Abstract
A randomized, double-blind, placebo-controlled, 4-period crossover study was conducted in 52 healthy adults to assess the effect of delafloxacin on the corrected QT (QTc) interval. The QT interval, corrected for heart rate using Fridericia's formula (QTcF), was determined predose and at 0.5, 1, 1.25, 1.5, 1.75, 2, 2.5, 3, 4, 5, 6, 12, 18, and 24 h after dosing with delafloxacin at 300 mg intravenously (i.v.; therapeutic), delafloxacin at 900 mg i.v. (supratherapeutic), moxifloxacin at 400 mg orally (p.o.; positive control), and placebo. The pharmacokinetic profile of delafloxacin was also evaluated. At each time point after delafloxacin administration, the upper limit of the 90% confidence interval (CI) for the placebo-corrected change from the predose baseline in QTcF (ΔΔQTcF) was less than 10 ms (maximum, 3.9 ms at 18 h after dosing), indicating an absence of a clinically meaningful increase in the QTc interval. The lower limit of the 90% CI of ΔΔQTcF for moxifloxacin versus placebo was longer than 5 ms at all 5 time points selected for assay sensitivity analysis, demonstrating that the study was adequately sensitive to assess QTc prolongation. There was no positive relationship between delafloxacin plasma concentrations and ΔΔQTcF. Treatment-emergent adverse events (AEs) were more frequent among subjects receiving a single supratherapeutic dose of 900 mg delafloxacin. There were no deaths, serious AEs, or AEs leading to study discontinuation and no clinically meaningful abnormalities in laboratory values or vital signs observed at any time point after any dose of the study drug.
INTRODUCTION
Delafloxacin (RX-3341, ABT-492, and WQ-3034) is an anionic investigational fluoroquinolone antibiotic, currently in phase III development for the treatment of acute bacterial skin and skin structure infections (ABSSSI) based on its activity against Gram-positive microorganisms, including methicillin-resistant Staphylococcus aureus, Gram-negative microorganisms, and anaerobes (1). Pharmacokinetic (PK) studies have shown that the increases in the maximum plasma concentration (Cmax) and total exposure (area under the concentration-time curve [AUC]) are dose proportional and greater than dose proportional, respectively, with a single intravenous (i.v.) dose of 300 to 1,200 mg in healthy volunteers (2). Both i.v. and oral formulations are expected to be available, allowing i.v.-to-oral “step-down” therapy in the appropriate patient populations. The proposed dose for the treatment of ABSSSI is 300 mg administered i.v. or 450 mg administered orally (p.o.) twice daily (BID).
QT interval prolongation caused by the inhibition of potassium channels encoded by the HERG gene has been described for a variety of drug classes, including the fluoroquinolone antibiotics (3, 4). QT prolongation is associated with an increased risk of life-threatening cardiac arrhythmias, most typically torsades de pointes (TdP). The degree to which the fluoroquinolones block the cardiac potassium channels, thereby prolonging the QT interval, varies by agent (5, 6). In preclinical studies, supratherapeutic concentrations of delafloxacin had no significant effect on the HERG current in stably transfected human embryonic kidney (HEK293) cells, on repolarization of canine Purkinje fibers in vitro, and on the QT interval corrected by Bazett's formula (QTcB) in anesthetized dogs in vivo (Melinta; data on file).
Thorough QT (TQT) studies are now a standard part of the drug development process and of guidelines which are designed to assess the effects of compounds on cardiac repolarization and proarrhythmic potential and have been issued and published by the International Conference on Harmonisation (ICH) (E14) (7). A prolongation of the corrected QT (QTc) interval in which the upper bound of the 1-sided 95% confidence interval (CI) for the mean effect on the QTc exceeds 10 ms suggests a clinically relevant effect (7).
The primary objective of this study was to assess the effect of single therapeutic (300 mg) and supratherapeutic (900 mg) doses of i.v. delafloxacin versus a positive control (moxifloxacin at 400 mg p.o.) on the placebo-adjusted, time-matched change from the baseline in QTc on the basis of Fridericia's correction (QTcF) method. The supratherapeutic dose of delafloxacin was chosen because this was the maximum tolerated dose for a single i.v. dose of delafloxacin in a phase I study (8). The secondary objective of the study was to evaluate the safety, tolerability, and pharmacokinetics of delafloxacin when administered as a single 300-mg i.v. therapeutic and single 900-mg i.v. supratherapeutic dose. A preliminary report of these results has been presented previously (9).
MATERIALS AND METHODS
Study design and subjects.
The present study was a randomized, placebo-controlled, 4-period crossover study. Subjects were healthy men or women between the ages of 18 and 45 years inclusive, with a body mass index (BMI) of between 19 and 30 kg/m2. Subjects were screened at up to 28 days and not less than 48 h prior to receipt of the first dose. Screening procedures included a thorough medical history and physical examination, standard 12-lead electrocardiograms (ECGs), and clinical laboratory testing (hematology, serum chemistry, and urinalysis). A total of 52 subjects were randomly assigned to 1 of 8 treatment sequences using a double Williams square schema; each sequence included all 4 study treatments.
The study was conducted in compliance with ICH Harmonised Tripartite Guideline E6 (R1) good clinical practice (10) and in accordance with all national, state, and local laws or regulations. Written informed consent was obtained prior to each subject entering the study, and the study protocol and amendments were approved by the institutional review board at the study center.
According to the predetermined sequence, subjects received delafloxacin at 300 mg i.v. (therapeutic) plus a placebo capsule p.o., delafloxacin at 900 mg i.v. (supratherapeutic) plus a placebo capsule p.o. (placebo for moxifloxacin), 5% dextrose–water (D5W; placebo for delafloxacin) i.v. plus a placebo capsule p.o., or D5W plus moxifloxacin at 400 mg p.o. Delafloxacin was provided as a sterile lyophilized powder and reconstituted in D5W. The 3 single parenteral doses were administered over 1 h, and all treatments were separated by a minimum 4-day washout interval. Delafloxacin was administered in a double-blind, double-dummy manner; moxifloxacin was administered in an open-label manner, because differences between the active tablet and placebo capsule could be observed. The subject, investigator, and site personnel involved in the study, with the exception of pharmacy personnel, were blind to the treatment.
The study considered 4 different patient populations (Fig. 1).
FIG 1.
Patient populations used for analysis. Safetya, all subjects who received at least 1 dose of study drug; PKb, all subjects who received at least 1 dose of study drug and had sufficient plasma concentration data to calculate the primary PK parameters; ECGc, all subjects who were randomly assigned, received at least 1 dose of study drug, and had at least 1 baseline (predose) ECG and 1 on-treatment (postdose) ECG within the same treatment period; PK/PDd, all subjects in the ECG population with time-matched plasma concentrations. ECG, electrocardiographic; PD, pharmacodynamic; PK, pharmacokinetic.
Electrocardiography.
All ECG data were obtained digitally using a Mortara Instrument (Milwaukee, WI) H12+ ECG continuous 12-lead digital recorder, stored continuously on a flash card, and read centrally by eResearch Technology, Inc. (Philadelphia, PA) using a high-resolution manual on-screen caliper semiautomatic method with annotations. The ECG data were obtained in triplicate at predetermined time points in each of the 4 treatment periods. Baseline time points were −45 min, −30 min, and −15 min; postdose time points, based on the start of infusion for delafloxacin or ingestion for moxifloxacin, were 0.5, 1, 1.25, 1.5, 1.75, 2, 2.5, 3, 4, 5, 6, 12, 18, and 24 h. The extractions were time matched with the collection of PK samples. Subjects who dropped out of the study were not replaced.
Statistical analyses of QT/QTc.
The sample size calculation was based on an assessment of the noninferiority of delafloxacin to placebo in the primary analysis and on the assumption of a true difference of 3 ms in time-matched changes from the baseline QTcF between delafloxacin and placebo with a standard deviation of 9 ms (11). A sample size of 42 subjects would provide more than 80% power to show that the upper limit of the 90% CI (2 sided) for the comparison of delafloxacin with placebo would fall below 10 ms, as specified in the ICH E14 guideline (7). The enrollment target of 52 subjects was established to control for the possibility that some subjects might drop out of the study.
Moxifloxacin at 400 mg was included as a positive control to validate the sensitivity of the assay to detect small increases in the QTcF duration from the baseline based on studies showing mean increases in QTc of 6 ms using a time-averaged analysis (12) and of 10 to 15 ms using a time-matched analysis (13). Assay sensitivity was established based on baseline-corrected change in QTcF at 1, 1.5, 2, 3, and 4 h after ingestion, which correspond with the times during which the plasma concentration is highest (14, 15).
The primary ECG analysis was of the placebo-adjusted change from the predose baseline of the QTcF interval (ΔΔQTcF), with the purpose of determining whether the upper bound of the 2-sided 90% CI exceeded 10 ms, per the ICH E14 guideline (7); ECGs were recorded at the same time of the day (time-matched analysis) for each treatment. Descriptive analysis of the time-matched means for the ECG interval parameters of heart rate (HR), PR interval, QRS, and QTcF was performed.
Outlier values were defined as those in which the QTcF was >450 ms, >480 ms, or >500 ms, for which the predose baseline was ≤450 ms, ≤480 ms, or ≤500 ms, respectively, or the ΔQTcF was >30 to 60 ms or >60 ms.
Pharmacokinetics.
Blood samples for PK analysis were collected from all subjects on the first day of each treatment period. However, because only plasma levels for delafloxacin were of interest for comparison with the ECG QTcF duration (pharmacodynamic [PD] effect), only samples from subjects receiving the therapeutic or supratherapeutic doses of delafloxacin were analyzed. The ECG time points on treatment days (days 1, 5, 9, and 13) that were used for PK sampling were as follows: before dosing and at 0.5, 1, 1.25, 1.5, 1.75, 2, 2.5, 3, 4, 5, 6, 12, 18, and 24 h after the start of the delafloxacin infusion. The ECG extractions were time matched to the PK samples but were obtained before the actual plasma sampling time to avoid changes in autonomic tone resulting from bloodletting.
Blood samples (6 ml each) were collected into chilled (2°C to 8°C) tubes containing dipotassium EDTA acid, gently mixed, and centrifuged under refrigeration (2°C to 8°C) at 900 × g for 15 min. Plasma was removed, divided into aliquots, and stored at −70°C until analyses were performed. Samples were analyzed at Intertek Pharmaceutical Services (El Dorado Hills, CA). Plasma delafloxacin concentrations were assessed by liquid chromatography coupled with tandem mass spectrometry (Sciex API-4000; Applied Biosystems, Inc., Foster City, CA) (data available upon request).
PK data were calculated using noncompartmental analyses with Phoenix WinNonlin Version 5.1 or higher (Pharsight, St. Louis, MO). PK parameters calculated for both doses of delafloxacin were the area under the concentration-time curve from time zero to 24 h (AUC0–24) by the linear trapezoidal rule, Cmax, and the time to achieve the maximum observed plasma concentration. All plasma concentrations that were below the level of quantitation (BLQ) prior to the first measurable concentration were set to zero. The BLQ values obtained between the measurable concentrations were treated as missing, and those obtained after the last quantifiable concentration were not used.
Pharmacokinetic and pharmacodynamic analyses.
Changes from the predose baseline in the QTcF interval at each time point with delafloxacin treatment were placebo adjusted using the equation ΔQTcFD(i) − ΔQTcFP(i), where ΔQTcFD(i) and ΔQTcFP(i) are the changes from the baseline at time point i for QTcF with delafloxacin and placebo, respectively. This varied slightly from the modeled values used in the time-matched analyses of the central tendency analyses specified above. The relationship between the ΔΔQTcF interval and plasma delafloxacin concentration was assessed using a linear mixed-effect model (data available upon request).
Safety analyses.
Safety assessments performed throughout the study included monitoring and recording of adverse events (AEs), vital sign measurements (blood pressure, HR, respiratory rate, and oral temperature), clinical laboratory results (including hematology, coagulation, serum chemistry, and urinalysis), and physical examination findings.
The occurrences of treatment-emergent AEs (TEAEs), serious AEs (SAEs), discontinuations because of AEs, or deaths were recorded, as was information on the intensity of AEs and relationship to the study drug. Descriptive statistics of clinical laboratory results and changes from the baseline, as well as potentially clinically significant laboratory values, were presented.
RESULTS
Demographics.
A total of 52 healthy subjects (29 men and 23 women) were enrolled and assigned to 1 of 8 treatment sequences in the 4-period crossover design. The mean age of study participants was 28.5 years (range, 18 to 43 years), and the mean BMI was 24.65 kg/m2 (range, 19.2 to 29.0 kg/m2). Most subjects were white (61.5%) and non-Hispanic (53.8%). Fifty-one subjects completed all dosing. One subject withdrew consent after 3 of 4 treatment periods because of a family emergency; this subject did not receive the supratherapeutic dose of delafloxacin.
Electrocardiography.
A plot of the ΔΔQTcF interval for the 2 doses of delafloxacin and 1 of moxifloxacin is shown in Fig. 2. Neither of the delafloxacin dose groups demonstrated an upper bound that approached or exceeded 10 ms, demonstrating no signal of any effect of this agent on cardiac repolarization. The time-matched analysis for the QTcF endpoint revealed that the moxifloxacin group met the assay sensitivity criteria outlined in the statistical plan, with all 5 predefined time points having a lower confidence bound of ≥5 ms. Placebo (D5W) had no significant effect on the QTcF interval; ΔQTcFP(i) was negative at all but 4 time points and was greater than 1 ms at 2 time points (for 5 h, ΔQTcFP(i) = 2.4 ms; for 12 h, ΔQTcFP(i) = 3.2 ms).
FIG 2.
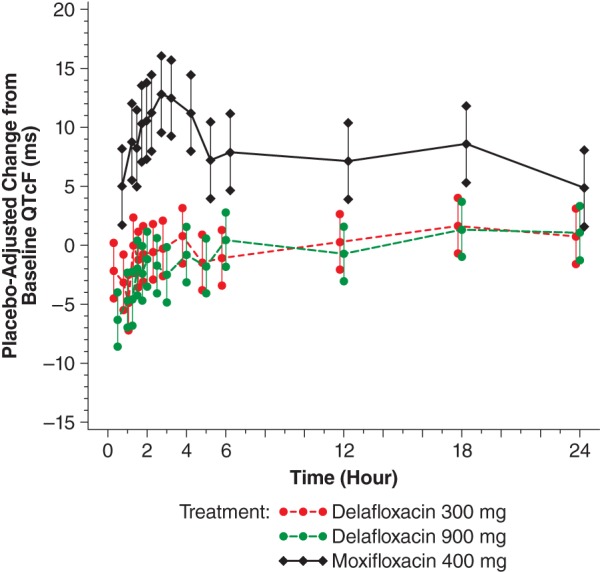
Placebo-adjusted change from the baseline QTcF (ms) versus time (electrocardiographic [PD] population). A mixed-effect general linear model (placebo adjusted and baseline corrected) was fitted for QTcF (ms) and included terms for treatment, gender, time, baseline value, time-by-treatment interaction, gender-by-baseline interaction of QTcF (ms), and time-by-treatment-by-gender interaction. Gender was a significant effect. Lower and upper bounds = lower and upper 2-sided 90% (1-sided 95%) model-based confidence limits. P values for gender effect were gender main effect = 0.6949, treatment-by-gender interaction = 0.2919, gender-by-baseline interaction = 0.5339, and treatment-by-gender-by-time interaction ≤ 0.0001. PD, pharmacodynamic; QTcF, corrected QT interval using Fridericia's formula.
Peak placebo-adjusted changes from the baseline for HR (14.7 beats/min) and PR interval (8.1 ms) with 900 mg delafloxacin occurred 1 and 1.5 h, respectively, after the start of infusion (data not shown). Both resolved quickly and were of minor clinical concern. Placebo-adjusted changes from the baseline in HR and PR interval with 300 mg were of no clinical concern at any time point. There were no placebo-adjusted changes from the baseline in the QRS interval of clinical concern with either dose of delafloxacin.
There were no subjects who met specific outlier criteria for QTcF, i.e., a new abnormal U wave, a new >500-ms absolute QTc duration, or a >60-ms change from the baseline. Similarly, no subjects in any group met the nonspecific outlier criterion of a >30-to-60-ms change from the baseline.
Pharmacokinetic and pharmacodynamic analysis.
The mean plasma PK parameters for delafloxacin at both therapeutic and supratherapeutic doses are presented in Table 1. The relationship between the plasma concentration of delafloxacin and the ΔΔQTcF interval for delafloxacin is presented in Fig. 3. The slope for the relationship between the ΔΔQTcF interval and the plasma concentration was −0.2128 (standard error, 0.0422; P < 0.0001) based on the estimates from the linear-mixed model. The predicted ΔΔQTcF intervals at Cmax were −1.9766 and −6.9672 for the therapeutic and supratherapeutic doses, respectively, with 1-sided upper 95% confidence bounds of −1.1418 and −4.8957, respectively. These data do not support the possibility of any effect of delafloxacin on QT interval prolongation.
TABLE 1.
Plasma pharmacokinetic parameters of delafloxacina
| Parameter | Mean (CV) or median (minimum, maximum) plasma PK values for delafloxacin (PK population) |
|
|---|---|---|
| 300 mg (therapeutic dose) (n = 52) | 900 mg (supratherapeutic dose) (n = 51) | |
| AUC0–24, μg · h/ml | 25.4 (28) | 98.7 (30) |
| Cmax, μg/ml | 9.82 (22) | 33.3 (23) |
| Tmax, hb | 1.00 (0.80, 1.10) | 1.00 (1.00, 1.12) |
AUC, area under the concentration-time curve; Cmax, maximum plasma concentration; CV, coefficient of variation; PK, pharmacokinetic; Tmax, time to achieve maximum observed plasma concentration.
For Tmax, the median (minimum, maximum) values are presented.
FIG 3.

QTcF placebo-corrected change from the baseline (ΔΔQTcF) versus delafloxacin plasma concentration (PK/PD population). The red line represents the mixed-effect regression prediction line. The gray line is a reference line at the predicted ΔΔQTcF interval for the supratherapeutic dose. These 2 lines cross at the mean Cmax value for the supratherapeutic dose (33.27 μg/ml). Conc., concentration; Cmax, maximum plasma concentration; PD, pharmacodynamic; PK, pharmacokinetic; QTcF, corrected QT interval using Fridericia's formula.
Safety.
No deaths, SAEs, severe TEAEs, or AEs leading to study discontinuation occurred during the study. Overall, 34 subjects (65%) reported TEAEs at any time. Subjects who received 900 mg delafloxacin reported TEAEs most frequently; a similar percentage of subjects had TEAEs after administration of moxifloxacin, 300 mg delafloxacin, and placebo (Table 2). Gastrointestinal TEAEs (23 subjects [44.2%]) were the most frequently experienced events, followed by nervous system disorders (14 patients [26.9%]) and general disorders and administration site conditions (13 patients [25.0%]). Seventy-eight percent of TEAEs were judged to be mild in severity. The patterns of the incidences of TEAE assessed by the investigator as possibly or probably related to the study drug were similar (Table 2). All TEAEs resolved by the end of the study, with the exception of a single event of skin depigmentation. No clinically meaningful abnormalities in laboratory values or vital signs were observed at any time point after any dose of the study drug.
TABLE 2.
Summary of adverse events (safety population)
| MedDRA preferred terma | No. (%) of subjects in indicated treatment group |
||||
|---|---|---|---|---|---|
| Overall (n = 52) | Placebo (n = 52) | Delafloxacin 300 mg (n = 52) | Delafloxacin 900 mg (n = 51) | Moxifloxacin 400 mg (n = 52) | |
| ≥1 TEAE | 34 (65.4) | 12 (23.1) | 12 (23.1) | 29 (56.9) | 13 (25.0) |
| Nausea | 20 (38.5) | 1 (1.9) | 2 (3.8) | 20 (39.2) | 4 (7.7) |
| Vomiting | 13 (25.0) | 0 | 0 | 13 (25.5) | 1 (1.9) |
| Headache | 10 (19.2) | 3 (5.8) | 3 (5.8) | 1 (2.0) | 3 (5.8) |
| Infusion-site pain | 8 (15.4) | 4 (7.7) | 1 (1.9) | 5 (9.8) | 0 |
| Myalgia | 6 (11.5) | 3 (5.8) | 2 (3.8) | 2 (3.9) | 0 |
| Dizziness | 5 (9.6) | 1 (1.9) | 0 | 4 (7.8) | 1 (1.9) |
| Abdominal pain | 4 (7.7) | 1 (1.9) | 0 | 1 (2.0) | 2 (3.8) |
| Feeling hot | 4 (7.7) | 0 | 0 | 4 (7.8) | 0 |
| ≥1 treatment-related AE | 30 (57.7) | 9 (17.3) | 5 (9.6) | 26 (51.0) | 7 (13.5) |
| Nausea | 20 (38.5) | 0 | 2 (3.8) | 20 (39.2) | 3 (5.8) |
| Vomiting | 13 (25.0) | 0 | 0 | 13 (25.5) | 0 |
| Headache | 6 (11.5) | 2 (3.8) | 1 (1.9) | 1 (2.0) | 2 (3.8) |
| Infusion-site pain | 8 (15.4) | 4 (7.7) | 1 (1.9) | 5 (9.8) | 0 |
| Myalgia | 1 (1.9) | 0 | 1 (1.9) | 0 | 0 |
| Dizziness | 4 (7.7) | 1 (1.9) | 0 | 4 (7.8) | 0 |
| Abdominal pain | 1 (1.9) | 1 (1.9) | 0 | 0 | 0 |
| Feeling hot | 4 (7.7) | 0 | 0 | 4 (7.8) | 0 |
MedDRA, Medical Dictionary for Regulatory Activities (http://www.meddra.org/); AE, adverse event; TEAE, treatment-emergent AE.
DISCUSSION
This randomized, double-blind, placebo-controlled, 4-period crossover study in healthy volunteers evaluated the effect on the QTcF interval of i.v. delafloxacin administered at both therapeutic (300-mg) and supratherapeutic (900-mg) doses. The principal finding is that neither of the 2 delafloxacin dose groups demonstrated an upper bound that approached or exceeded 10 ms, indicating no clinically meaningful increase in the QTcF interval. The results of the PK/PD model show that the slope for the QTcF interval for delafloxacin was negative, and the predicted ΔΔQTcF interval at Cmax was less than 0 ms for both the therapeutic and supratherapeutic dose groups. These changes were not clinically significant. Together, these data do not support the idea of any effect of delafloxacin on QT interval prolongation.
The Cmax and AUC values observed for the supratherapeutic dose of delafloxacin in this study were approximately 1.7 and 1.9 times greater, respectively, than the values reported for subjects with severe renal impairment (estimated glomerular filtration rate, ≤30 ml/min/1.73 m2) who received a single 300-mg dose of i.v. delafloxacin (8, 16). The supratherapeutic dose of delafloxacin used in the present study thus provided greater systemic concentrations of delafloxacin for QTc evaluation than those likely to be observed during routine clinical use of delafloxacin.
Our finding that delafloxacin, even at a supratherapeutic dose, does not prolong the QTcF interval in healthy volunteers is consistent with results from preclinical studies, suggesting that delafloxacin did not inhibit the HERG current in vitro at a concentration approximately 33-fold the estimated therapeutic plasma level when dosed at 400 mg/day (Melinta, data on file). Randomized prospective studies with healthy individuals given ciprofloxacin or levofloxacin have shown that these also do not cause QT prolongation. Noel and colleagues (17) reported that a single dose of levofloxacin administered at 500 mg p.o. had no effect on the QTc interval, whereas higher doses—1,000 mg p.o. and 1,500 mg p.o.—caused significant prolongation. Results from a study by Tsikouris and colleagues (18) suggest that repeated dosing of up to 7 days of ciprofloxacin at 500 mg BID or levofloxacin at 500 mg once daily also has no effect on the QTc interval; administered as a positive control, moxifloxacin at 400 mg once daily caused a significant increase. Despite the widespread use of fluoroquinolones, the overall incidence of TdP remains low (19).
This study demonstrated the lack of effect of delafloxacin on prolongation of the QTc interval and was designed in accordance with current regulatory guidelines for evaluating the potential of new drugs to prolong the QTc interval; however, several limitations of this type of study should be noted. The study was conducted in a population both younger and healthier than the populations that would receive delafloxacin for the treatment of infections, populations likely to have comorbid diseases and to receive concomitant medications. The use of healthy subjects in a TQT study, such as this one, reduces variability in the measurement of QTc intervals.
Another limitation is that the current study looked at the effects on the QTc interval after just a single 300-mg dose of i.v. delafloxacin, while the recommended i.v. dose for the treatment of infections is 300 mg BID for 5 to 14 days. In a phase I study, however, there was no appreciable accumulation of delafloxacin after multiple days of twice-daily i.v. dosing. Mean plasma clearance levels were similar on days 1 and 14, and PK parameters were similar after single and multiple doses (8).
Finally, the present study utilized a supratherapeutic dose (900 mg) of delafloxacin in addition to the clinical dose (300 mg). Given that there was no increase of the QTc interval at the supratherapeutic dose, it can be expected that there will not be an effect of delafloxacin on the QTc interval at the dose targeted for use in the clinic. There was a small but significant inverse relationship between delafloxacin plasma concentrations and ΔΔQTcF. The clinical relevance of this small degree of QTc shortening remains unknown. However, the small decline in ΔΔQTcF found in the present study is not expected to be clinically meaningful.
In conclusion, delafloxacin, at both therapeutic and supratherapeutic doses, had no clinically relevant effect on the QT/QTc interval in a population of healthy male and female adult volunteers. There were no SAEs, deaths, or AEs leading to discontinuation during the study, and there were no clinically meaningful abnormalities in laboratory values or vital signs observed at any time point after any dose of the study drug.
ACKNOWLEDGMENTS
This study was funded by Melinta Therapeutics (formerly Rib-X Pharmaceuticals).
We thank Scott Hopkins and Jarrod Longcor for their contributions as program lead and medical monitor, respectively.
L.E.L., S.K.C., and E.S. are employees of Melinta Therapeutics. J.S.L., M.S.B., and M.D.T. declare that we have no conflicts of interest.
Editorial assistance was provided by The Curry Rockefeller Group, LLC, Tarrytown, NY, and was funded by Melinta Therapeutics.
REFERENCES
- 1.Remy JM, Tow-Keogh CA, McConnell TS, Dalton JM, Devito JA. 2012. Activity of delafloxacin against methicillin-resistant Staphylococcus aureus: resistance selection and characterization. J Antimicrob Chemother 67:2814–2820. doi: 10.1093/jac/dks307. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence L, Benedict M, Hart J, Hawkins A, Li D, Medlock M, Hopkins S, Burak E. 2011. Pharmacokinetics (PK) and safety of single doses of delafloxacin administered intravenously in healthy human subjects, abstr A2-045a Abstr 51st Intersci Conf Antimicrob Agents Chemother. [Google Scholar]
- 3.Kang J, Wang L, Chen XL, Triggle DJ, Rampe D. 2001. Interactions of a series of fluoroquinolone antibacterial drugs with the human cardiac K+ channel HERG. Mol Pharmacol 59:122–126. doi: 10.1124/mol.59.1.122. [DOI] [PubMed] [Google Scholar]
- 4.Roden DM. 2004. Drug-induced prolongation of the QT interval. N Engl J Med 350:1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 5.Ball P. 2000. Quinolone-induced QT interval prolongation: a not-so-unexpected class effect. J Antimicrob Chemother 45:557–559. doi: 10.1093/jac/45.5.557. [DOI] [PubMed] [Google Scholar]
- 6.Crouch MA, Limon L, Cassano AT. 2003. Clinical relevance and management of drug-related QT interval prolongation. Pharmacotherapy 23:881–908. doi: 10.1592/phco.23.7.881.32730. [DOI] [PubMed] [Google Scholar]
- 7.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. May 2005. The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs, E14. ICH harmonised tripartite guideline. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Guideline.pdf Accessed 29 August 2013.
- 8.Melinta Therapeutics. 2013. Investigator's brochure. Melinta Therapeutics, New Haven, CT. [Google Scholar]
- 9.Lawrence L, Benedict M, Litwin J, Thorn M, Medlock M, Hopkins S, Longcor J. 2012. A thorough phase 1 QTc study of delafloxacin (DLX) compared with placebo and moxifloxacin (MXF), abstr A2-1958 Abstr 52nd Intersci Conf Antimicrob Agents Chemother. [Google Scholar]
- 10.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. 1996. June Guideline for good clinical practice, E6 (R1). ICH harmonised tripartite guideline. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf Accessed 29 August 2013.
- 11.Agin MA, Kazierad DJ, Abel R, Anziano R, Billing CB, Layton G, Mancuso J, Strieter D, Xu W, Blum RA, Jorkasky DK. 2003. Assessing QT variability in healthy volunteers. J Clin Pharmacol 43:1028. doi: 10.1177/0091270003043009010. [DOI] [Google Scholar]
- 12.Merck & Co., Inc. 14 August 2013. Avelox (moxifloxacin) package insert. Merck & Co., Inc., Whitehouse Station, NJ. [Google Scholar]
- 13.Garnett CE, Beasley N, Bhattaram VA, Jadhav PR, Madabushi R, Stockbridge N, Tornoe CW, Wang Y, Zhu H, Gobburu JV. 2008. Concentration-QT relationships play a key role in the evaluation of proarrhythmic risk during regulatory review. J Clin Pharmacol 48:13–18. doi: 10.1177/0091270007307881. [DOI] [PubMed] [Google Scholar]
- 14.Stass H, Kubitza D. 1999. Pharmacokinetics and elimination of moxifloxacin after oral and intravenous administration in man. J Antimicrob Chemother 43(Suppl B):83–90. [DOI] [PubMed] [Google Scholar]
- 15.Wise R, Andrews JM, Marshall G, Hartman G. 1999. Pharmacokinetics and inflammatory-fluid penetration of moxifloxacin following oral or intravenous administration. Antimicrob Agents Chemother 43:1508–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoover R, Lawrence L, Smith C, Longcor J. 2013. Pharmacokinetics (PK) of delafloxacin (DLX) in patients with varying degrees of renal impairment. abstr A-017e Abstr 53rd Intersci Conf Antimicrob Agents Chemother, Denver, CO. [Google Scholar]
- 17.Noel GJ, Goodman DB, Chien S, Solanki B, Padmanabhan M, Natarajan J. 2004. Measuring the effects of supratherapeutic doses of levofloxacin on healthy volunteers using four methods of QT correction and periodic and continuous ECG recordings. J Clin Pharmacol 44:464–473. doi: 10.1177/0091270004264643. [DOI] [PubMed] [Google Scholar]
- 18.Tsikouris JP, Peeters MJ, Cox CD, Meyerrose GE, Seifert CF. 2006. Effects of three fluoroquinolones on QT analysis after standard treatment courses. Ann Noninvasive Electrocardiol 11:52–56. doi: 10.1111/j.1542-474X.2006.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frothingham R. 2001. Rates of torsades de pointes associated with ciprofloxacin, ofloxacin, levofloxacin, gatifloxacin, and moxifloxacin. Pharmacotherapy 21:1468–1472. doi: 10.1592/phco.21.20.1468.34482. [DOI] [PubMed] [Google Scholar]


