 1997. Hepatitis B virus infection. N Engl J Med 337:1733–1745. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 3.Lok AS, Lai CL. 1990. Acute exacerbations in Chinese patients with chronic hepatitis B virus (HBV) infection. Incidence, predisposing factors and etiology. J Hepatol 10:29–34. [DOI] [PubMed] [Google Scholar]
- 4.Chien RN, Lin CH, Liaw YF. 2003. The effect of lamivudine therapy in hepatic decompensation during acute exacerbation of chronic hepatitis B. J Hepatol 38:322–327. doi: 10.1016/S0168-8278(02)00419-1. [DOI] [PubMed] [Google Scholar]
- 5.Wong VW, Chan HL. 2009. Severe acute exacerbation of chronic hepatitis B: a unique presentation of a common disease. J Gastroenterol Hepatol 24:1179–1186. doi: 10.1111/j.1440-1746.2009.05924.x. [DOI] [PubMed] [Google Scholar]
- 6.Lok AS, McMahon BJ. 2009. Chronic hepatitis B: update 2009. Hepatology 50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 7.Liaw YF, Kao JH, Piratvisuth T, Chan HL, Chien RN, Liu CJ, Gane E, Locarnini S, Lim SG, Han KH, Amarapurkar D, Cooksley G, Jafri W, Mohamed R, Hou JL, Chuang WL, Lesmana LA, Sollano JD, Suh DJ, Omata M. 2012. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int 6:531–561. doi: 10.1007/s12072-012-9365-4. [DOI] [PubMed] [Google Scholar]
- 8.EASL Clinical Practice Guidelines. 2012. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. European Association for the Study of the Liver. J Hepatol 57:167–185. [DOI] [PubMed] [Google Scholar]
- 9.Jindal A, Kumar M, Sarin SK. 2013. Management of acute hepatitis B and reactivation of hepatitis B. Liver Int 33(Suppl 1):S164–S175. doi: 10.1111/liv.12081. [DOI] [PubMed] [Google Scholar]
- 10.Chan HL, Wong VW, Hui AY, Tsang SW, Chan JL, Chan HY, Wong GL, Sung JJ. 2006. Long-term lamivudine treatment is associated with a good maintained response in severe acute exacerbation of chronic HBeAg-negative hepatitis B. Antivir Ther 11:465–471. [PubMed] [Google Scholar]
- 11.Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, Dienstag JL, Heathcote EJ, Little NR, Griffiths DA, Gardner SD, Castiglia M. 2003. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology 125:1714–1722. doi: 10.1053/j.gastro.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 12.Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, Wichroski MJ, Xu D, Yang J, Wilber RB, Colonno RJ. 2009. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology 49:1503–1514. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 13.Wong VW, Wong GL, Yiu KK, Chim AM, Chu SH, Chan HY, Sung JJ, Chan HL. 2011. Entecavir treatment in patients with severe acute exacerbation of chronic hepatitis B. J Hepatol 54:236–242. doi: 10.1016/j.jhep.2010.06.043. [DOI] [PubMed] [Google Scholar]
- 14.Lange CM, Bojunga J, Hofmann WP, Wunder K, Mihm U, Zeuzem S, Sarrazin C. 2009. Severe lactic acidosis during treatment of chronic hepatitis B with entecavir in patients with impaired liver function. Hepatology 50:2001–2006. doi: 10.1002/hep.23346. [DOI] [PubMed] [Google Scholar]
- 15.Hsu YC, Mo LR, Chang CY, Perng DS, Tseng CH, Lo GH, Tai CM, Lin CW, Hsu CC, Hsu CY, Huang SC, Lin JT. 2012. Entecavir versus lamivudine in the treatment of chronic hepatitis B patients with hepatic decompensation. Antivir Ther 17:605–612. doi: 10.3851/IMP2027. [DOI] [PubMed] [Google Scholar]
- 16.Chen T, He Y, Liu X, Yan Z, Wang K, Liu H, Zhang S, Zhao Y. 2012. Nucleoside analogues improve the short-term and long-term prognosis of patients with hepatitis B virus-related acute-on-chronic liver failure. Clin Exp Med 12:159–164. doi: 10.1007/s10238-011-0160-7. [DOI] [PubMed] [Google Scholar]
- 17.Chen CH, Lin CL, Hu TH, Hung CH, Tseng PL, Wang JH, Chang JY, Lu SN, Chien RN, Lee CM. 2014. Entecavir vs. lamivudine in chronic hepatitis B patients with severe acute exacerbation and hepatic decompensation. J Hepatol 60:1127–1134. doi: 10.1016/j.jhep.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K, Manns M, Kotzev I, Tchernev K, Buggisch P, Weilert F, Kurdas OO, Shiffman ML, Trinh H, Washington MK, Sorbel J, Anderson J, Snow-Lampart A, Mondou E, Quinn J, Rousseau F. 2008. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med 359:2442–2455. doi: 10.1056/NEJMoa0802878. [DOI] [PubMed] [Google Scholar]
- 19.Wong SN, Lok ASF. 2006. Tenofovir disoproxil fumarate: role in hepatitis B treatment. Hepatology 44:309–313. doi: 10.1002/hep.21307. [DOI] [PubMed] [Google Scholar]
- 20.Garg H, Sarin SK, Kumar M, Garg V, Sharma BC, Kumar A. 2011. Tenofovir improves the outcome in patients with spontaneous reactivation of hepatitis B presenting as acute-on-chronic liver failure. Hepatology 53:774–780. doi: 10.1002/hep.24109. [DOI] [PubMed] [Google Scholar]
- 21.Hung CH, Lu SN, Wang JH, Lee CM, Chen TM, Tung HD, Chen CH, Huang WS, Changchien CS. 2003. Correlation between ultrasonographic and pathologic diagnoses of hepatitis B and C virus-related cirrhosis. J Gastroenterol 38:153–157. doi: 10.1007/s005350300025. [DOI] [PubMed] [Google Scholar]
- 22.Hung CH, Chen CH, Lu SN, Wang JH, Hu TH, Huang CM, Tsai MC, Lee CM. 2012. Precore/core promoter mutations and hepatitis B virus genotype in hepatitis B and C dually infected patients treated with interferon-based therapy. Antiviral Res 93:55–63. doi: 10.1016/j.antiviral.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 23.Shouval D. 2014. The pros and cons of lamivudine vs. entecavir in decompensated or severe acute exacerbation of chronic hepatitis B. J Hepatol 60:1108–1109. doi: 10.1016/j.jhep.2014.03.004.. [DOI] [PubMed] [Google Scholar]
- 24.Tillmann HL, Zachou K, Dalekos GN. 2012. Management of severe acute to fulminant hepatitis B: to treat or not to treat or when to treat? Liver Int 32:544–553. doi: 10.1111/j.1478-3231.2011.02682.x. [DOI] [PubMed] [Google Scholar]
- 25.Sun LJ, Yu JW, Zhao YH, Kang P, Li SC. 2010. Influential factors of prognosis in lamivudine treatment for patients with acute-on-chronic hepatitis B liver failure. J Gastroenterol Hepatol 25:583–590. doi: 10.1111/j.1440-1746.2009.06089.x. [DOI] [PubMed] [Google Scholar]
- 26.Ceylan B, Yardimci C, Fincanci M, Eren G, Tozalgan U, Muderrisoglu C, Akkoyunlu Y. 2013. Comparison of tenofovir and entecavir in patients with chronic HBV infection. Eur Rev Med Pharmacol Sci 17:2467–2473. [PubMed] [Google Scholar]
- 27.Doğan ÜB, Kara B, Gümürdülü Y, Soylu A, Akin MS. 2012. Comparison of the efficacy of tenofovir and entecavir for the treatment of nucleos(t)ide-naive patients with chronic hepatitis B. Turk J Gastroenterol 23:247–252. [DOI] [PubMed] [Google Scholar]
- 28.Ke W, Liu L, Zhang C, Ye X, Gao Y, Zhou S, Yang Y. 2014. Comparison of efficacy and safety of tenofovir and entecavir in chronic hepatitis B virus infection: a systematic review and meta-analysis. PLoS One 9:e98865. doi: 10.1371/journal.pone.0098865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, Chan HY, Chan FK, Sung JJ, Chan HL. 2009. Metabolic syndrome increases the risk of liver cirrhosis in chronic hepatitis B. Gut 58:111–117. doi: 10.1136/gut.2008.157735. [DOI] [PubMed] [Google Scholar]
- 30.Jin X, Chen YP, Yang YD, Li YM, Zheng L, Xu CQ. 2012. Association between hepatic steatosis and entecavir treatment failure in Chinese patients with chronic hepatitis B. PLoS One 7:e34198. doi: 10.1371/journal.pone.0034198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duarte-Rojo A, Heathcote EJ. 2010. Efficacy and safety of tenofovir disoproxil fumarate in patients with chronic hepatitis B. Therap Adv Gastroenterol 3:107–119. doi: 10.1177/1756283X09354562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heathcote EJ, Gane EJ, de Man RA. 2010. Long-term (4 year) efficacy and safety of tenofovir disoproxil fumarate (TDF) treatment in HBeAg- positive patients with chronic hepatitis B (study 103). Hepatology 52(Suppl 1):Abstract 477. [Google Scholar]
- 33.Moore JK, Love E, Craig DG, Hayes PC, Simpson KJ. 2013. Acute kidney injury in acute liver failure: a review. Expert Rev Gastroenterol Hepatol 7:701–712. doi: 10.1586/17474124.2013.837264. [DOI] [PubMed] [Google Scholar]


