Abstract
Changing treatment practices may be selecting for changes in the drug sensitivity of malaria parasites. We characterized ex vivo drug sensitivity and parasite polymorphisms associated with sensitivity in 459 Plasmodium falciparum samples obtained from subjects enrolled in two clinical trials in Tororo, Uganda, from 2010 to 2013. Sensitivities to chloroquine and monodesethylamodiaquine varied widely; sensitivities to quinine, dihydroartemisinin, lumefantrine, and piperaquine were generally good. Associations between ex vivo drug sensitivity and parasite polymorphisms included decreased chloroquine and monodesethylamodiaquine sensitivity and increased lumefantrine and piperaquine sensitivity with pfcrt 76T, as well as increased lumefantrine sensitivity with pfmdr1 86Y, Y184, and 1246Y. Over time, ex vivo sensitivity decreased for lumefantrine and piperaquine and increased for chloroquine, the prevalences of pfcrt K76 and pfmdr1 N86 and D1246 increased, and the prevalences of pfdhfr and pfdhps polymorphisms associated with antifolate resistance were unchanged. In recurrent infections, recent prior treatment with artemether-lumefantrine was associated with decreased ex vivo lumefantrine sensitivity and increased prevalence of pfcrt K76 and pfmdr1 N86, 184F, and D1246. In children assigned chemoprevention with monthly dihydroartemisinin-piperaquine with documented circulating piperaquine, breakthrough infections had increased the prevalence of pfmdr1 86Y and 1246Y compared to untreated controls. The noted impacts of therapy and chemoprevention on parasite polymorphisms remained significant in multivariate analysis correcting for calendar time. Overall, changes in parasite sensitivity were consistent with altered selective pressures due to changing treatment practices in Uganda. These changes may threaten the antimalarial treatment and preventive efficacies of artemether-lumefantrine and dihydroartemisinin-piperaquine, respectively.
INTRODUCTION
Malaria, in particular disease caused by Plasmodium falciparum, remains an overwhelming problem in most of sub-Saharan Africa (1, 2). Malaria control was greatly limited by resistance to chloroquine (CQ) and sulfadoxine-pyrimethamine (SP), leading to adoption of artemisinin-based combination therapy (ACT) as the standard treatment for uncomplicated falciparum malaria in the last decade (3). ACT consists of a rapid-acting artemisinin derivative plus a longer-acting partner drug that clears parasites not eliminated by the artemisinin component and limits selection of artemisinin resistance (4, 5). In nearly all countries in sub-Saharan Africa, either artemether-lumefantrine (AL) or artesunate-amodiaquine (AS-AQ) is recommended to treat uncomplicated malaria (6). Other ACTs are dihydroartemisinin (DHA)-piperaquine (DP), a first-line therapy in some countries in Asia, with particular promise for malaria prevention due to the extended half-life of piperaquine (7), and artesunate-mefloquine (AS-MQ), which is used in some countries in Asia and South America. In Uganda, AL was named the national malaria treatment regimen in 2004; its implementation began in 2006 but was fairly slow. The proportion of children <5 years of age with a fever treated with an ACT (AL is the only widely available ACT) within 24 h rose from an estimated 1% in 2006 to 14% in 2009 to 30% in 2011 (8). Although treatment of all fevers as malaria is no longer national policy, these statistics indicate increasing access to AL for treating malaria. Thus, both the appropriate treatment of malaria with AL and increased selective pressure for resistance to AL appear to have increased markedly in Uganda in recent years.
Intermittent preventive therapy (IPT) with SP to decrease malaria incidence and the risk of placental malaria is recommended for pregnant women in Uganda at least once per trimester (9). IPT is not recommended in children because of the limited efficacy of SP due to drug resistance, although in the Sahel subregion of West Africa, where malaria is highly seasonal and the prevalence of SP resistance is lower than that in other areas, seasonal malaria chemoprevention with the combination of SP and amodiaquine is now recommended (10). DP was recently studied for use as chemoprevention in Uganda. Children were randomized to monthly DP administered from 6 to 24 months of age. Compared to untreated controls, the DP chemoprevention arm experienced a 58% decrease in the incidence of malaria (11). Treatment was not directly observed, and piperaquine serum levels were consistent with poor treatment compliance in many children (11). Thus, the true preventive efficacy of monthly DP was likely higher. This conclusion is supported by a second trial in which schoolchildren were randomized to directly observed monthly DP or no therapy for 1 year, and the preventive efficacy of DP was a remarkable 96% (12).
Of obvious concern with increasing utilization of newer malaria therapies for treatment or chemoprevention is potential selection of drug-resistant parasites. Resistance to the aminoquinolines chloroquine and amodiaquine is mediated principally by well-defined polymorphisms in two putative drug transporters encoded by pfcrt and pfmdr1 (3, 13), and these polymorphisms are selected in new infections that emerge soon after therapy with AS-AQ (14, 15). Piperaquine is a related bisaminoquinoline, but although resistance was widely reported in the preartemisinin era from China (16), the mechanisms of resistance are uncertain. Use of DP for treatment (17) or chemoprevention (18) did not select for the polymorphisms associated with chloroquine resistance in Burkina Faso, but, in Uganda, recent treatment with DP selected for pfmdr1 mutations associated with decreased sensitivity to aminoquinolines (19). Interestingly, a number of other antimalarials exert the opposite selective pressure. In particular, new infections emerging within 2 months of treatment with AL show selection of wild-type sequences at the pfcrt K76T and pfmdr1 N86Y and D1246Y alleles (19–22); mutant sequences are selected at these same alleles by aminoquinolines. Of great recent concern has been resistance to artemisinins, manifest as delayed parasite clearance after therapy, in Southeast Asia (23, 24). This phenomenon was recently linked to a laboratory phenotype, with enhanced survival of ring-stage parasites after pulse exposure to artemisinins (25) and with polymorphisms in the newly identified K13 gene (26). However, delayed parasite clearance (27), K13 polymorphisms associated with resistance (28), and enhanced ring survival (R. Cooper, unpublished data) have not been seen in Uganda, and, consistent with these results, a recent survey suggested that artemisinin resistance is to date confined to Southeast Asia (29).
With recent changes in malaria treatment practices in Uganda, it is of interest to assess whether parasite resistance mediators have been selected. In two studies from Tororo, the prevalence of the wild-type pfcrt and pfmdr1 alleles noted above increased significantly, both in parasites not under selective pressure (30) and in those from children treated for each episode of malaria with AL (19). However, limited data have been available on the actual drug sensitivity of parasites in Uganda, with only one prior report from Kampala showing good efficacy of lumefantrine, piperaquine, and dihydroartemisinin (31). The goal of this study was to consider changes in drug sensitivity over time and the selective pressures of ACTs for treatment and chemoprevention, utilizing both parasitological and molecular assessments.
MATERIALS AND METHODS
Clinical trials.
All studied parasites were from subjects enrolled in two clinical trials conducted in Tororo, Uganda, a region with very high malaria transmission intensity, with an entomological inoculation rate recently estimated at 125 infectious bites per year (32). In the PROMOTE-1 trial (registered at ClinicalTrials.gov under registration no. NCT00978068), 170 HIV-infected children were randomized to antiretroviral therapy with a protease inhibitor-based or nonnucleoside reverse transcriptase inhibitor-based regimen and followed for the incidence of malaria over 6 to 24 months (33); all of these children were provided with daily trimethoprim-sulfamethoxazole (TS). In the PROMOTE-3 trial (ClinicalTrials.gov no. NCT00948896), 393 HIV-uninfected children were randomized to no intervention or malaria chemoprevention at 6 to 24 months of age with monthly SP, daily TS, or monthly DP (11). In both trials, subjects received free medical care, including transport to our study clinic, throughout the course of the trials. Use of antimalarial drugs outside the study protocols was uncommon. When patients presented with symptoms suggestive of malaria, either upon routine monthly assessment or at unscheduled visits, Giemsa-stained thick blood smears were performed. Malaria was defined as any parasitemia in the setting of fever or history of fever in the last 24 h. In both trials, uncomplicated malaria was treated with AL and complicated malaria with quinine. The clinical trials and analyses of cultured parasites were approved by the Uganda National Council of Science and Technology, the Makerere University Research and Ethics Committee, and the University of California, San Francisco Committee on Human Research.
Parasite culture.
At the time of diagnosis of a new episode of malaria and before the initiation of therapy, blood was collected in heparinized tubes and transported promptly to our laboratory, located adjacent to the study clinic. Giemsa-stained thin blood smears were examined, and if P. falciparum infection with >1% parasitemia and a lack of other plasmodial species was confirmed, culture was initiated as previously described (31). Briefly, blood was centrifuged, plasma and buffy coat were removed, and the erythrocyte pellet was washed three times with RPMI 1640 medium. A 200-μl aliquot of the washed pellet was added to 10 ml of RPMI 1640 medium supplemented with 25 mM HEPES, 0.2% NaHCO3, 0.1 mM hypoxanthine, 100 μg/ml gentamicin, and 0.5% AlbuMAX II serum substitute to produce a packed cell volume of 2%. Higher parasite densities were diluted with 2% uninfected erythrocytes to obtain a density of 0.05% to limit inoculum effects on drug susceptibility assay results.
Measurement of ex vivo drug sensitivity.
Sensitivities were measured against fresh isolates and control strains (acquired from the Malaria Research and Reference Reagent Resource Center) for chloroquine (Sigma-Aldrich), monodesethylamodiaquine (MDAQ) (BD Gentest), quinine (Sigma-Aldrich), DHA (Dafra Pharma), lumefantrine (Porton International), and piperaquine (Porton International), as described previously (31). Multiple wells of 96-well culture plates were predosed with six duplicate 2-fold serial dilutions of each drug of interest (31.3 to 2,004 nM chloroquine, 12.5 to 801 nM MDAQ, 30.8 to 1,971 nM quinine, 0.13 to 8.4 nM DHA, 0.2 to 12.5 nM lumefantrine, 3.1 to 200 nM piperaquine). Plates were dried in an incubator and stored at 4°C in sealed plastic bags. Wells without drug served as controls. Aliquots (200 μl) of cultured parasites were added to each well and maintained at 5% CO2, 3% O2, and 37°C for 72 h. After the incubation, a blood smear was prepared to confirm healthy growth of controls and determine the level of parasitemia. Samples were then frozen (−20°C) and thawed three times to allow for complete hemolysis before analysis. Drug sensitivity was assessed using an enzyme-linked immunosorbent assay (ELISA) to quantify parasite histidine-rich protein-2 in treated and control cultures (34). Samples were diluted 1:10 in water, and 100 μl of each was added to an ELISA plate precoated with mouse anti-horseradish peroxidase (HRP)-2 IgM capture antibody (Immunology Consultant Laboratory) and incubated at room temperature for 1 h. Each well was then washed three times with 0.05% Tween 20 in phosphate-buffered saline, incubated with 100 μl of secondary antibody (horseradish peroxidase-conjugated anti-mouse IgG) for 1 h at room temperature, washed again three times, and incubated with 100 μl of 3,3′,5,5′-tetramethylbenzidine chromogen for 10 min before adding 50 μl of 1 M sulfuric acid to stop the reaction. Absorbance at 450 nm was then read with a Multiskan ELISA plate reader. ELISA results for experimental and control cultures were used to construct dose-response curves for each drug, and the 50% inhibitory concentrations (IC50s) were calculated using HN-NonLin software (34), with data fitted by nonlinear regression to a variable-slope sigmoidal dose-response formula, and with attention to test validity based on adequate readings above background (35). In addition, visual examination of each curve was performed on two separate occasions by two investigators (P.T. and P.J.R.), with elimination of data for which curve fits were not straightforward.
Analysis of parasite polymorphisms associated with drug resistance.
At the time of each new malaria diagnosis, blood was also spotted onto filter paper (Whatman 3MM) for molecular studies. DNA was extracted with Chelex (36). We evaluated polymorphisms in the pfcrt, pfmdr1, pfmrp1, pfdhfr, and pfdhps genes. Initial assessments were performed by amplification of flanking sequences by PCR, digestion with sequence-specific restriction endonucleases, and evaluation of digested fragments by agarose gel electrophoresis, as previously described (31, 37, 38). The formal assessments described here were performed with a newer technique, utilizing a ligase detection reaction-fluorescent microsphere assay. Assays were performed as previously described (39), with the modifications of nested-PCR for all reactions, and with a new primer to detect pfdhfr 164 polymorphisms (see Tables S1 and S2 in the supplemental material).
Measurement of piperaquine drug levels.
Piperaquine levels were measured at the time of malaria diagnosis in subjects assigned to monthly DP, as previously reported (11). Subjects were separated into three categories: DPlow (plasma piperaquine, ≤10 ng/ml [limit of detection]), DPmed (plasma piperaquine, >10 ng/ml and ≤20 ng/ml), and DPhigh (plasma piperaquine, >20 ng/ml) to approximate compliance with chemoprevention.
Statistical analysis.
The analyses used Stata version 12 (StataCorp). The outcome variables of interest were IC50s for six antimalarial drugs and genotype (wild-type versus mixed or mutant at each allele of interest). The exposure variables of interest were calendar time (date of treatment), time since prior malaria treatment, or chemoprevention arm. Calendar time was evaluated as a categorical variable, with each year as an independent category. Time since last treatment was evaluated as a categorical variable, comparing four intervals since the previous therapy. The significance of changes over time or differences between categories was evaluated using univariate and multivariate analyses executed using generalized estimating equations with exchangeable correlations and robust standard errors to account for repeated measures in the same child. For associations between genotypes and drug sensitivity, the geometric means of the IC50s were compared between wild-type, mixed, or mutant malaria episodes, and significant differences between categories were identified using t tests. Differences were considered significant at a P value of <0.05.
RESULTS
Clinical trials providing samples for study.
Samples were obtained from two clinical trials conducted in Tororo, Uganda, and were collected from 2010 to 2013 (Table 1). PROMOTE-1 compared impacts on malaria of two different antiretroviral regimens in 170 children age 2 months to 5 years; treatment with a regimen that included the protease inhibitor lopinavir/ritonavir was associated with a 41% decreased incidence of malaria compared to that in children treated with a nonnucleoside reverse transcriptase inhibitor-based regimen (33). PROMOTE-3 compared the incidence of malaria in 393 children assigned to one of 3 chemoprevention regimens or no chemoprevention from age 6 to 24 months; the incidence of malaria was decreased by 58% with monthly DP, 28% with daily TS, and an insignificant 7% with monthly SP (11). In both trials, episodes of uncomplicated malaria were treated with AL.
TABLE 1.
Trials providing parasite samples for analysis
| Trial | Dates | Age (median [range]) (mo) | Treatment for uncomplicated malaria | Chpra | No. of samples for which data were available for: |
HIV status | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| IC50s | SNPs | IC50 and SNPs | |||||||
| PROMOTE-1 | May 2010–November 2012 | 43 (10–100) | AL | TS | 64 | 38 | 37 | Infected | 33 |
| PROMOTE-3 | June 2010–June 2013 | 18 (4–36) | AL | None | 232 | 249 | 162 | Not infected | 11 |
| TS | 62 | 134 | 54 | ||||||
| SP | 64 | 151 | 60 | ||||||
| DP | 37 | 116 | 34 | ||||||
| Total | 459 | 688 | 347 | ||||||
Chpr, chemoprevention; TS, trimethoprim-sulfamethoxazole; SP, sulfadoxine-pyrimethamine; DP, dihydroartemisinin-piperaquine. None, samples from subjects in chemoprevention arms collected before or after the intervention.
Ex vivo drug sensitivity of P. falciparum isolates.
Samples collected at the time of malaria diagnosis were placed into culture, and ex vivo sensitivities to 6 antimalarial drugs were determined (Table 2; see also Table S3 in the supplemental material). Sensitivities of control laboratory strains were as expected based on earlier studies (see Table S4 in the supplemental material) and did not show any consistent changes over time (see Fig. S1 in the supplemental material). Formal in vitro cutoffs for resistance have not been established for antimalarials, and sensitivities vary depending on the assay utilized. To facilitate analysis, we modified the resistance cutoffs established by others (40, 41), setting the cutoffs for the aminoquinolines at 100 nM and at 600 nm for quinine (40), and choosing conservative cutoffs of 10 nM for DHA and 50 nM for lumefantrine. Sensitivities to chloroquine and MDAQ (the active metabolite of amodiaquine) varied widely. Based on the chosen cutoffs, 78% of isolates were resistant to chloroquine, and 37% were resistant to MDAQ. Parasites were generally sensitive to quinine, DHA, lumefantrine, and piperaquine. However, 9.2% of tested isolates had a piperaquine IC50 of >100 nM.
TABLE 2.
Ex vivo drug sensitivity data of isolated parasitesa
| Drug used | No. of samples | Geometric mean IC50 (nM) | 95% CI (nM)b | Range (nM) | Cutoff for resistance (nM) | No. (%) resistant |
|---|---|---|---|---|---|---|
| Chloroquine | 408 | 247.9 | 223.1–275.4 | 31.0–1,398 | 100 | 317 (77.7) |
| Quinine | 419 | 126.7 | 115.5–138.9 | 30.7–1,339 | 600 | 37 (8.8) |
| MDAQ | 421 | 76.9 | 70.2–84.1 | 12.5–564.6 | 100 | 157 (37.3) |
| DHA | 442 | 1.4 | 1.3–1.5 | 0.3–16.9 | 10 | 8 (1.8) |
| Lumefantrine | 378 | 3.0 | 2.6–3.3 | 0.4–24.4 | 50 | 0 (0) |
| Piperaquine | 381 | 19.1 | 17.1–21.4 | 3.1–188.9 | 100 | 35 (9.2) |
This table includes results for all samples studied. Results are stratified into different trials and study arms in Table S3 in the supplemental material.
95% CI, 95% confidence interval.
Associations between ex vivo drug sensitivity and parasite transporter polymorphisms.
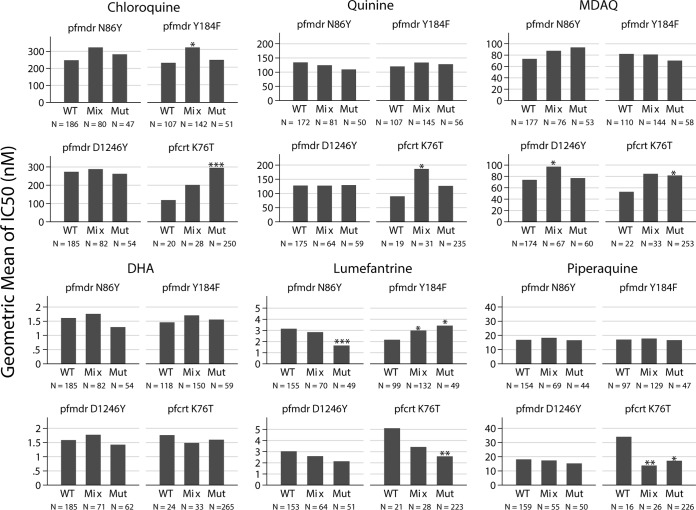
Samples were tested for polymorphisms in pfcrt, pfmdr1, prmrp1, pfdhfr, and pfdhps known or suspected to be associated with altered responses to antimalarial drugs. Analyses searching for associations between parasite genetics and ex vivo drug sensitivity are complicated by the high multiplicity of infection that is typical in isolates from Tororo. Thus, measured ex vivo sensitivities represent averages of cocirculating clones, and genotyping often identifies mixed infections. Nonetheless, the analysis offers a valuable summary (Fig. 1). The most convincing associations showed a dose-response relationship, with mixed genotypes having intermediate drug sensitivity between wild-type and mutant genotypes. Sensitivities to chloroquine and MDAQ correlated with the pfcrt K76T polymorphism, with wild type more sensitive than mutant parasites (chloroquine geometric mean IC50s, 119 versus 295 nM, respectively, P = 0.0002; MDAQ mean IC50s, 52.8 versus 81.6 nM, respectively, P = 0.031). Parasites with the wild-type pfcrt K76 genotype were less sensitive to chloroquine than in many other reports, perhaps due to the effects of mixed isolates; other studies of field isolates have also identified relatively high IC50s in field isolates with the wild-type K76 genotype (42). Sensitivities to lumefantrine and piperaquine showed the opposite trend, with wild-type parasites being less sensitive to the drugs (lumefantrine geometric mean IC50, 5.1 versus 2.6 nM, respectively, P = 0.0088; piperaquine IC50, 34.0 versus 17.1 nM, respectively, P = 0.022). Considering polymorphisms in pfmdr1, the most noteworthy associations were decreased sensitivity to MDAQ and increased sensitivity to lumefantrine with the 86Y and 1246Y mutant genotypes, with the opposite association occurring with the 184F mutant genotype. These results were all consistent with prior studies showing selection by AL of the N86, 184F, and D1246 genotypes (19–22). For most other comparisons, pfmdr1 alleles were not associated with drug sensitivity. Considering other polymorphisms identified primarily outside Africa, only wild-type pfmdr1 S1034 and N1042 alleles were detected. A third putative drug transporter, pfmrp1, is highly polymorphic, and the I876 wild-type allele was selected by prior AL therapy in Tanzania (43). However, we found no association between the pfmrp1 polymorphisms I876V and K1466R and sensitivity to any of the tested antimalarial drugs (data not shown).
FIG 1.
Association between P. falciparum genetic polymorphisms and ex vivo drug sensitivity. Geometric mean IC50s are shown for parasites with wild-type (WT), mixed (Mix), or mutant (Mut) genotypes at the indicated alleles. The number of samples in each category is indicated by N. Values for each allele were compared between wild-type and mixed or mutant genotypes using t tests, and comparisons with P values of <0.05 (*), <0.01 (**), and <0.001 (***) are labeled.
Changes in drug sensitivity over time.
Ex vivo sensitivities to most drugs changed significantly over the 4-year course of the study (Fig. 2; see also Table S5 in the supplemental material). Sensitivities to chloroquine and DHA increased, although for chloroquine, most parasites were resistant (IC50, >100 nM) throughout the course of the study (80% in 2010, 85% in 2011, 73% in 2012, and 65% in 2013); for DHA, changes were modest, with nearly all IC50s being <10 nM throughout the study. Sensitivities to lumefantrine and piperaquine decreased from 2010 to 2012, as AL was established as the national malaria treatment regimen.
FIG 2.
Ex vivo sensitivity of P. falciparum over time. For longitudinal assessments of IC50 data, drug sensitivities were plotted as IC50s over time. Each point represents a single isolate. A representation of IC50 over time was plotted using the lowess smoothing function in Stata.
Considering resistance-mediating parasite polymorphisms, we recently showed that P. falciparum underwent marked changes in the prevalence of some key single-nucleotide polymorphisms (SNPs) in Tororo over the last decade (30), and changes were markedly affected by the choice of antimalarial treatment regimen (28). An evaluation of samples from children in the two PROMOTE trials identified similar trends (Fig. 3; see also Table S6 in the supplemental material). Most notably, the prevalence of the pfmdr1 N86 wild-type genotype, which is selected by AL use (19–22), increased over 2 years of observation, and the prevalence of the pfcrt K76T mutation, which is associated with resistance to chloroquine (37) and was fixed at a high prevalence in older studies from Uganda (44, 45), decreased markedly in 2012. In all cases with the chloroquine-resistant 76T mutation, the pfcrt 72-76 haplotype, which identifies the geographic origins of parasites, was CVIET, consistent with an Asian origin (46). These results are consistent with the expected selective pressures of decreasing use of chloroquine and increasing use of AL in Uganda. Considering polymorphisms associated with altered responses to antifolates, the prevalence of key mutations was stable across the course of the study (see Fig. S2 in the supplemental material). Specifically, the pfdhfr 51I, 59R, and 108N, and pfdhps 437G and 540E mutations were all common, but other mutations associated with higher levels of drug resistance (pfdhfr 164L, and pfdhps 581G and 613T/S), which have been uncommon in earlier surveys (39, 45, 47), were also rare in these samples. Factors that likely contribute to the continued high prevalence of resistance-mediating antifolate mutations include use of antifolates to treat malaria (although this is no longer national policy), as intermittent preventive therapy against malaria, to treat bacterial infections, and as prophylaxis against opportunistic infections in those with HIV infection.
FIG 3.
Allele prevalences over time. Prevalences of wild-type (WT), mixed (Mix), and mutant (Mut) alleles during the indicated intervals are shown. The number of samples in each category is indicated by N. Changes in wild-type allele prevalences compared to those in 2010 that were significant in univariate analyses are labeled for P values of <0.05 (*), <0.01 (**), and <0.001 (***). With multivariate analysis adjusting for the amount of time since last AL treatment and chemoprevention arms, changes in the prevalences of pfmdr1 86 and 184 mutations remained significant.
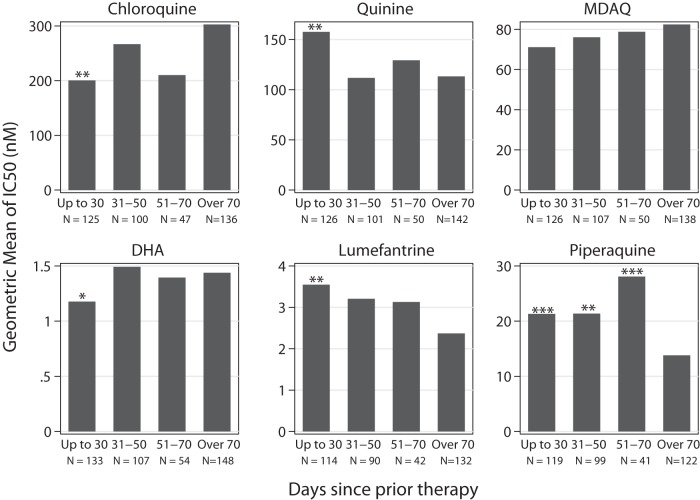
Impact of prior therapy with AL on drug sensitivity.
Treatment with AL has been shown to select for polymorphisms associated with decreased lumefantrine sensitivity (19–22), but the impact of prior treatment on ex vivo drug sensitivity has not previously been assessed. We compared the sensitivities of parasites collected from children without recent prior therapy with those of parasites from children treated previously with AL for ≤30 days, 31 to 50 days, or 51 to 70 days prior to an episode of malaria. Compared to samples from patients without recent therapy, those from subjects previously treated with AL had decreased lumefantrine sensitivity, with the impact of prior therapy being greatest in those with parasites emerging soonest after a previous therapy (Fig. 4; see also Table S7 in the supplemental material). However, in multivariate analysis, the identified differences in lumefantrine sensitivity were not significant, suggesting that the changes were mostly explained by changing lumefantrine sensitivity over time (see Table S7). Associations were very similar when we considered samples from the PROMOTE-3 trial only (not shown), suggesting that selection was not notably impacted by the use of antiretroviral protease inhibitors, which extend lumefantrine exposure (33), in some PROMOTE-1 subjects. Considering other drugs, prior therapy with AL was also associated with increased sensitivity to chloroquine and decreased sensitivity to quinine and piperaquine; these differences were generally also not significant in multivariate analysis (see Table S7).
FIG 4.
Impact of prior therapy with AL on ex vivo drug sensitivities. Samples were categorized based on the time since patients had received a prior therapy with AL, and geometric mean IC50s were calculated. The number of samples in each category is indicated by N. Values for each drug were compared with those for children who did not receive prior therapy within 70 days (over 70), and comparisons with P values of <0.05 (*), <0.01 (**), and <0.001 (***) in univariate analysis are labeled. With multivariate analysis, only the median piperaquine IC50 for 51 to 70 days since prior treatment was statistically significant.
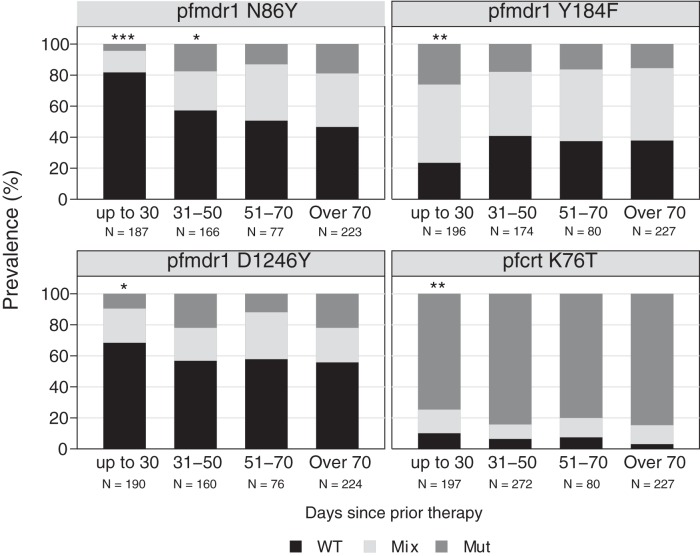
Considering resistance-mediating parasite polymorphisms, consistent with prior studies (19–21) and the results for ex vivo drug sensitivity (Fig. 4), prior use of AL impacted parasite polymorphisms. The prevalences of wild-type alleles at pfcrt K76T, pfmdr1 N86Y, and pfmdr1 D1246Y were all greater in samples collected from children with recurrent infections within 30 days of a prior treatment with AL than in samples from children without recent prior therapy (Fig. 5; see also Table S8 in the supplemental material). For pfmdr1 Y184F, the trend was in the opposite direction, with selection of the mutant allele, as reported previously (19). With multivariate analysis, these identified differences in polymorphism prevalences remained significant (see Table S8). As expected, prior treatment with AL did not impact the prevalence of resistance-mediating SNPs in pfdhfr or pfdhps (see Fig. S3 in the supplemental material).
FIG 5.
Impact of prior therapy with AL on polymorphism prevalence. Samples were categorized based on the time since patients had received a prior therapy with AL, and prevalences of wild-type (WT), mixed (Mix), and mutant (Mut) alleles were determined. The number of samples in each category is indicated by N. Prevalences for each polymorphism were compared with those for children who did not receive prior therapy within 70 days (over 70), and comparisons with P values of <0.05 (*), <0.01 (**), and <0.001 (***) in univariate analysis are labeled. With multivariate analysis, the labeled comparisons remained statistically significant.
Impact of chemoprevention on drug sensitivity.
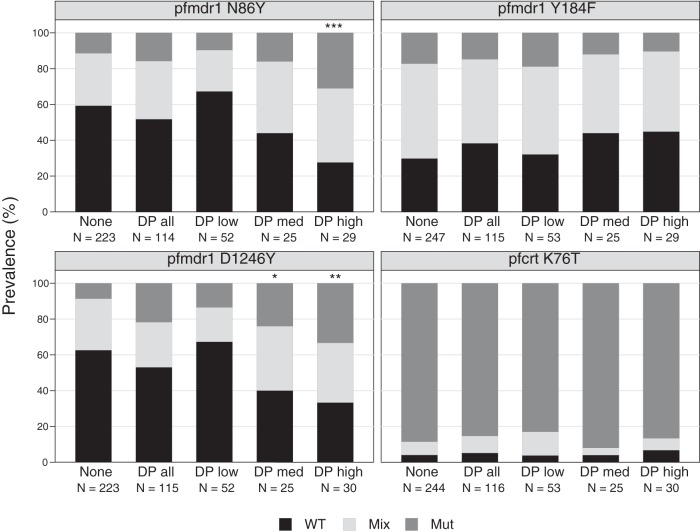
For samples collected from children in the PROMOTE-3 chemoprevention trial, it was of interest to determine if drug sensitivity varied among chemoprevention arms. Of note, this analysis is complicated by the understanding that without directly observed therapy, many breakthrough episodes of malaria occurred in children not receiving their assigned regimen and who were therefore not actually under drug pressure. Overall, no association was seen between chemoprevention regimen and sensitivity to any tested drug (see Table S3 in the supplemental material). In particular, the piperaquine and DHA sensitivities of parasites from children assigned to monthly DP and those demonstrated to be compliant with monthly DP did not differ from those of parasites from children in the control arm of the trial. Considering resistance-mediating polymorphisms, only minor differences in the prevalence of SNPs of interest were seen between subjects assigned to different chemoprevention regimens (Fig. 6). Notably, assignment to the monthly DP arm was not associated with pfcrt or pfmdr1 polymorphisms previously associated with sensitivity to aminoquinolines, and assignment to either antifolate regimen was not associated with polymorphism prevalences. However, when samples from the DP treatment arm were sorted based on DP exposure by measuring circulating piperaquine levels at the time of malaria, parasites from subjects compliant with monthly DP demonstrated selection for the pfmdr1 86Y and 1246Y mutations. These results showed a dose-response relationship, with subjects with the highest piperaquine levels at the time of malaria (and thus presumably most compliant with monthly DP) demonstrating the highest prevalence of mutant genotypes. With multivariate analysis correcting for calendar time and time since prior therapy with AL, selection for pfmdr1 86Y and 1246Y remained significant.
FIG 6.
Impact of chemoprevention regimen on polymorphisms. The prevalences of wild-type (WT), mixed (Mix), and mutant (Mut) alleles are shown for parasites isolated from subjects in the different study arms. The number of samples in each category is indicated by N. For the DP arm, results for all subjects (DPall) and those classified based on drug-level analysis as DPlow (PQ levels, <10 ng/ml), DPmed (PQ levels, 10 to <20 ng/ml), and DPhigh (PQ levels, >20 ng/ml) are shown. Studied polymorphisms with significant differences in prevalences of WT alleles between samples from children receiving the indicated chemoprevention regimen and those not receiving chemoprevention are labeled for P values of <0.05 (*) and <0.01 (**). With multivariate analysis, the labeled comparisons remained statistically significant.
DISCUSSION
We characterized the drug sensitivity of P. falciparum, utilizing both parasitological and molecular assessments, in samples from two recent trials of children in Tororo, Uganda. Assessments of ex vivo drug sensitivity of ACT components showed that sensitivity to MDAQ, the active metabolite of amodiaquine, is not optimal, and that sensitivity to lumefantrine and piperaquine is decreasing. In addition, therapy with AL, the national treatment regimen in Uganda, selected for parasites in subsequent infections with decreased lumefantrine sensitivity. Evaluations of parasite polymorphisms showed related trends, with increases over time and after AL treatment in the prevalence of polymorphisms associated with decreased lumefantrine sensitivity. Thus, even with apparent absence of artemisinin resistance in Africa, the antimalarial efficacies of the three leading ACTs, and in particular of AL, may be in jeopardy.
We recently showed that parasites in Tororo have changed remarkably in recent years, with increased prevalence of wild-type pfcrt K76, pfmdr1 N86, and pfmdr1 D1246 polymorphisms associated with decreased sensitivity to lumefantrine (19, 30), and in this report, we describe similar trends in samples from additional trials. Similar results were described recently in Kenya (42), Tanzania (48), and Ghana (49). As seen previously (42, 50), the wild-type alleles at pfcrt K76 and pfmdr1 N86 were associated with decreased lumefantrine sensitivity. We also found an association between the wild-type pfmdr1 D1246 and mutant pfmdr1 184F genotypes and decreased lumefantrine sensitivity. Surprisingly, results for piperaquine were not consistent with those for the other aminoquinolines, chloroquine and amodiaquine, with the pfcrt K76 wild-type sequence associated with decreased piperaquine sensitivity. In a longitudinal analysis of ex vivo sensitivity, we identified increasing sensitivity to chloroquine and decreasing sensitivity to lumefantrine and piperaquine; more modest changes were seen for other tested drugs. In the only other study that assessed changes in antimalarial drug sensitivity over time, increasing sensitivity to chloroquine and amodiaquine and decreasing sensitivity to lumefantrine were seen in Kenya from 2008 to 2011, although for lumefantrine, the changes were not significant (42). In Uganda, our data consistently show that with the change to AL as the national malaria treatment regimen, lumefantrine sensitivity is decreasing.
Earlier, elimination of chloroquine use in Malawi was accompanied by the reestablishment of parasites with the wild-type pfcrt K76 genotype (51, 52) and excellent treatment efficacy of chloroquine (53). Thus, changes in parasite drug sensitivity have had clinical consequences. Recent changes suggest that we should reconsider the use of aminoquinolines to treat malaria, including AS-AQ, which showed inferior efficacy to AL in Tanzania (54) and Uganda (55, 56) some years ago but excellent recent efficacy in West and Central Africa (57–61); DP, which has shown excellent efficacy (7, 62–66); and perhaps combinations that include chloroquine (52, 67). Further, we should be cautious regarding the long-term antimalarial efficacy of AL, as although both the clinical efficacy of AL (57–61, 64–66) and ex vivo activity of DHA and lumefantrine (31, 42, 68–70) have remained good in recent studies, current results suggest that the antimalarial potency of lumefantrine is decreasing.
Our data show that antimalarial treatment regimens rapidly select for parasites with decreased drug sensitivity. With both amodiaquine-containing regimens (71) and AL (this study), recent treatment was associated with significantly diminished ex vivo drug sensitivity in subsequent infections. Similarly, treatment with AS-AQ or AL led to marked changes in genotypes in a subsequent infection, with opposite selective pressures of amodiaquine and lumefantrine (14, 15, 19–22). Interestingly, results for piperaquine differed from those for the other aminoquinolines: prior therapy with AL selected for increased chloroquine sensitivity but decreased piperaquine sensitivity in subsequent infections. Also of note, a higher recent prevalence of the chloroquine resistance pfcrt 76T mutation in Uganda compared to that in Malawi (51, 52), Tanzania (48), or Kenya (42) suggests that there is stronger selective pressure from continued chloroquine use in Uganda than in the other African countries. Nonetheless, with the establishment of AL as the treatment of choice for malaria, the prevalence of chloroquine-resistant parasites has decreased, and selection of parasites with decreased responsiveness to AL is now occurring.
We also explored the impact of antimalarial chemoprevention on parasite drug sensitivity. Regular use of daily TS, monthly SP, or monthly DP did not select for alterations in drug sensitivity. However, our study did not include directly observed therapy, and many episodes of malaria occurred in subjects not adhering to their assigned chemoprevention regimen. This conclusion is supported by a lack of detectable circulating piperaquine in 52% of subjects from the DP chemoprevention arm at the time of malaria diagnosis (11) and by the much better chemopreventive efficacy of directly observed DP in a different trial in the same region (12). To further explore this issue, we characterized parasites isolated from children deemed compliant or noncompliant with monthly DP based on circulating piperaquine levels at the time of malaria diagnosis. In this subgroup analysis, compliance with monthly DP was associated with increased prevalence of pfmdr1 86Y and 1246Y mutations, which were previously shown to be selected by therapy with amodiaquine-containing regimens (14, 15).
Associations between specific parasite pfcrt and pfmdr1 polymorphisms and sensitivity to the aminoquinolines chloroquine and MDAQ, as well as opposite associations with lumefantrine, have been consistent in many studies, and treatment with amodiaquine and lumefantrine-containing regimens has demonstrated expected selective pressure (14, 15, 19–21). In contrast, results for piperaquine have been perplexing. As noted above, in a subset of children in whom compliance with monthly DP was documented, parasites were more likely to have the same polymorphisms selected by prior therapy with amodiaquine, and similar selection by DP was seen in a recent treatment study from Uganda (19). However, this selection was not seen in treatment (17) and chemoprevention (18) trials in Burkina Faso. Further, ex vivo piperaquine sensitivity was not associated with polymorphisms in pfmdr1, and for pfcrt position 76, associations were opposite of those seen for chloroquine and MDAQ, with mutant parasites being more sensitive to piperaquine. The reasons for the differences between piperaquine and other aminoquinolines and between studies in East and West Africa are unknown, but they suggest the importance of factors in addition to the studied pfcrt and pfmdr1 polymorphisms affecting piperaquine sensitivity. Concerns about piperaquine sensitivity are urgent, as the drug has a long history of resistance (16), and recent trials have demonstrated decreased ex vivo piperaquine sensitivity and decreased treatment efficacy of DP in Cambodia (72, 73).
With changes in drug sensitivity, new strategies for the treatment and chemoprevention of malaria may be warranted. One option is to rotate drugs for treatment and/or chemoprevention. As discussed above, regimens containing chloroquine, amodiaquine, and possibly piperaquine select for parasites with increased sensitivity to lumefantrine and mefloquine, while AL and AS-MQ select for parasites with increased sensitivity to aminoquinolines. Rotating regimens might allow an optimal balance, with parasites retaining sensitivity to all leading ACTs. Considering the artemisinin component of ACTs, the resistance phenotype recently described in Southeast Asia (23, 24) appears to be mediated by an unrelated mechanism (26), but pfcrt and/or pfmdr1 polymorphisms also impact artemisinin sensitivity, with sensitivities correlated between artemisinin, lumefantrine, and mefloquine, and inversely correlated with aminoquinolines (31, 50, 74). Thus, both components of AL and AS-MQ select toward decreased drug sensitivity, but the components of AS-AQ and possibly DP select in opposite directions, potentially facilitating maintenance of effectiveness of these ACTs.
Our study has some limitations. First, due to past challenges with low-level parasitemia samples, we limited our assessments to cultures from patients with parasitemias of >1%, and ex vivo assessments were not successful for all isolates. Thus, our results may not be representative of all circulating P. falciparum in Tororo. Second, due to logistical challenges, some samples were not assessed for parasite polymorphisms, again challenging the representativeness of results. Third, we analyzed a limited number of P. falciparum genetic polymorphisms. The studied polymorphisms in pfcrt and pfmdr1 appear to play primary roles in mediating altered sensitivity to a number of relevant drugs, but nonetheless, our assessments likely missed additional mediators of altered drug sensitivity. Fourth, assessments of both ex vivo drug sensitivity and the prevalence of genetic polymorphisms are necessarily complex in an area of very high transmission intensity, such as Tororo, where infections are typically polyclonal. Thus, measures of ex vivo drug sensitivity are usually averages of multiple circulating strains, and measures of polymorphism prevalence are complicated by arbitrary cutoffs for characterization of mixed infections. Despite these limitations, our large sample size affords a good understanding of drug sensitivity in Tororo, with definitive changes occurring in the context of increasing use of AL to treat malaria.
In summary, we surveyed the drug sensitivity of P. falciparum in Tororo using parasitological and molecular methods and showed that parasites have changed remarkably in recent years. Specifically, parasites show ex vivo and molecular evidence of decreasing sensitivity to lumefantrine, a component of the first-line national malaria regimen AL. The changes to date have not led to high-level resistance to lumefantrine, but our data and other recent studies suggest that the antimalarial efficacy of AL may be at risk and that consideration of treatment with other ACTs or of rotating different regimens over time is warranted.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (AI075045, HD059454, AI089674, and TW007375) and the Doris Duke Charitable Foundation.
Control strains of P. falciparum were from the Malaria Research and Reference Reagent Resource Center.
We thank the participants in the clinical trials from which the samples were collected, their parents and guardians, and our clinical study team. We also thank Frederick Baliraine and Greta Tam for preliminary work leading to the analyses described here and Francesca Aweeka and Liusheng Huang for drug-level analyses.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.05141-14.
REFERENCES
- 1.White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM. 2014. Malaria. Lancet 383:723–735. doi: 10.1016/S0140-6736(13)60024-0. [DOI] [PubMed] [Google Scholar]
- 2.Yeka A, Gasasira A, Mpimbaza A, Achan J, Nankabirwa J, Nsobya S, Staedke SG, Donnelly MJ, Wabwire-Mangen F, Talisuna A, Dorsey G, Kamya MR, Rosenthal PJ. 2012. Malaria in Uganda: challenges to control on the long road to elimination: I. Epidemiology and current control efforts. Acta Trop 121:184–195. doi: 10.1016/j.actatropica.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenthal PJ. 2013. The interplay between drug resistance and fitness in malaria parasites. Mol Microbiol 89:1025–1038. doi: 10.1111/mmi.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nosten F, White NJ. 2007. Artemisinin-based combination treatment of falciparum malaria. Am J Trop Med Hyg 77(6 Suppl):181–192. [PubMed] [Google Scholar]
- 5.Eastman RT, Fidock DA. 2009. Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nat Rev Microbiol 7:864–874. doi: 10.1038/nrmicro2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. 2010. Guidelines for the treatment of malaria. Second edition. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf?ua=1. [Google Scholar]
- 7.Yeka A, Dorsey G, Kamya MR, Talisuna A, Lugemwa M, Rwakimari JB, Staedke SG, Rosenthal PJ, Wabwire-Mangen F, Bukirwa H. 2008. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for treating uncomplicated malaria: a randomized trial to guide policy in Uganda. PLoS One 3:e2390. doi: 10.1371/journal.pone.0002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.President's Malaria Initiative. 2010. President's Malaria Initiative Uganda malaria operational plan for FY 2010. President's Malaria Initiative. United States Agency for International Development, Washington, DC: http://www.pmi.gov/docs/default-source/default-document-library/malaria-operational-plans/fy10/uganda_mop-fy10.pdf?sfvrsn=6. [Google Scholar]
- 9.ter Kuile FO, van Eijk AM, Filler SJ. 2007. Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. JAMA 297:2603–2616. doi: 10.1001/jama.297.23.2603. [DOI] [PubMed] [Google Scholar]
- 10.Konaté AT, Yaro JB, Ouédraogo AZ, Diarra A, Gansané A, Soulama I, Kangoyé DT, Kaboré Y, Ouédraogo E, Ouédraogo A, Tiono AB, Ouédraogo IN, Chandramohan D, Cousens S, Milligan PJ, Sirima SB, Greenwood B, Diallo DA. 2011. Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide-treated bednet in Burkina Faso: a randomised, double-blind, placebo-controlled trial. PLoS Med 8:e1000408. doi: 10.1371/journal.pmed.1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bigira V, Kapisi J, Clark TD, Kinara S, Mwangwa F, Muhindo MK, Osterbauer B, Aweeka FT, Huang L, Achan J, Havlir DV, Rosenthal PJ, Kamya MR, Dorsey G. 2014. Protective efficacy and safety of three antimalarial regimens for the prevention of malaria in young Ugandan children: a randomized controlled trial. PLoS Med 11:e1001689. doi: 10.1371/journal.pmed.1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nankabirwa JI, Wandera B, Amuge P, Kiwanuka N, Dorsey G, Rosenthal PJ, Brooker SJ, Staedke SG, Kamya MR. 2014. Impact of intermittent preventive treatment with dihydroartemisinin-piperaquine on malaria in Ugandan schoolchildren: a randomized, placebo-controlled trial. Clin Infect Dis 58:1404–1412. doi: 10.1093/cid/ciu150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ecker A, Lehane AM, Clain J, Fidock DA. 2012. PfCRT and its role in antimalarial drug resistance. Trends Parasitol 28:504–514. doi: 10.1016/j.pt.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nsobya SL, Dokomajilar C, Joloba M, Dorsey G, Rosenthal PJ. 2007. Resistance-mediating Plasmodium falciparum pfcrt and pfmdr1 alleles after treatment with artesunate-amodiaquine in Uganda. Antimicrob Agents Chemother 51:3023–3025. doi: 10.1128/AAC.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humphreys GS, Merinopoulos I, Ahmed J, Whitty CJ, Mutabingwa TK, Sutherland CJ, Hallett RL. 2007. Amodiaquine and artemether-lumefantrine select distinct alleles of the Plasmodium falciparum mdr1 gene in Tanzanian children treated for uncomplicated malaria. Antimicrob Agents Chemother 51:991–997. doi: 10.1128/AAC.00875-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis TM, Hung TY, Sim IK, Karunajeewa HA, Ilett KF. 2005. Piperaquine: a resurgent antimalarial drug. Drugs 65:75–87. doi: 10.2165/00003495-200565010-00004. [DOI] [PubMed] [Google Scholar]
- 17.Somé AF, Séré YY, Dokomajilar C, Zongo I, Rouamba N, Greenhouse B, Ouédraogo JB, Rosenthal PJ. 2010. Selection of known Plasmodium falciparum resistance-mediating polymorphisms by artemether-lumefantrine and amodiaquine-sulfadoxine-pyrimethamine but not dihydroartemisinin-piperaquine in Burkina Faso. Antimicrob Agents Chemother 54:1949–1954. doi: 10.1128/AAC.01413-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Some AF, Zongo I, Compaoré YD, Sakandé S, Nosten F, Ouédraogo JB, Rosenthal PJ. 2014. Selection of drug resistance-mediating Plasmodium falciparum genetic polymorphisms by seasonal malaria chemoprevention in Burkina Faso. Antimicrob Agents Chemother 58:3660–3665. doi: 10.1128/AAC.02406-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conrad MD, LeClair N, Arinaitwe E, Wanzira H, Kakuru A, Bigira V, Muhindo M, Kamya MR, Tappero JW, Greenhouse B, Dorsey G, Rosenthal PJ. 2014. Comparative impacts over 5 years of artemisinin-based combination therapies on Plasmodium falciparum polymorphisms that modulated drug sensitivity in Ugandan children. J Infect Dis 210:344–353. doi: 10.1093/infdis/jiu141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sisowath C, Strömberg J, Mårtensson A, Msellem M, Obondo C, Björkman A, Gil JP. 2005. In vivo selection of Plasmodium falciparum pfmdr1 86N coding alleles by artemether-lumefantrine (Coartem). J Infect Dis 191:1014–1017. doi: 10.1086/427997. [DOI] [PubMed] [Google Scholar]
- 21.Zongo I, Dorsey G, Rouamba N, Tinto H, Dokomajilar C, Guiguemde RT, Rosenthal PJ, Ouedraogo JB. 2007. Artemether-lumefantrine versus amodiaquine plus sulfadoxine-pyrimethamine for uncomplicated falciparum malaria in Burkina Faso: a randomised non-inferiority trial. Lancet 369:491–498. doi: 10.1016/S0140-6736(07)60236-0. [DOI] [PubMed] [Google Scholar]
- 22.Baliraine FN, Rosenthal PJ. 2011. Prolonged selection of pfmdr1 polymorphisms after treatment of falciparum malaria with artemether-lumefantrine in Uganda. J Infect Dis 204:1120–1124. doi: 10.1093/infdis/jir486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM, Artemisinin Resistance in Cambodia 1 (ARC1) Study Consortium. 2008. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med 359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 24.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Witkowski B, Khim N, Chim P, Kim S, Ke S, Kloeung N, Chy S, Duong S, Leang R, Ringwald P, Dondorp AM, Tripura R, Benoit-Vical F, Berry A, Gorgette O, Ariey F, Barale JC, Mercereau-Puijalon O, Menard D. 2013. Reduced artemisinin susceptibility of Plasmodium falciparum ring stages in western Cambodia. Antimicrob Agents Chemother 57:914–923. doi: 10.1128/AAC.01868-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Menard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale JC, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O, Menard D. 2014. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muhindo MK, Kakuru A, Jagannathan P, Talisuna A, Osilo E, Orukan F, Arinaitwe E, Tappero JW, Kaharuza F, Kamya MR, Dorsey G. 2014. Early parasite clearance following artemisinin-based combination therapy among Ugandan children with uncomplicated Plasmodium falciparum malaria. Malar J 13:32. doi: 10.1186/1475-2875-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conrad MD, Bigira V, Kapisi J, Muhindo M, Kamya MR, Havlir DV, Dorsey G, Rosenthal PJ. 2014. Polymorphisms in K13 and falcipain-2 associated with artemisinin resistance are not prevalent in Plasmodium falciparum isolated from Ugandan children. PLoS One 9:e105690. doi: 10.1371/journal.pone.0105690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han KT, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, et al. . 2014. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mbogo GW, Nankoberanyi S, Tukwasibwe S, Baliraine FN, Nsobya SL, Conrad MD, Arinaitwe E, Kamya M, Tappero J, Staedke SG, Dorsey G, Greenhouse B, Rosenthal PJ. 2014. Temporal changes in prevalence of molecular markers mediating antimalarial drug resistance in a high malaria transmission setting in Uganda. Am J Trop Med Hyg 91:54–61. doi: 10.4269/ajtmh.13-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nsobya SL, Kiggundu M, Nanyunja S, Joloba M, Greenhouse B, Rosenthal PJ. 2010. In vitro sensitivities of Plasmodium falciparum to different antimalarial drugs in Uganda. Antimicrob Agents Chemother 54:1200–1206. doi: 10.1128/AAC.01412-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kilama M, Smith DL, Hutchinson R, Kigozi R, Yeka A, Lavoy G, Kamya MR, Staedke SG, Donnelly MJ, Drakeley C, Greenhouse B, Dorsey G, Lindsay SW. 2014. Estimating the annual entomological inoculation rate for Plasmodium falciparum transmitted by Anopheles gambiae s.l. using three sampling methods in three sites in Uganda. Malar J 13:111. doi: 10.1186/1475-2875-13-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Achan J, Kakuru A, Ikilezi G, Ruel T, Clark TD, Nsanzabana C, Charlebois E, Aweeka F, Dorsey G, Rosenthal PJ, Havlir D, Kamya MR. 2012. Antiretroviral agents and prevention of malaria in HIV-infected Ugandan children. N Engl J Med 367:2110–2118. doi: 10.1056/NEJMoa1200501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noedl H, Bronnert J, Yingyuen K, Attlmayr B, Kollaritsch H, Fukuda M. 2005. Simple histidine-rich protein 2 double-site sandwich enzyme-linked immunosorbent assay for use in malaria drug sensitivity testing. Antimicrob Agents Chemother 49:3575–3577. doi: 10.1128/AAC.49.8.3575-3577.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basco L. 2007. Field application of in vitro assays for the sensitivity of human malaria parasites to antimalarial drugs. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2007/9789241595155_eng.pdf?ua=1. [Google Scholar]
- 36.Plowe CV, Djimde A, Bouare M, Doumbo O, Wellems TE. 1995. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am J Trop Med Hyg 52:565–568. [DOI] [PubMed] [Google Scholar]
- 37.Djimdé A, Doumbo OK, Cortese JF, Kayentao K, Doumbo S, Diourté Y, Coulibaly D, Dicko A, Su XZ, Nomura T, Fidock DA, Wellems TE, Plowe CV. 2001. A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med 344:257–263. doi: 10.1056/NEJM200101253440403. [DOI] [PubMed] [Google Scholar]
- 38.Duraisingh MT, Jones P, Sambou I, von Seidlein L, Pinder M, Warhurst DC. 2000. The tyrosine-86 allele of the pfmdr1 gene of Plasmodium falciparum is associated with increased sensitivity to the anti-malarials mefloquine and artemisinin. Mol Biochem Parasitol 108:13–23. doi: 10.1016/S0166-6851(00)00201-2. [DOI] [PubMed] [Google Scholar]
- 39.Leclair NP, Conrad MD, Baliraine FN, Nsanzabana C, Nsobya SL, Rosenthal PJ. 2013. Optimization of a ligase detection reaction fluorescent microsphere assay for the characterization of resistance-mediating polymorphisms in African samples of Plasmodium falciparum. J Clin Microbiol 51:2564–2570. doi: 10.1128/JCM.00904-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ringwald P, Bickii J, Basco LK. 1996. In vitro activity of antimalarials against clinical isolates of Plasmodium falciparum in Yaounde, Cameroon. Am J Trop Med Hyg 55:254–258. [DOI] [PubMed] [Google Scholar]
- 41.Kaddouri H, Djimde A, Dama S, Kodio A, Tekete M, Hubert V, Kone A, Maiga H, Yattara O, Fofana B, Sidibe B, Sangare CP, Doumbo O, Le Bras J. 2008. Baseline in vitro efficacy of ACT component drugs on Plasmodium falciparum clinical isolates from Mali. Int J Parasitol 38:791–798. doi: 10.1016/j.ijpara.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Eyase FL, Akala HM, Ingasia L, Cheruiyot A, Omondi A, Okudo C, Juma D, Yeda R, Andagalu B, Wanja E, Kamau E, Schnabel D, Bulimo W, Waters NC, Walsh DS, Johnson JD. 2013. The role of Pfmdr1 and Pfcrt in changing chloroquine, amodiaquine, mefloquine and lumefantrine susceptibility in western-Kenya P. falciparum samples during 2008–2011. PLoS One 8:e64299. doi: 10.1371/journal.pone.0064299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dahlström S, Ferreira PE, Veiga MI, Sedighi N, Wiklund L, Martensson A, Farnert A, Sisowath C, Osorio L, Darban H, Andersson B, Kaneko A, Conseil G, Björkman A, Gil JP. 2009. Plasmodium falciparum multidrug resistance protein 1 and artemisinin-based combination therapy in Africa. J Infect Dis 200:1456–1464. doi: 10.1086/606009. [DOI] [PubMed] [Google Scholar]
- 44.Dorsey G, Kamya MR, Singh A, Rosenthal PJ. 2001. Polymorphisms in the Plasmodium falciparum pfcrt and pfmdr-1 genes and clinical response to chloroquine in Kampala, Uganda. J Infect Dis 183:1417–1420. doi: 10.1086/319865. [DOI] [PubMed] [Google Scholar]
- 45.Francis D, Nsobya SL, Talisuna A, Yeka A, Kamya MR, Machekano R, Dokomajilar C, Rosenthal PJ, Dorsey G. 2006. Geographic differences in antimalarial drug efficacy in Uganda are explained by differences in endemicity and not by known molecular markers of drug resistance. J Infect Dis 193:978–986. doi: 10.1086/500951. [DOI] [PubMed] [Google Scholar]
- 46.Wootton JC, Feng X, Ferdig MT, Cooper RA, Mu J, Baruch DI, Magill AJ, Su XZ. 2002. Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature 418:320–323. doi: 10.1038/nature00813. [DOI] [PubMed] [Google Scholar]
- 47.Gasasira AF, Kamya MR, Ochong EO, Vora N, Achan J, Charlebois E, Ruel T, Kateera F, Meya DN, Havlir D, Rosenthal PJ, Dorsey G. 2010. Effect of trimethoprim-sulphamethoxazole on the risk of malaria in HIV-infected Ugandan children living in an area of widespread antifolate resistance. Malar J 9:177. doi: 10.1186/1475-2875-9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malmberg M, Ngasala B, Ferreira PE, Larsson E, Jovel I, Hjalmarsson A, Petzold M, Premji Z, Gil JP, Bjorkman A, Martensson A. 2013. Temporal trends of molecular markers associated with artemether-lumefantrine tolerance/resistance in Bagamoyo district, Tanzania. Malar J 12:103. doi: 10.1186/1475-2875-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duah NO, Matrevi SA, de Souza DK, Binnah DD, Tamakloe MM, Opoku VS, Onwona CO, Narh CA, Quashie NB, Abuaku B, Duplessis C, Kronmann KC, Koram KA. 2013. Increased pfmdr1 gene copy number and the decline in pfcrt and pfmdr1 resistance alleles in Ghanaian Plasmodium falciparum isolates after the change of anti-malarial drug treatment policy. Malar J 12:377. doi: 10.1186/1475-2875-12-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mwai L, Kiara SM, Abdirahman A, Pole L, Rippert A, Diriye A, Bull P, Marsh K, Borrmann S, Nzila A. 2009. In vitro activity of piperaquine, lumefantrine and dihydroartemisinin in Kenyan Plasmodium falciparum isolates and polymorphisms in Pfcrt and Pfmdr1. Antimicrob Agents Chemother 53:5069–5073. doi: 10.1128/AAC.00638-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kublin JG, Cortese JF, Njunju EM, Mukadam RA, Wirima JJ, Kazembe PN, Djimde AA, Kouriba B, Taylor TE, Plowe CV. 2003. Reemergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. J Infect Dis 187:1870–1875. doi: 10.1086/375419. [DOI] [PubMed] [Google Scholar]
- 52.Frosch AE, Laufer MK, Mathanga DP, Takala-Harrison S, Skarbinski J, Claassen CW, Dzinjalamala FK, Plowe CV. 2014. Return of Widespread Chloroquine-Sensitive Plasmodium falciparum to Malawi. J Infect Dis 210:1110–1114. doi: 10.1093/infdis/jiu216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laufer MK, Thesing PC, Eddington ND, Masonga R, Dzinjalamala FK, Takala SL, Taylor TE, Plowe CV. 2006. Return of chloroquine antimalarial efficacy in Malawi. N Engl J Med 355:1959–1966. doi: 10.1056/NEJMoa062032. [DOI] [PubMed] [Google Scholar]
- 54.Mutabingwa TK, Anthony D, Heller A, Hallett R, Ahmed J, Drakeley C, Greenwood BM, Whitty CJ. 2005. Amodiaquine alone, amodiaquine+sulfadoxine-pyrimethamine, amodiaquine+artesunate, and artemether-lumefantrine for outpatient treatment of malaria in Tanzanian children: a four-arm randomised effectiveness trial. Lancet 365:1474–1480. doi: 10.1016/S0140-6736(05)66417-3. [DOI] [PubMed] [Google Scholar]
- 55.Bukirwa H, Yeka A, Kamya MR, Talisuna A, Banek K, Bakyaita N, Rwakimari JB, Rosenthal PJ, Wabwire-Mangen F, Dorsey G, Staedke SG. 2006. Artemisinin combination therapies for treatment of uncomplicated malaria in Uganda. PLoS Clin Trials 1:e7. doi: 10.1371/journal.pctr.0010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dorsey G, Staedke S, Clark TD, Njama-Meya D, Nzarubara B, Maiteki-Sebuguzi C, Dokomajilar C, Kamya MR, Rosenthal PJ. 2007. Combination therapy for uncomplicated falciparum malaria in Ugandan children: a randomized trial. JAMA 297:2210–2219. doi: 10.1001/jama.297.20.2210. [DOI] [PubMed] [Google Scholar]
- 57.Espié E, Lima A, Atua B, Dhorda M, Flevaud L, Sompwe EM, Palma Urrutia PP, Guerin PJ. 2012. Efficacy of fixed-dose combination artesunate-amodiaquine versus artemether-lumefantrine for uncomplicated childhood Plasmodium falciparum malaria in Democratic Republic of Congo: a randomized non-inferiority trial. Malar J 11:174. doi: 10.1186/1475-2875-11-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Faye B, Kuete T, Kiki-Barro CP, Tine RC, Nkoa T, Ndiaye JL, Kakpo CA, Sylla K, El Menan H, Gaye O, Faye O, Same-Ekobo A, Moussa K. 2012. Multicentre study evaluating the non-inferiority of the new paediatric formulation of artesunate/amodiaquine versus artemether/lumefantrine for the management of uncomplicated Plasmodium falciparum malaria in children in Cameroon, Ivory Coast and Senegal. Malar J 11:433. doi: 10.1186/1475-2875-11-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schramm B, Valeh P, Baudin E, Mazinda CS, Smith R, Pinoges L, Dhorda M, Boum Y Jr, Sundaygar T, Zolia YM, Jones JJ, Comte E, Houze P, Jullien V, Carn G, Kiechel JR, Ashley EA, Guerin PJ. 2013. Efficacy of artesunate-amodiaquine and artemether-lumefantrine fixed-dose combinations for the treatment of uncomplicated Plasmodium falciparum malaria among children aged six to 59 months in Nimba County, Liberia: an open-label randomized non-inferiority trial. Malar J 12:251. doi: 10.1186/1475-2875-12-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Djallé D, Njuimo SP, Manirakiza A, Laganier R, Le Faou A, Rogier C. 2014. Efficacy and safety of artemether + lumefantrine, artesunate + sulphamethoxypyrazine-pyrimethamine and artesunate + amodiaquine and sulphadoxine-pyrimethamine + amodiaquine in the treatment of uncomplicated falciparum malaria in Bangui, Central African Republic: a randomized trial. Malar J 13:9. doi: 10.1186/1475-2875-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tinto H, Diallo S, Zongo I, Guiraud I, Valea I, Kazienga A, Kpoda H, Sorgho H, Ouedraogo JB, Guiguemde TR, D'Alessandro U. 2014. Effectiveness of artesunate-amodiaquine vs. artemether-lumefantrine for the treatment of uncomplicated falciparum malaria in Nanoro, Burkina Faso: a non-inferiority randomised trial. Trop Med Int Health 19:469–475. doi: 10.1111/tmi.12274. [DOI] [PubMed] [Google Scholar]
- 62.Kamya MR, Yeka A, Bukirwa H, Lugemwa M, Rwakimari JB, Staedke SG, Talisuna AO, Greenhouse B, Nosten F, Rosenthal PJ, Wabwire-Mangen F, Dorsey G. 2007. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for treatment of malaria: a randomized trial. PLoS Clin Trials 2:e20. doi: 10.1371/journal.pctr.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zongo I, Dorsey G, Rouamba N, Dokomajilar C, Sere Y, Rosenthal PJ, Ouedraogo JB. 2007. Randomized comparison of amodiaquine plus sulfadoxine-pyrimethamine, artemether-lumefantrine, and dihydroartemisinin-piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria in Burkina Faso. Clin Infect Dis 45:1453–1461. doi: 10.1086/522985. [DOI] [PubMed] [Google Scholar]
- 64.Agarwal A, McMorrow M, Onyango P, Otieno K, Odero C, Williamson J, Kariuki S, Kachur SP, Slutsker L, Desai M. 2013. A randomized trial of artemether-lumefantrine and dihydroartemisinin-piperaquine in the treatment of uncomplicated malaria among children in western Kenya. Malar J 12:254. doi: 10.1186/1475-2875-12-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ogutu BR, Onyango KO, Koskei N, Omondi EK, Ongecha JM, Otieno GA, Obonyo C, Otieno L, Eyase F, Johnson JD, Omollo R, Perkins DJ, Akhwale W, Juma E. 2014. Efficacy and safety of artemether-lumefantrine and dihydroartemisinin-piperaquine in the treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children aged less than five years: results of an open-label, randomized, single-centre study. Malar J 13:33. doi: 10.1186/1475-2875-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wanzira H, Kakuru A, Arinaitwe E, Bigira V, Muhindo MK, Conrad M, Rosenthal PJ, Kamya MR, Tappero JW, Dorsey G. 2014. Longitudinal outcomes in a cohort of Ugandan children randomized to artemether-lumefantrine versus dihydroartemisinin-piperaquine for the treatment of malaria. Clin Infect Dis 59:509–516. doi: 10.1093/cid/ciu353. [DOI] [PubMed] [Google Scholar]
- 67.Laufer MK, Thesing PC, Dzinjalamala FK, Nyirenda OM, Masonga R, Laurens MB, Stokes-Riner A, Taylor TE, Plowe CV. 2012. A longitudinal trial comparing chloroquine as monotherapy or in combination with artesunate, azithromycin or atovaquone-proguanil to treat malaria. PLoS One 7:e42284. doi: 10.1371/journal.pone.0042284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fall B, Pascual A, Sarr FD, Wurtz N, Richard V, Baret E, Dieme Y, Briolant S, Bercion R, Wade B, Tall A, Pradines B. 2013. Plasmodium falciparum susceptibility to anti-malarial drugs in Dakar, Senegal, in 2010: an ex vivo and drug resistance molecular markers study. Malar J 12:107. doi: 10.1186/1475-2875-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Issaka M, Salissou A, Arzika I, Guillebaud J, Maazou A, Specht S, Zamanka H, Fandeur T. 2013. Ex vivo responses of Plasmodium falciparum clinical isolates to conventional and new antimalarial drugs in Niger. Antimicrob Agents Chemother 57:3415–3419. doi: 10.1128/AAC.02383-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tinto H, Bonkian LN, Nana LA, Yerbanga I, Lingani M, Kazienga A, Valea I, Sorgho H, Kpoda H, Guiguemde TR, Ouedraogo JB, Mens PF, Schallig H, D'Alessandro U. 2014. Ex vivo anti-malarial drugs sensitivity profile of Plasmodium falciparum field isolates from Burkina Faso five years after the national policy change. Malar J 13:207. doi: 10.1186/1475-2875-13-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nawaz F, Nsobya SL, Kiggundu M, Joloba M, Rosenthal PJ. 2009. Selection of parasites with diminished drug susceptibility by amodiaquine-containing antimalarial regimens in Uganda. J Infect Dis 200:1650–1657. doi: 10.1086/647988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leang R, Barrette A, Bouth DM, Menard D, Abdur R, Duong S, Ringwald P. 2013. Efficacy of dihydroartemisinin-piperaquine for treatment of uncomplicated Plasmodium falciparum and Plasmodium vivax in Cambodia, 2008 to 2010. Antimicrob Agents Chemother 57:818–826. doi: 10.1128/AAC.00686-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saunders DL, Vanachayangkul P, Lon C, U.S. Army Military Malaria Research Program, National Center for Parasitology, Entomology, and Malaria Control (CNM), Royal Cambodian Armed Forces. 2014. Dihydroartemisinin-piperaquine failure in Cambodia. N Engl J Med 371:484–485. doi: 10.1056/NEJMc1403007. [DOI] [PubMed] [Google Scholar]
- 74.Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF. 2000. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature 403:906–909. doi: 10.1038/35002615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.