Abstract
Antibiotic-resistant bacterial infections represent an emerging health concern in clinical settings, and a lack of novel developments in the pharmaceutical pipeline is creating a “perfect storm” for multidrug-resistant bacterial infections. Antimicrobial peptides (AMPs) have been suggested as future therapeutics for these drug-resistant bacteria, since they have potent broad-spectrum activity, with little development of resistance. Due to the unique structure of the lung, bacterial pneumonia has the additional problem of delivering antimicrobials to the site of infection. One potential solution is coadministration of AMPs with exogenous surfactant, allowing for distribution of the peptides to distal airways and opening of collapsed lung regions. The objective of this study was to test various surfactant-AMP mixtures with regard to maintaining pulmonary surfactant biophysical properties and bactericidal functions. We compared the properties of four AMPs (CATH-1, CATH-2, CRAMP, and LL-37) suspended in bovine lipid-extract surfactant (BLES) by assessing surfactant-AMP mixture biophysical and antimicrobial functions. Antimicrobial activity was tested against methillicin-resistant Staphylococcus aureus and Pseudomonas aeruginosa. All AMP/surfactant mixtures exhibited an increase of spreading compared to a BLES control. BLES+CATH-2 mixtures had no significantly different minimum surface tension versus the BLES control. Compared to the other cathelicidins, CATH-2 retained the most bactericidal activity in the presence of BLES. The BLES+CATH-2 mixture appears to be an optimal surfactant-AMP mixture based on in vitro assays. Future directions involve investigating the potential of this mixture in animal models of bacterial pneumonia.
INTRODUCTION
The emergence of highly resistant strains of bacteria currently represents a significant public health issue for patients due to the pervasive use of antibiotics on a global scale. For patients with acute or chronic pulmonary infections where antibiotic use is widespread, such as in cases with cystic fibrosis and ventilator-associated pneumonia, antimicrobial resistance is particularly problematic and is a strong predictor of poor outcomes (1). Furthermore, the distinct structure of the lung, combined with the potential of inaccessible areas due to collapse and edema arising from infections, significantly impairs effective antimicrobial drug delivery in this organ. New, innovative therapeutic approaches to combat lung infections are desperately needed.
Antimicrobial peptides (AMPs) form part of the innate immune system and are evolutionarily conserved across a wide variety of organisms, including humans (2). One class of antimicrobial peptides, cathelicidins, possess antimicrobial function through a variety of mechanisms, including direct interaction with bacterial cell membranes and interference of intracellular bacterial targets (2). The net positive charge of these peptides ensures that they are more likely to interact with the negative cell walls of bacteria than the neutral cellular membranes of eukaryotic cells. Importantly, it has been demonstrated that they remain functional against microbes with antibiotic resistance and, considering their mechanism of action, it is less likely that resistance will develop due to the changes in membrane structure that would be required for effective resistance (2). It has been shown in vitro that AMPs can induce transient resistance, but there is no evidence that this occurs in vivo (3). Based on these properties, antimicrobial peptides have received a lot of attention as alternatives to antibiotics, particularly for topical therapies (4). Utilization for pulmonary infections has been investigated but is complicated by delivery issues and to date has not been successful in clinical trials (5–7).
To address the pulmonary delivery issue, exogenous surfactant has been proposed as carrier for a variety of biological agents (8). Exogenous surfactant is derived from endogenous pulmonary surfactant, a lipoprotein complex naturally produced by type II alveolar cells that is made up of ca. 80 to 85% phospholipids, 5 to 10% neutral lipids (including cholesterol), and 10% proteins (9). The main function of surfactant is to reduce surface tension at the air-liquid interface of the alveoli. To accomplish this goal, surfactant adsorbs and spreads rapidly to form a surface film consisting of lipid-condensed and lipid-expanded regions at the air-liquid interface, which upon lateral compression forms a stable multilayer structure capable of reducing surface tension values to near 0 mN/m (10). Through its ability to spread throughout the lung and open up collapsed lung areas, exogenous surfactant therapy has been shown to reduce mortality of premature infants afflicted with neonatal respiratory distress syndrome (10, 11).
The general characteristics of surfactant and positive findings of exogenous surfactant therapy led to a number of laboratory studies to investigate the possibility of exogenous surfactant as a pulmonary delivery vehicle for other drugs (12–16). The rationale for this approach is that the spreading properties of surfactant would improve therapeutic distribution throughout the lung, while opening collapsed lung regions to improve drug availability directly at the site of an infection (8). Imperative for this approach is that the drug of choice does not impact surfactant's ability to spread throughout the lung and reduce surface tension and, vice versa, that surfactant does not interfere with the therapeutic efficacy of the drug.
Based on this information our objective was to evaluate the spreading, biophysical capabilities, and bactericidal function of four cathelicidin peptides—CATH-1, CATH-2, CRAMP, and LL-37—combined with a clinical exogenous surfactant, bovine lipid-extract surfactant (BLES). Maintenance of surfactant and cathelicidin function when the two compounds are combined would provide a first proof of principle toward the hypothesis that a combination treatment of cathelicidins with an exogenous surfactant vehicle would be effective in treating antibiotic-resistant lung infections.
MATERIALS AND METHODS
Surfactant/peptide compounds.
BLES (BLES Biochemicals, London, Ontario, Canada) is a commercially available clinical preparation that is stored in 100 mM sodium chloride and 1.5 mM calcium chloride with a phospholipid concentration of 27 mg/ml. BLES contains all natural phospholipids found in bovine surfactant, along with surfactant-specific proteins SP-B and SP-C and a small percentage of cholesterol.
Antimicrobial peptides were synthesized using Fmoc (9-fluorenylmethoxy carbonyl) solid-phase synthesis as described previously (17). All peptides were purified to a minimum purity of 95% by reversed-phase high-performance liquid chromatography prior to biological testing. The sequences of the peptides used in the present study are shown in Table S1 in the supplemental material. Four AMPs were tested in this experiment: chicken cathelicidins 1 and 2 (CATH-1 and CATH-2), mouse cathelicidin CRAMP, and human cathelicidin LL-37. All peptides were suspended in nonbuffered sterile saline. BLES (suspended in phosphate-buffered saline [PBS]) and peptides were mixed to concentrations of 10 mg/ml from BLES and 10, 40, 100, and 200 μM for the peptides.
Spreading.
Adsorption of BLES and the various peptides were measured using the Wilhelmy probe and FilmWare 2.51 software of the Langmuir balance. A petri dish with a diameter of 100 mm, and surface area of 78.54 cm2 was used. It was filled with 25 ml of 10% sucrose, with a 15-ml layer of double-distilled H2O (ddH2O) layered on top. The probe of the Langmuir balance was placed on the surface of the ddH2O subphase, and 50 μl of each the various peptide surfactant samples was pipetted into the water phase. The surface tension was recorded over the subsequent 600 s.
Minimal surface tension over multiple compression-expansion cycles.
To investigate the minimum achievable surface tension over 10 compression-expansion cycles, a constrained sessile drop surfactometer (CDS; BioSurface Instruments, HI) was used (18–20). A 10-μl drop of sample was placed upon the drop pedestal and, using an external stepper motor, the drop was cyclically compressed and expanded for 10 cycles at a rate of 5 s/cycle and a compression ratio of ca. 27%. Images of the droplet were recorded at a rate of one image per second and analyzed. The image with the lowest surface tension throughout each cycle was determined to be the minimum surface tension (MST) for that cycle. Minimum surface tensions for all 10 cycles were measured.
AFM imaging of surface films.
In order to examine the effect of peptides on the surfactant surface films, Langmuir-Blodgett films were prepared using a Kibron Trough SE (Helsinki, Finland). Briefly, films were spread by depositing droplets of surfactant samples uniformly throughout the air-water interface. Deposits were taken at surface pressure of 30 mN/m on freshly cleaved mica. Topographical atomic force microscope (AFM) images were obtained using a Nanoscope III scanning force multimode microscope (Digital Instruments, Santa Barbara, CA). Samples were scanned with a J-type scanner using contact mode in air. A silicon nitride cantilever with a spring constant of 0.12 N/m was used. Image analysis was performed using the Nanoscope III software (version 5.12r3). All AFM images were subjected to quantitative analysis by using ImageJ software (National Institutes of Health) to determine the surface area of condensed domains. At least nine AFM images were used for each sample, and the results are shown as averages and standard errors.
Antimicrobial assays.
An overnight culture of either methillicin-resistant Staphylococcus aureus WKZ-2 or Pseudomonas aeruginosa VW178 (isolated from a cystic fibrosis patient) was diluted 1/1,000 in tryptone soy broth and incubated for 3 h at 37°C in order to reach exponential growth phase. The optical density was measured, and bacteria were further diluted in PBS to reach an initial concentration of approximately 2 × 106 CFU/ml. From there, 25 μl of peptides at various concentrations, with or without BLES, were added to polypropylene coated round-bottom 96-well plates, along with 25 μl of bacteria, followed by incubation at 37°C for 3 h with no shaking. Subsequently, 200 μl of minimal medium was added, and the colonies were serially diluted 10- to 1,000-fold. After the serial dilution, 100 μl of each dilution was plated on tryptone soy agar plates and left to incubate overnight at 37°C. After overnight incubation, the colonies on the plates were counted. MICs were defined for these experiments as the concentration of surfactant/peptide compound at which fewer than 10 colonies were counted at the highest dilution, corresponding to fewer than 100 CFU/ml.
In vivo analysis of safety and tolerability of BLES+CATH-2 treatment.
Male C57BL/6 mice (Charles River, Sherbrooke, Quebec, Canada), weighing 22 to 30 g, were used for this experiment. All animal procedures were approved by the Animal Use Subcommittee at the University of Western Ontario and followed the approved guidelines described by the Canadian Council of Animal Care. Mice were anesthetized by intraperitoneal injection of a ketamine (130 mg/kg of body weight [BW]) and dexmedetomidine (0.5 mg/kg BW) and then intubated using an 20 G catheter, with the aid of a fiber-optic stylet (BioLite intubation system for small rodents, BioTex, Inc., Houston, TX). Once intubated, the mice were instilled with 50 μl of one of five treatments: (i) air bolus, (ii) sterile saline, (iii) BLES (10 mg of phospholipid/ml), (iv) CATH-2 (100 μM, suspended in sterile saline), or (v) BLES+CATH-2 (10 mg of phospholipid/ml, 100 μM peptide). Mice were extubated after successful instillation and were subsequently injected with the reversal agent for dexmedetomidine, Antisedan, and allowed to breathe spontaneously for the following 4 h. After 4 h, the mice were euthanized by intraperitoneal injection of sodium pentobarbital and dissection of the descending aorta. The animals were placed on a FlexiVent (SCIREQ, Montreal, Quebec, Canada) in order to measure physiologic parameters, such as lung capacity, compliance, and airway resistance. Following these measurements, whole-lung lavage was collected in three 1-ml aliquots of sterile saline. The whole-lung lavage was immediately centrifuged at 150 × g at 4°C, and the pellet was collected for cell analysis, while the supernatant was used to measure protein and interleukin-6 (IL-6) content. Differential cell analysis of the cells obtained in the lavage was performed as previously described (21). The protein content within the lavage fluid was measured by using a Micro-BCA protein assay kit (Pierce, Rockford, IL), according to the manufacturer's instructions. IL-6 levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit (BD Biosciences, San Diego, CA), according to the manufacturer's instructions.
Statistical analysis.
The effect of various cathelicidin peptides on the surface tension-reducing capabilities of BLES, as well as its adsorptive properties, were calculated by one-way measure analysis of variance (ANOVA), followed by a Dunnett post hoc test using BLES as the control group. Analysis of CFU assays was performed by paired Student t test at each peptide concentration. Analyses of in vivo assays were performed using one-way ANOVA, followed by Tukey post hoc test. Means are reported ± the standard errors of the mean (SEM), and values were considered significantly different at a probability value (P) of <0.05.
RESULTS
Surface tension after 600 s.
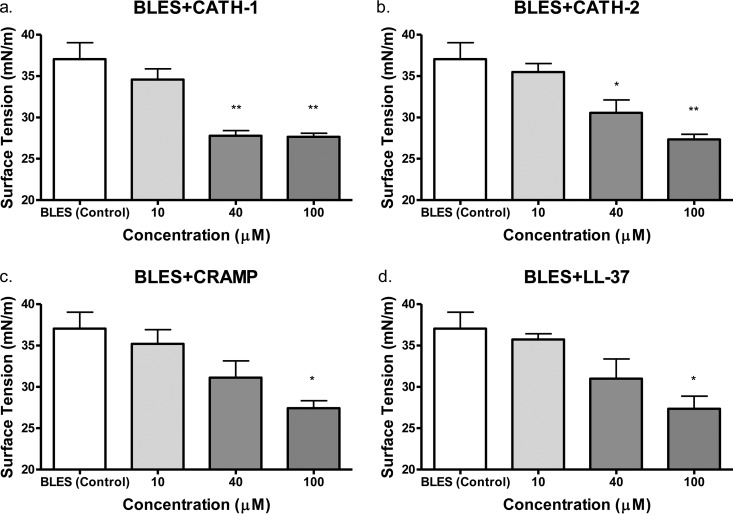
Initial assessment involved an analysis of the ability of the surfactant plus peptides to form a surface film on a clean air-water interface. These data reflect the spreadability of the surfactant which, extrapolating to a clinical scenario, is an important indicator of the ability of this material to distribute throughout the lung and reach the distal airways and alveoli. Each sample was allowed to form a film for 600 s, and the surface tension reached was recorded. As shown in Fig. 1, there were no significant differences in the end surface tensions between any of the surfactant-cathelicidin mixtures at 10 μM concentrations and BLES (control). However, at a cathelicidin concentration of 40 μM, a significant decrease in the surface tension at the end of 600 s of spreading was observed for BLES+CATH-1 (P < 0.01) and BLES+CATH-2 (P < 0.05) mixtures compared to BLES (control), indicating improved spreading characteristics. All four surfactant-cathelicidin mixtures tested resulted in significantly lower surface tension at the end of 600 s spreading at 100 μM concentrations compared to BLES controls.
FIG 1.
Adsorption assay of exogenous surfactant BLES in mixture with cathelicidin peptides. CATH-1, CATH-2, CRAMP, and LL-37 were evaluated at concentrations of 0, 10, 40, and 100 μM. Values represent the mean surface tensions ± the SEM after 600 s. *, P < 0.05 versus BLES (control); **, P < 0.01 versus BLES (control).
Minimum surface tension over multiple compression cycles.
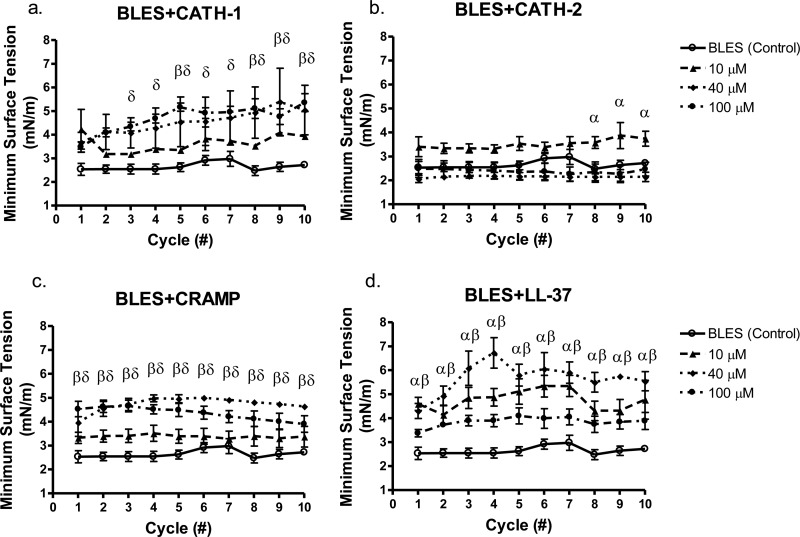
In order to examine the surface tension reducing function of the peptide-surfactant mixtures, the samples underwent 10 cyclic compressions at a rate of 5 s/cycle via the CDS, as a physiologically relevant model of multiple expansion-contraction cycles during respiration. Minimum achievable surface tension (MST) values were recorded for 10 cycles, as a marker of surfactant activity which, in the in vivo situation, would reflect the ability of this material to stabilize the alveoli and allow for ease of inflation. BLES alone was able to reach MST values of ∼2.5 mN/m consistently over all 10 cycles. It was observed that all concentrations of BLES+CATH-2 had a robust surface tension reducing activity, consistently reaching MST values below 4 mN/m. BLES+CATH-2 only showed significantly higher MST values at cycles 8, 9, and 10 at a concentration of 10 μM compared to BLES (control). BLES+CATH-1 mixtures at 40 and 100 μM concentrations showed significantly higher MST values compared to BLES (control) at cycles 2 through 10 (Fig. 2a), increasing slightly from 4 mN/m to an MST of roughly 5 mN/m at the end of the 10 compressions. BLES+CRAMP mixtures had consistently higher MSTs of ∼4.5 mN/m over all cycles at 40 and 100 μM compared to BLES alone (Fig. 2c). BLES+LL-37 mixtures had significantly increased MSTs between 4.5 and 5.5 mN/m over all cycles versus BLES (control), but the values were only significantly different at concentrations of 10 and 40 μM (Fig. 2d). Minimum surface tensions of BLES+LL-37 mixtures at 100 μM were not statistically different from the BLES (control). Mixtures of BLES+CATH-2 were the only surfactant-cathelicidin mixture to show no significant difference versus BLES (control) at 40 and 100 μM concentrations.
FIG 2.
Measurement of minimum surface tension during cyclic compressions of exogenous surfactant BLES in mixture with cathelicidin peptides. CATH-1 (a), CATH-2 (b), CRAMP (c), and LL-37 (d) were evaluated at concentrations of 0 (○), 10 (▲), 40 (⧫), and 100 μM (●). Values are mean minimal achievable surface tensions ± the SEM. α, 10 μM < 0.05 versus BLES (control); β, 40 μM < 0.05 versus BLES (control); δ, 100 μM < 0.05 versus BLES (control).
Atomic force microscopy images.
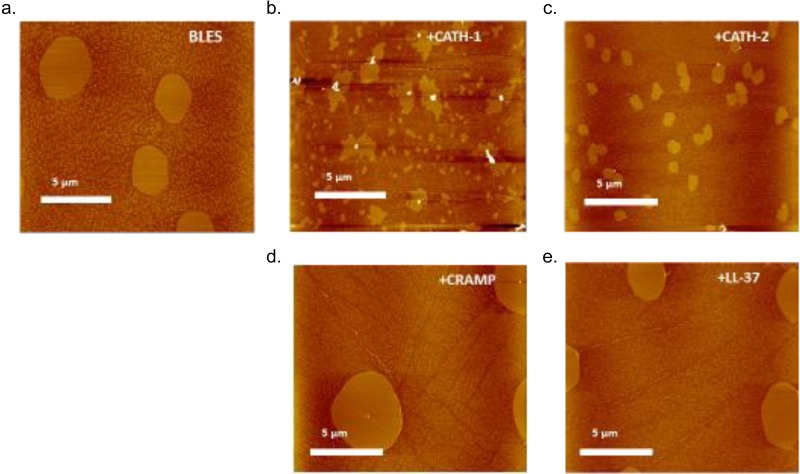
To assess the influence of peptides on surfactant film structure, AFM images were analyzed focusing specifically on the formation of liquid condensed domains, since these domains have been suggested to be important in surfactant function (22). Characteristic AFM topographic images of BLES films in the absence or presence of 100 μM peptides (CATH-2, CATH-1, LL-37, and CRAMP) are shown in Fig. 3. The brightness in these images is proportional to the height. Phase separation, as indicated by the presence of lighter, condensed domains, is evident in all samples at a surface pressure of 30 mN/m (Fig. 3). Quantitative analysis of these images shows that addition of CATH-1 and CATH-2 to BLES leads to a change in its lateral organization. More specifically, the addition of CATH-1 and CATH-2 to BLES leads to an increase in the number of condensed domains and a decrease in the average surface area of the individual domains (Fig. 4). The addition of LL37 and CRAMP to BLES has no significant effect on average surface area of condensed domains at surface pressure 30 mN/m (Fig. 4).
FIG 3.
Characteristic AFM topographic images showing the effect of peptide addition on the lateral organization of BLES monolayers. The scan area is 20 by 20 μm2. All films were deposited at a surface pressure of 30 mN/m. (a) BLES; (b) BLES + 100 μM CATH-1; (c) BLES + 100 μM CATH-2; (d) BLES + 100 μM CRAMP; (e) BLES + 100 μM LL-37.
FIG 4.
Quantification results showing the effect of peptide addition (CATH-1, CATH-2, CRAMP, and LL-37) on the size (i.e., surface area) of the condensed domains in BLES monolayers at a surface pressure of 30 mN/m. These results were obtained from four unique AFM topographic images of different samples.
Antimicrobial assays.
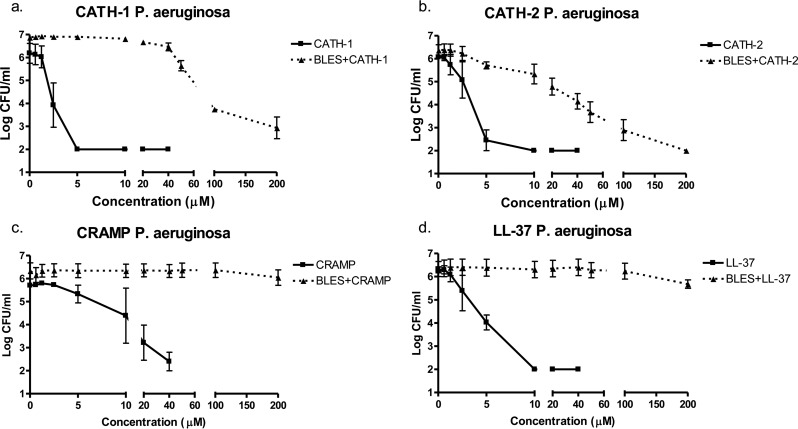
In order to determine the bactericidal properties of the surfactant-cathelicidin mixtures, bactericidal assays of each peptide were performed in both the presence and the absence of BLES. CATH-1, CATH-2, and LL-37 in PBS were found to have MIC values of 5 μM, reducing the CFU/ml values of methicillin-resistant Staphylococcus aureus by >4 log units (Fig. 5a, b, and d), while CRAMP reduced levels by three log units at 40 μM concentrations (Fig. 5c). In the presence of BLES (Fig. 5), CATH-2 had an MIC of 200 μM but had considerable antimicrobial activity, resulting in a 2-log reduction in viability counts at 50 μM. CATH-1 reduced CFU/ml levels by 2 log units at 200 μM, while CRAMP and LL-37 mixtures resulted in either a 1-log reduction or no reduction at 200 μM concentrations in the presence of BLES.
FIG 5.
Antimicrobial activities of cathelicidins. CATH-1 (a), CATH-2 (b), CRAMP (c), and LL-37 (d) were evaluated in the absence (square, solid line) or presence (triangle, dotted line) of BLES at 10 mg/ml against methicillin-resistant S. aureus WKZ-2 in PBS after 3 h at 37°C.
Similar results were seen against P. aeruginosa, where CATH-1, CATH-2, and LL-37 peptides (in the absence of BLES) had MIC values from 5 to 10 μM (Fig. 6), while CRAMP reduced bacterial viability by 3 log units at 40 μM (Fig. 6c). In the presence of BLES, CATH-2 again had an MIC of 200 μM, and a 3-log reduction at 50 μM. CATH-1 reduced P. aeruginosa viability by 3 log units at 200 μM. CRAMP and LL-37 showed negligible bactericidal activity in BLES up to 200 μM concentrations.
FIG 6.
Antimicrobial assay of cathelicidin peptides. CATH-1 (a), CATH-2 (b), CRAMP (c), and LL-37 (d) were evaluated in the absence (square, solid line) or presence (triangle, dotted line) of BLES at 10 mg/ml against P. aeruginosa VW178 in PBS after 3 h at 37°C.
In vivo analysis of BLES+CATH-2 treatment.
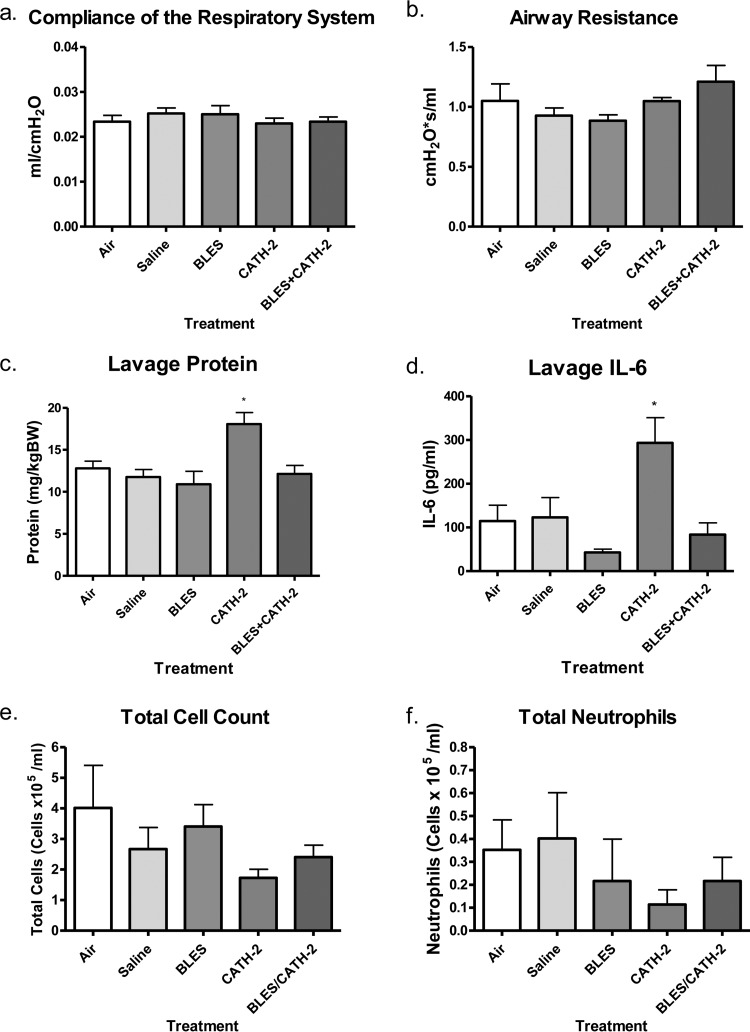
The results of the experiment testing safety and tolerability are shown in Fig. 7. The experiment focused on CATH-2 and included two control groups (air- and saline-instilled groups) and three experimental groups (CATH-2-, BLES+CATH-2-, and BLES-instilled groups). Analysis of lung physiology included lung compliance and airway resistance (Fig. 7a and b), which showed no significant difference among the groups. Mice that were instilled with CATH-2 alone had a significantly higher protein content within the BAL fluid versus animals in other treatment groups and had significantly increased IL-6 levels versus animals treated with BLES alone (Fig. 7c and d). BLES+CATH-2-treated animals showed no significant difference in protein or IL-6 levels compared to air, saline, or BLES controls. Cell count analyses found no significant difference in total cell counts (Fig. 7e) or neutrophil recruitment (Fig. 7f) for any treatment group.
FIG 7.
In vivo tolerance model. (a and b) Compliance of the respiratory system (a) and airway resistance (b), as measured by using a FlexiVent. The protein content (c), IL-6 content (d), total immune cell numbers (e), and total number of recruited neutrophils (f) were also determined in animal BAL fluid. *, P < 0.05.
DISCUSSION
The goal of the present study was to investigate the biophysical and antimicrobial properties of four novel surfactant/cathelicidin mixtures and to determine whether combining such elements together impacts native function. Four cathelicidin peptides were tested in combination with an exogenous surfactant, BLES. The presence of all cathelicidin peptides accelerated surface film formation of BLES as indicated by the spreading capabilities of BLES plus peptides over 600 s. Although all other cathelicidin peptides decreased the ability of BLES to achieve low surface tensions during cyclic compressions on the CDS, the addition of CATH-2 to BLES had a minimal effect on the biophysical function of the exogenous surfactant even at high concentrations. AFM images suggested that the peptides did incorporate into the film and were capable of altering the film structure. Although there was mitigation of the bactericidal capabilities of BLES+CATH-2 compared to CATH-2 activity alone, the BLES+CATH-2 compound could consistently reduce bacterial CFU to below detectable levels, unlike all other surfactant-cathelicidin mixtures, which showed either a large decrease or complete loss of bactericidal function in the presence of BLES. Lastly, when intratracheally instilled into naive mice, BLES+CATH-2 showed no effect on animal physiology, lung edema (as measured by protein leak), or IL-6 inflammatory response. Taken together, we conclude that it would be feasible to develop a surfactant-cathelicidin compound that is capable of maintaining surfactant and AMP properties and that, among the current peptides tested, BLES+CATH-2 appears to be the most promising candidate for future studies to investigate the efficacy of this therapy as an antimicrobial treatment in vivo.
The overall rationale for our study was that surfactant can assist in the delivery of an antimicrobial peptide into the lung, thereby overcoming some of the limitations of local delivery of drugs in pulmonary bacterial infections. For such compounds to be effective, a direct interaction between the surfactant and the peptide would be essential to facilitate codistribution of the two compounds when delivered to the lung. Our data, including the AFM images of surfactant films and experiments related to the spreading of the surfactant, indicate that such direct interaction occurs, as observed by the definitive changes in microdomain size and formation, particularly for CATH-1 and CATH-2 addition. The peptide-surfactant interaction is likely related to the amphipathic, cationic nature of cathelicidins, and their ability to interact with negatively charged phospholipids. Pulmonary surfactant contains ca. 10 to 15% acidic phospholipids, mainly phosphatidylglycerol (PG), with which the cathelicidins could electrostatically interact (23). Although surfactant composition is more complex than simple lipid mixtures, the idea of the interaction with PG is supported by studies by Sevcsik et al. (24). This group utilized binary lipid mixtures to investigate interactions between lipid mixtures and LL-37 and found that even in binary mixtures the interaction between the cathelicidin and lipid mixture is dictated by one of the lipids present. Although the lipid ratios in their experimental model and those found within pulmonary surfactant are different, it is still reasonable to conclude that the interaction observed is between the cathelicidin peptides and PG since it appears this interaction with negatively charged phospholipids contributes to its basic mechanism of antimicrobial activity (2). We suggest that the interaction occurring between the surfactant phospholipids and cathelicidin peptides are affecting the individual properties of each component, such as the surface tension-reducing functions of BLES, and bactericidal capabilities of the cathelicidin peptides.
While the general mechanism of interaction between cathelicidins and surfactant is discussed above, we observed substantial variations among the peptides in terms of their behavior when mixed with surfactant. Specifically, marked differences were obtained when analyzing the antimicrobial activity when peptides were combined with BLES, with only CATH-2 maintaining a relatively robust activity. Differences were also observed in the microdomain shape and size via AFM when CATH-1 or CATH-2 were added to BLES, but this was not observed for CRAMP or LL-37. Although these differences did not correlate with surfactant activity, the changes do provide evidence of direct interaction, and possibly incorporation, of the peptides in the surfactant film. Other studies have also found different lipid interactions among various AMPs. Studies by Neville et al. (25) demonstrated that the addition of LL-37 to a monolayer of DPPG caused monolayer collapse at high surface pressures, but that the addition of SMAP-29, a sheep cathelicidin, actually improved DPPG stability upon compression. This group suggested that this was due to the differences in peptide structures and hydrophobicity and that SMAP-29 was more likely to interact with the head groups of the phospholipids, whereas LL-37 was more likely to insert into the fatty acid groups of the lipid monolayers. This may help explain the differences observed within our study, since the peptides tested here have different structures. It is possible that CATH-2 interacts with PG similar to SMAP-29, allowing CATH-2 to maintain its activity as more cationic residues are exposed to the external environment and able to interact with bacterial membranes. Other peptides, where the residues may be shielded by the interaction with the monolayer, would be less readily available to interact with the targeted bacteria, and therefore have reduced bactericidal capabilities. One interesting feature of CATH-2 that could be related to this difference in activity is the presence of a proline residue at amino acid position 14 that forms a kink region between the two alpha-helical segments of the CATH-2 peptide. This kink is not present in the other tested AMPs. It was shown previously that this hinge region is essential for antibacterial activity, since substitution of the proline residue, straightening the peptide, resulted in highly reduced activity (26, 27). Whether this structural difference between CATH-2 and the other AMPs is the basis of the observed differences in activity would be an interesting future investigation and could potentially lead to the development of improved designer peptides for use in surfactant mixtures.
It has been repeatedly shown that intratracheal surfactant administration results in better pulmonary distribution than aerosol and saline administration (28, 29). Exogenous surfactant has been shown to enhance the pulmonary delivery and bioavailability of other potential therapeutic agents such as antioxidants, antibiotics, corticosteroids, and adenoviral vectors (8, 30). Similar to the current experiments, several studies have investigated the bactericidal and biophysical functions of antibiotics in the presence of exogenous surfactant. In general, it was found using in vitro techniques that some, but not all, of the antibiotics tested were inhibited in the presence of surfactant (31). A subsequent in vivo study in Klebsiella pneumoniae-infected mice demonstrated that surfactant with the antibiotic tobramycin had significantly reduced mortality compared to surfactant or tobramycin by alone (32). These studies provide support for the concept of surfactant as a vehicle for drug delivery, including in the setting of bacterial infection. Our concept of utilizing AMP-based surfactant expands on these studies, since cathelicidin peptides have the added advantage of lacking microbial resistance toward them, while maintaining broad-spectrum bactericidal functions against drug-resistant bacteria (2).
Whereas the main focus of our in vitro experiments was the maintenance of surfactant and antimicrobial activities of potential AMP-based surfactant preparations, it should be noted that it has also been reported that AMP can have cytotoxic effects toward mammalian cells (10, 26, 33). Such effects could potentially limit the therapeutic applicability of this approach. Our in vivo experiment tested this issue for the most promising AMP-surfactant preparation, BLES+CATH-2, by instilling a therapeutic dose in healthy mice and assessing lung physiology and inflammation. Consistent with the reported effects for AMP, instillation of CATH-2 by itself did result in slightly elevated lavage protein levels and IL-6 concentrations, results indicative of mild pulmonary edema and inflammation. Importantly, no such deleterious effects were observed by administration of BLES+CATH-2. These data suggest that BLES+CATH-2 is well tolerated and safe when instilled in a healthy lung and that mild negative side effects of the instillation of a high dose of CATH-2 by itself are mitigated by coadministration with surfactant.
Although our main interpretation of our spreading data is that peptides did not inhibit this property of surfactant, it was interesting that the addition of all cathelicidin peptides actually improved the adsorptive function of surfactant, as seen by a reduced surface tension after 600 s, representing more surface-active material available at the air-liquid interface. The addition of all four peptides to exogenous surfactant led to significantly lower surface tension values reached after 600 s. It is possible that these findings are related to the ability of cathelicidin peptides to penetrate bacterial membranes. In the context of surfactant, this would imply that the peptides act as “bilayer breakers,” thereby enhancing the ability of surfactant lipids to adsorb to the interface. This is similar to the proposed mechanisms by which the two hydrophobic surfactant proteins, SP-B and SP-C, are thought to enhance surfactant adsorption (34, 35). Interestingly, isolated SP-B has been reported to have antimicrobial activity as well (36). Although this activity was completely mitigated by the interaction with surfactant lipids, the finding does support the concept that there may be shared biophysical properties between AMPs and surfactant properties in terms of lipid interaction (37).
In conclusion, our results support the proof of concept that AMP-based surfactant can be utilized for the therapy of bacterial infections. It appears that the interaction between cathelicidins and surfactant varies and, among the four peptides tested, CATH-2 would be optimal for further testing. The current observations have been limited to in vitro studies and in vivo safety studies. Future studies are required to test the efficacy of this compound, by itself as well as in combination with other drugs, in an animal model of bacterial pneumonia.
Supplementary Material
ACKNOWLEDGMENTS
We thank BLES Biochemicals, Inc., for generously providing BLES. We acknowledge the assistance of Johanna L. M. Tjeerdsma-van Bokhoven with the antimicrobial assays and Floris J. Bikker with the synthesis and supply of antimicrobial peptides. We also acknowledge Fred Possmayer for helpful discussions and Valeria Puntorieri for proofreading the manuscript.
This research was locally funded by the Lawson Internal Research Fund, operating grants from the Canadian Institutes of Health Research, the Ontario Thoracic Society, and Physician Services Incorporated, as well as by the Program of Experimental Medicine.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.04937-14.
REFERENCES
- 1.Cosgrove SE, Qi Y, Kaye KS, Karchmer AW, Carmeli Y. 2014. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol 26:166–174. [DOI] [PubMed] [Google Scholar]
- 2.Zasloff M. 2002. Antimicrobial peptides of organisms. Nature 415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 3.Peschel A, Sahl H-G. 2006. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol 4:529–536. doi: 10.1038/nrmicro1441. [DOI] [PubMed] [Google Scholar]
- 4.Fjell CD, Hiss JA, Hancock RE, Schneider G. 2012. Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov 11:37–51. doi: 10.1038/nrd3591. [DOI] [PubMed] [Google Scholar]
- 5.Beaumont PE, McHugh B, Gwyer Findlay E, Mackellar A, Mackenzie KJ, Gallo RL, Govan JRW, Simpson AJ, Davidson DJ. 2014. Cathelicidin host defense peptide augments clearance of pulmonary Pseudomonas aeruginosa infection by its influence on neutrophil function in vivo. PLoS One 9:e99029. doi: 10.1371/journal.pone.0099029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kovach MA, Ballinger MN, Newstead MW, Zeng X, Bhan U, Yu F, Moore BB, Gallo RL, Standiford TJ. 2012. Cathelicidin-related antimicrobial peptide is required for effective lung mucosal immunity in Gram-negative bacterial pneumonia. J Immunol 189:304–311. doi: 10.4049/jimmunol.1103196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipsky BA, Holroyd KJ, Zasloff M. 2008. Topical versus systemic antimicrobial therapy for treating mildly infected diabetic foot ulcers: a randomized, controlled, double-blinded, multicenter trial of pexiganan cream. Clin Infect Dis 47:1537–1545. doi: 10.1086/593185. [DOI] [PubMed] [Google Scholar]
- 8.Haitsma JJ, Lachmann U, Lachmann B. 2001. Exogenous surfactant as a drug delivery agent. Adv Drug Deliv Rev 47:197–207. doi: 10.1016/S0169-409X(01)00106-5. [DOI] [PubMed] [Google Scholar]
- 9.Goerke J. 1974. Lung surfactant. Biochim Biophys Acta 344:241–261. doi: 10.1016/0304-4157(74)90009-4. [DOI] [PubMed] [Google Scholar]
- 10.Forde E, Humphreys H, Greene CM, Fitzgerald-Hughes D, Devocelle M. 2014. Potential of host defense peptide prodrugs as neutrophil elastase-dependent anti-infective agents for cystic fibrosis. Antimicrob Agents Chemother 58:978–985. doi: 10.1128/AAC.01167-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stotland PK, Radzioch D, Stevenson MM. 2000. State of the art mouse models of chronic lung infection with Pseudomonas aeruginosa: models for the study of cystic fibrosis. Pediatr Pulmonol 30:413–424. [DOI] [PubMed] [Google Scholar]
- 12.van't Veen A, Gommers D, Mouton JW, Kluytmans JAJW, Krijt EJ, Lachmann B. 1996. Exogenous pulmonary surfactant as a drug delivering agent: influence of antibiotics on surfactant activity. Br J Pharmacol 118:593–598. doi: 10.1111/j.1476-5381.1996.tb15442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwameis R, Erdogan-Yildirim Z, Manafi M, Zeitlinger MA, Strommer S, Sauermann R. 2013. Effect of pulmonary surfactant on antimicrobial activity in vitro. Antimicrob Agents Chemother 57:5151–5154. doi: 10.1128/AAC.00778-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birkun A. 2014. Exogenous pulmonary surfactant as a vehicle for antimicrobials: assessment of surfactant-antibacterial interactions in vitro. Scientifica 2014:930318. doi: 10.1155/2014/930318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang YE, Zhang H, Fan Q, Neal CR, Zuo YY. 2012. Biophysical interaction between corticosteroids and natural surfactant preparation: implications for pulmonary drug delivery using surfactant as a carrier. Soft Matter 8:504. doi: 10.1039/C1SM06444D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Wang YE, Neal CR, Zuo YY. 2012. Differential effects of cholesterol and budesonide on biophysical properties of clinical surfactant. Pediatr Res 71:316–323. doi: 10.1038/pr.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bikker FJ, Kaman-van Zanten WE, de Vries-van de Ruit A-MBC, Voskamp-Visser I, van Hooft PAV, Mars-Groenendijk RH, de Visser PC, Noort D. 2006. Evaluation of the antibacterial spectrum of drosocin analogues. Chem Biol Drug Des 68:148–153. doi: 10.1111/j.1747-0285.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- 18.Yu LMY, Lu JJ, Chan YW, Ng A, Zhang L, Hoorfar M, Policova Z, Grundke K, Neumann AW. 2004. Constrained sessile drop as a new configuration to measure low surface tension in lung surfactant systems. J Appl Physiol 97:704–715. doi: 10.1152/japplphysiol.00089.2003. [DOI] [PubMed] [Google Scholar]
- 19.Valle RP, Huang CL, Loo JSC, Zuo YY. 2014. Increasing hydrophobicity of nanoparticles intensifies lung surfactant film inhibition and particle retention. ACS Sustain Chem Eng 2:1574–1580. doi: 10.1021/sc500100b. [DOI] [Google Scholar]
- 20.Goetzman ES, Alcorn JF, Bharathi SS, Uppala R, McHugh KJ, Kosmider B, Chen R, Zuo YY, Beck ME, McKinney RW, Skilling H, Suhrie KR, Karunanidhi A, Yeasted R, Otsubo C, Ellis B, Tyurina YY, Kagan VE, Mallampalli RK, Vockley J. 2014. Long-chain acyl-CoA dehydrogenase deficiency as a cause of pulmonary surfactant dysfunction. J Biol Chem 289:10668–10679. doi: 10.1074/jbc.M113.540260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker MG, Tessolini JM, Mccaig L, Yao L, James F, Veldhuizen RAW. 2009. Elevated endogenous surfactant reduces inflammation in an acute lung injury model. Exp Lung Res 35:591–604. doi: 10.1080/01902140902780460. [DOI] [PubMed] [Google Scholar]
- 22.Zuo YY, Keating E, Zhao L, Tadayyon SM, Veldhuizen RA, Petersen NO, Possmayer F. 2008. Atomic force microscopy studies of functional and dysfunctional pulmonary surfactant films. I. Micro- and nanostructures of functional pulmonary surfactant films and the effect of SP-A. Biophys J 94:3549–3564. doi: 10.1529/biophysj.107.122648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Gendy N, Kaviratna A, Berkland C, Dhar P. 2013. Delivery and performance of surfactant replacement therapies to treat pulmonary disorders. Ther Deliv 4:951–980. doi: 10.4155/tde.13.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sevcsik E, Pabst G, Richter W, Danner S, Amenitsch H, Lohner K. 2008. Interaction of LL-37 with model membrane systems of different complexity: influence of the lipid matrix. Biophys J 94:4688–4699. doi: 10.1529/biophysj.107.123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neville F, Ivankin A, Konovalov O, Gidalevitz D. 2010. A comparative study on the interactions of SMAP-29 with lipid monolayers. Biochim Biophys Acta 1798:851–860. doi: 10.1016/j.bbamem.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Dijk A, Molhoek EM, Veldhuizen EJA, Bokhoven JLMT, Wagendorp E, Bikker F, Haagsman HP. 2009. Identification of chicken cathelicidin-2 core elements involved in antibacterial and immunomodulatory activities. Mol Immunol 46:2465–2473. doi: 10.1016/j.molimm.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Xiao Y, Herrera AI, Bommineni YR, Soulages JL, Prakash O, Zhang G. 2009. The central kink region of fowlicidin-2, an alpha-helical host defense peptide, is critically involved in bacterial killing and endotoxin neutralization. J Innate Immun 1:268–280. doi: 10.1159/000174822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukhopadhyay S, Staddon GE, Eastman C, Palmers M, Davies ER. 1994. The quantitative distribution of nebulized antibiotic in the lung in cystic fibrosis. Respir Med 88:203–211. doi: 10.1016/S0954-6111(05)80348-8. [DOI] [PubMed] [Google Scholar]
- 29.Kharasch VS, Sweeney TD, Fredberg J, Lehr J, Damokosh AI, Avery ME, Brain JD. 1991. Pulmonary surfactant as a vehicle for intratracheal delivery of technetium sulfur colloid and pentamidine in hamster lungs. Am Rev Respir Dis 144:909–913. doi: 10.1164/ajrccm/144.4.909. [DOI] [PubMed] [Google Scholar]
- 30.Katkin JP, Husser RC, Langston C, Welty SE. 1997. Exogenous surfactant enhances the delivery of recombinant adenoviral vectors to the lung. Hum Gene Ther 8:171–185. doi: 10.1089/hum.1997.8.2-171. [DOI] [PubMed] [Google Scholar]
- 31.Van 't Veen A, Mouton JW, Gommers D, Kluytmans JA, Dekkers P, Lachmann B. 1995. Influence of pulmonary surfactant on in vitro bactericidal activities of amoxicillin, ceftazidime, and tobramycin. Antimicrob Agents Chemother 39:329–333. doi: 10.1128/AAC.39.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van't Veen A, Mouton JW, Gommers D, Lachmann B. 1996. Pulmonary surfactant as vehicle for intratracheally instilled tobramycin in mice infected with Klebsiella pneumoniae. Br J Pharmacol 119:1145–1148. doi: 10.1111/j.1476-5381.1996.tb16016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haney EF, Hancock REW. 2013. Peptide design for antimicrobial and immunomodulatory applications. Biopolymers 100:572–583. doi: 10.1002/bip.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pérez-Gil J. 2008. Structure of pulmonary surfactant membranes and films: the role of proteins and lipid-protein interactions. Biochim Biophys Acta 1778:1676–1695. doi: 10.1016/j.bbamem.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Veldhuizen EJ, Haagsman HP. 2000. Role of pulmonary surfactant components in surface film formation and dynamics. Biochim Biophys Acta 1467:255–270. [DOI] [PubMed] [Google Scholar]
- 36.Yang L, Johansson J, Ridsdale R, Willander H, Fitzen M, Henry T, Weaver TE. 2010. Surfactant protein B propeptide contains a saposin-like protein (SAPLIP) domain with antimicrobial activity at low pH. J Immunol 184:975–983. doi: 10.4049/jimmunol.0900650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryan MA, Akinbi HT, Serrano AG, Perez-Gil J, Wu H, McCormack FX, Weaver TE. 2005. Antimicrobial activity of native and synthetic surfactant protein B peptides. J Immunol 176:416–425. doi: 10.4049/jimmunol.176.1.416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.