Abstract
The increasing resistance of malaria parasites to almost all available drugs calls for the characterization of novel targets and the identification of new compounds. Carotenoids are polyisoprenoids from plants, algae, and some bacteria, and they are biosynthesized by Plasmodium falciparum but not by mammalian cells. Biochemical and reverse genetics approaches were applied to demonstrate that phytoene synthase (PSY) is a key enzyme for carotenoid biosynthesis in P. falciparum and is essential for intraerythrocytic growth. The known PSY inhibitor squalestatin reduces biosynthesis of phytoene and kills parasites during the intraerythrocytic cycle. PSY-overexpressing parasites showed increased biosynthesis of phytoene and its derived product phytofluene and presented a squalestatin-resistant phenotype, suggesting that this enzyme is the primary target of action of this drug in the parasite.
INTRODUCTION
Malaria is one of the most important infectious diseases in the world, causing about 200 million clinical cases and over 600,000 deaths every year. Lethal forms of the disease are caused mostly by Plasmodium falciparum, and there is an increasing resistance of this parasite to virtually all current drugs, including artemisinin, in five southeast Asian countries and probably in South America (1). This calls for the identification of new therapeutic targets and the development of new drugs (2). The causative agent of malaria is a protozoan of the genus Plasmodium, transmitted by the female Anopheles mosquito host, in which occurs the sexual phase of the parasite's life cycle. Five species of Plasmodium infect humans, and P. falciparum causes most cases of morbidity and mortality (3). Given the genetic flexibility and the resulting development of resistance to almost every drug, a comprehensive understanding of plasmodial metabolic pathways is essential for the development of new chemotherapeutic strategies. An important target for the development of new antimalarial drugs is isoprenoid biosynthesis (Fig. 1), which occurs via the 2-C-methyl-d-erythritol-4-phosphate pathway (MEP) (4–8) in P. falciparum, some plants, and bacteria (9, 10). In contrast, most animal cells, certain eubacteria, archaea, and fungi synthesize isoprenoid precursors through the mevalonate pathway (11). In the case of malaria parasites, especially the most virulent species, P. falciparum, a series of new plantlike enzymes from this metabolic pathway was discovered recently. Some of these enzymes are associated with the apicoplast (12), whereas the nature of the others and the pathways they are involved in remain elusive to current bioinformatics approaches.
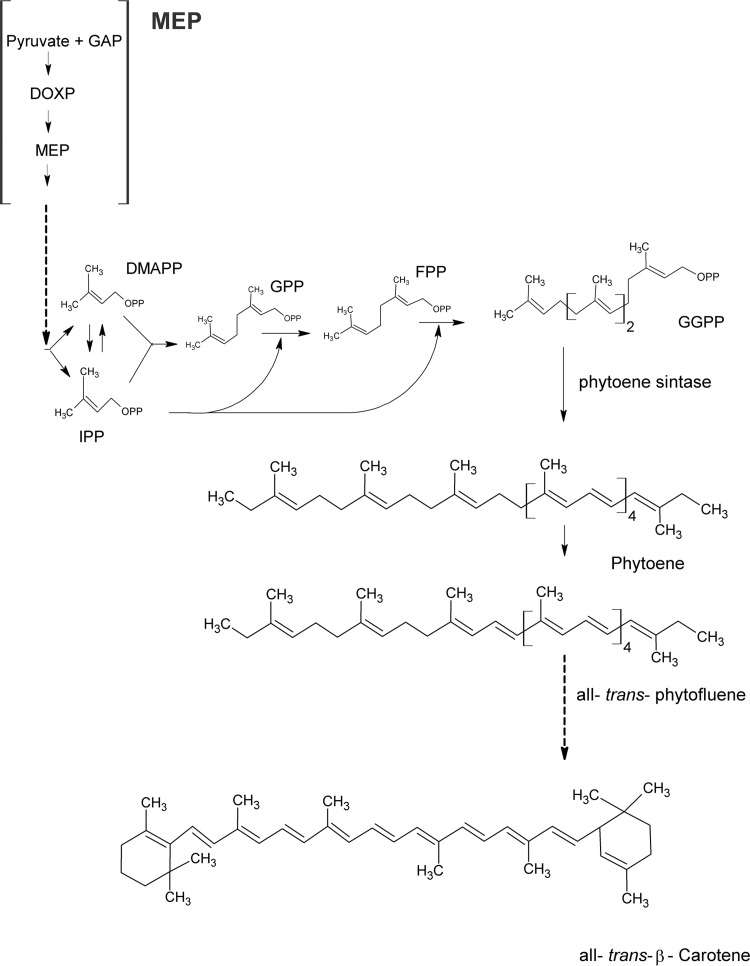
FIG 1.
Carotenoid biosynthesis pathway in P. falciparum. Downstream of the MEP pathway, the carotenoid biosynthesis starts with the condensation of two molecules of geranylgeranyl pyrophosphate (GGPP) by phytoene synthase enzyme (PSY) to form phytoene, the initial C40 carotenoid skeleton.
Many isoprenoids from the MEP pathway, such as carotenoids and ubiquinones (biosynthesized by P. falciparum) (8, 13), are essential components of the cellular machinery of many organisms, participating in a variety of biological processes. All carotenoids possess a polyisoprenoid structure (Fig. 1), a long conjugated chain of double bonds, and an almost bilateral symmetry around the central double bond. Among these biochemical pathways, carotenoid biosynthesis is an attractive target for investigation, because it is essential in algae, higher plants, bacteria, and fungi, and it is absent from most animals, including mammals (14). Carotenoid biosynthesis in intraerythrocytic stages of P. falciparum has been demonstrated, suggesting it plays a role in parasite development and/or replication; therefore, it could be a drug target (13). Carotenoid biosynthesis starts with the condensation of two molecules of geranylgeranyl pyrophosphate (GGPP) to form phytoene, the initial C40 carotenoid skeleton (14). This reaction is catalyzed by the enzyme phytoene synthase (PSY). The gene encoding PSY in P. falciparum has been identified (PlasmoDB accession no. PF3D7_0202700) and its product characterized (13). It appears to be a bifunctional enzyme, since it exerts octaprenyl pyrophosphate synthase (OPP) activity, which is involved in the elongation of the isoprenic chain, which then is attached to the benzoquinone ring that comes from the shikimate pathway (15). The plasmodial enzyme is an example of a carotenogenic enzyme with a continuous line of evolution from archaea to bacteria (via cyanobacteria) and plants (16, 17) containing two activities.
Inhibiting Plasmodium PSY and, therefore, the first step in carotenoid biosynthesis could help to reveal the role of these isoprenoid compounds in the parasite intraerythrocytic cycle, demonstrating whether this metabolic pathway could be a drug target. The compound zaragozic acid, also known as squalestatin, is a carboxylic acid, with the molecular structure C35H43O14Na3 (2,8-dioxabicyclo-[3.2.1]-octan-3 core acid, 4,5-tricarboxylic), that was discovered by screening metabolites of filamentous fungi for inhibitors of squalene synthase, the enzyme responsible for the first step of sterol biosynthesis (18–21). Detailed analysis disclosed that it acts as a competitive inhibitor of squalene synthase by mimicking the farnesyl-PP substrate or the stable intermediate presqualene-PP with its bicyclic, highly acidic core (22). Neudert et al. showed that squalestatin also inhibits PSY from the enterobacterium Erwinia uredovora (23). The inhibition of phytoene synthase by an inhibitor of squalene synthase presumably can be explained by similar catalytic mechanisms proposed for both enzymes during conversion of two molecules of GGPP or farnesyl pyrophosphate ammonium salt (FPP), respectively (23, 24).
Recent data showed that squalestatin has an inhibitory effect on P. falciparum in vitro growth and a synergistic effect when combined with other drugs (M. F. da Silva, A. Y. Saito, V. J. Peres, A. C. Oliveira, and A. M. Katzin, submitted for publication), suggesting PSY activity is the target, since Plasmodium does not have squalene synthase or synthesize sterols (25). In this study, we applied biochemical and reverse genetics approaches to demonstrate that PSY is the main target of squalestatin in P. falciparum and that the first carotenoid, phytoene, is essential for parasite development during the intraerythrocytic cycle.
MATERIALS AND METHODS
Reagents.
[1-(n)-3H]geranylgeranyl pyrophosphate triammonium salt, [1-(n)-3H]GGPP (16.5 Ci/mmol), and [1-(n)-3H]farnesyl pyrophosphate triammonium salt, [1-(n)-3H]FPP (23 Ci/mmol), were obtained from GE Healthcare. Life Technologies supplied Albumax I. All solvents used were high-performance liquid chromatography (HPLC) grade or better. Sigma-Aldrich provided isopentenyl pyrophosphate (IPP), FPP, GGPP, all-trans-lutein, all-trans-β-carotene, squalestatin, and all other biochemical reagents. Carotenoid standards were donated by DSM Nutritional Products (Basel, Switzerland).
Plasmid construction.
The sequences for the oligonucleotides used are described in Table 1. The expression vector pRM2-GFP-HA-DD24 (26) was digested with NheI and SpeI and religated to delete the DD24 sequence, generating pRM2-GFP-HA. The P. falciparum genomic DNA (gDNA) sequence that encodes the bifunctional enzyme OPP/PSY (PlasmoDB no. PF3D7_0202700), nucleotides 687 to 1614, was PCR amplified with oligonucleotides F-int-PSY-Sma and R-PSY-Mlu, digested with SmaI and MluI, and cloned in pRM2-GFP-HA via the same sites, replacing the MSP2 promoter and the green fluorescent protein (GFP) gene and generating the integration vector pPSY/OPP-HA. DD24 was retrieved from pRM2-GFP-HA-DD24 digested with MluI and NotI and cloned via the same sites in pPSY/OPP-HA to generate the integration vector pPSY/OPP-HA-DD24.
TABLE 1.
Oligonucleotides utilized in PCR amplification
| Name | Sequencea |
|---|---|
| F-int-PSY-Sma | CCCGGGTTTCAAATCAAATAAACTCACGTC |
| R-PSY-Mlu | ACGCGTTTTGACGTTTCTTGATAACACGTTTAAG |
| F-PSY-Xho | CTCGAGATGGTTCACCTAAGTAAAAGAAATAATATT |
| F-PSY | TGGTACGGGTTCACCAAAAAT |
| R-PSY | CATTTTGAGTGCTTCTTCAACA |
Underlining denotes restriction sites.
The coding sequence of the P. falciparum OPP/PSY gene was PCR amplified from cDNA with oligonucleotides F-PSY-Xho and R-PSY-Mlu, digested with XhoI and MluI, and cloned in the same sites of pRM2-GFP-HA, replacing GFP and generating Plasmodium expression vector pRM2-PSY-HA.
P. falciparum culture.
Cultures of P. falciparum clone 3D7 were grown as described previously (27), except that human serum was replaced with Albumax I (0.5%; Invitrogen/Life Technologies). Parasite multiplication was monitored by microscopic evaluation of Giemsa-stained thin smears. Schizont stages were purified with magnetic columns (magnetically activated cell sorting [MACS] separation columns; CS; Miltenyi Biotec) (28). Column preequilibration, washing, and elution all were carried out at room temperature with RPMI 1640 (Sigma-Aldrich). For schizont purification, the culture was centrifuged (2,000 × g for 5 min) and the pellet resuspended in RMPI 1640 (1:10, vol/vol), and 10 ml of the 10% suspension of erythrocytes was applied to a CS column assembled in a magnetic unit, where only schizonts are retained. After washing the column with 50 ml of RMPI 1640, the column was removed from the magnetic field and its contents eluted with 50 ml of RMPI 1640, and the schizont-stage parasites were centrifuged at 2,000 × g for 5 min at room temperature. The supernatant was discarded, and the pellet of parasites was stored in liquid N2 for subsequent analysis.
Parasite transfection.
Parasites were transfected as previously described (29), using the electroporation conditions established elsewhere (30). Briefly, P. falciparum 3D7 was cultured in 4% hematocrit in RPMI-HEPES supplemented with 0.5% Albumax I (Invitrogen). Ring-stage parasites at 5 to 8% parasitemia were transfected with 150 μg of plasmid DNA. The culture media were changed on the second day, and parasites were subjected to drug pressure with 2.5 nM WR99210 (stable) on the third day. Transfected parasites were cultured under standard conditions until parasites reappeared and normal growth was reestablished. When necessary, the integration at the genomic OPP/PSY locus was selected by intermittent exposure and retrieval of WR99210 and detected by PCR.
cDNA preparation and qRT-PCR.
RNA was extracted using TRIzol LS (Invitrogen) by following instructions of the manufacturer. The washed RNA pellet was dried briefly at room temperature, dissolved in water, and stored at −80°C until use. About 5 μg of total RNA was used for cDNA synthesis. Briefly, total RNA was treated 3 times with DNase I (Fermentas) prior to synthesis to prevent genomic DNA contamination. The treated RNA was reverse transcribed using MuLV-Revert Aid reverse transcriptase (Fermentas) and random hexamer primers by following the manufacturers' instructions. Oligonucleotides that amplify the transcript that encodes the enzyme OPP/PSY were designed using Primer3 (F-PSY; R-PSY) (http://frodo.wi.mit.edu/). The internal control transcript used for calibration throughout the experiments was locus seryl t-RNA transferase (PlasmoDB no. PF3D7_0717700), previously shown as a reliable control (31), since its transcript level does not vary greatly during the intraerythrocytic cycle. Thus, the relative mRNA expression was obtained using the formula 2−ΔCT. All experiments were performed in duplicate.
Inhibition tests with zaragozic acid (squalestatin).
Squalestatin was dissolved in water, resulting in 10 mM stock solutions (23). We applied the method proposed by Desjardins et al. and Moneriz et al. (32, 33) to determine the 50% inhibitory concentrations after 48 h (IC50s). Briefly, synchronic ring-stage parasite cultures (5% hematocrit and 1% parasitemia) were exposed to increasing drug concentrations, and parasitemia was determined by Giemsa-stained smears after 2 days. All tests were performed in triplicates from three independent experiments. The IC50s for growth inhibition were calculated by nonlinear regression in GraphPad Prism (GraphPad Software, Inc., San Diego, CA).
For isoprenoid biosynthesis inhibition, schizont-stage parasites were treated with squalestatin at the IC50 for 48 h, and parasites were labeled with the metabolic precursors during the last 16 h.
Metabolic labeling and carotenoid extraction.
Synchronous cultures of P. falciparum in the trophozoite stage were labeled with [1-3H]GGPP (0.75 μCi/ml) or [1-(n)-3H]FPP (0.75 μCi/ml) diluted in complete RPMI 1640 medium for 16 h. Subsequently, infected red blood cells with parasites in the schizont stage were concentrated with magnetic columns. Extraction was performed by mixing the cell pellet with four volumes of ice-cold acetone, which was centrifuged at 8,000 × g for 5 min. The supernatant was collected and the extraction repeated 3 times. The pooled acetone phases were dried under a nitrogen stream and stored in liquid nitrogen (34).
RP-HPLC.
For reverse-phase HPLC (RP-HPLC), the acetone extracts were resuspended in 20 μl of methyl tertiary-butyl ether and then analyzed using a YMC C30 polymeric column (4.6 by 250 mm, 3 μm and/or 5 μm) (YMC Inc.) in a gradient system (13). A carotenoid extract mixture in methyl tertiary-butyl ether was coinjected and served as a standard. We carried out the analyses in an HPLC-photodiode array (PDA) equipped with a model 600 quaternary solvent delivery system (Waters, Milford, MA) and an on-line degasser, a Rheodyne injection valve with a 20-μl loop, and an external oven coupled to the model 996 PDA detector (Waters). We used Millenium Waters software for data acquisition and processing. The PDA was set at 450, 346, and 288 nm for the analysis of carotenoids (34).
Western blot analyses.
Synchronous cultures of transfected parasites were recovered in each stage. Ring, trophozoite, and schizont stages were treated with 0.15% saponin in RPMI media to release hemoglobin from the red blood cells. Proteins were extracted with 2D buffer (7 M urea, 2 M thiourea, 2% ASB-14) (35) for separation by SDS-PAGE. The gel then was transferred to nitrocellulose membrane (Amersham) for 1 h using a Trans-Blot semidry electroblotter (Bio-Rad) (36). After blocking with 1% casein in phosphate-buffered saline (PBS), the membranes were incubated with an α-hemagglutinin (HA) monoclonal antibody (1:500 dilution; Sigma-Aldrich) or with the control antibody α-PTEX150 (1:1,000) (37) or α-MSP2 (1:500) (38), diluted in blocking solution for 1 h at room temperature or 14 h at 4°C, washed in PBS, and incubated with an anti-mouse IgG-labeled, peroxidase-conjugated secondary antibody diluted in blocking solution. After PBS washing, blots were incubated with an ECL (enhanced chemiluminescence) detection kit according to the instructions of the manufacturer, and the signal was detected with the IQ350 apparatus (GE Healthcare).
RESULTS
Effect of squalestatin on P. falciparum carotenoid biosynthesis.
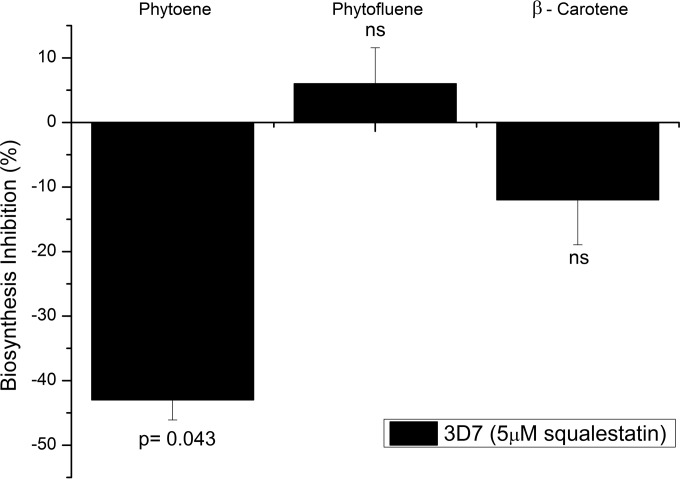
To determine the effect of squalestatin on carotenoid biosynthesis, parasites in schizont stage were cultured at the IC50 of the drug (5 μM) for 48 h and metabolically labeled with the carotenoid precursor [1-3H]GGPP during the last 16 h. Equal quantities of extracts from schizont-stage parasites, treated and untreated, were analyzed by RP-HPLC, which revealed that biosynthesis of the first carotenoid phytone had significantly diminished by 43.6% ± 5% in parasites treated with drug compared to the levels in untreated parasites (Fig. 2). Biosynthesis of the other carotenoids phytofluene and β-carotene were not significantly affected, suggesting specific inhibition of PSY rather than a general toxic effect of the drug.
FIG 2.
Effect of squalestatin on carotenoid biosynthesis. Parasites were treated with 5 μM squalestatin for 48 h, and carotenoid biosynthesis was determined by HPLC. The bars represent the relationship between squalestatin-treated parasites and controls (means ± standard deviations; n = 3 experiments). Significant differences comparing control parasites and parasites treated with squalestatin were determined by one-way analysis of variance (ANOVA). Significant P values (P < 0.05) are shown, and ns denotes nonsignificant P values (P > 0.05).
OPP/PSY is essential and constitutively expressed in the intraerythrocytic cycle.
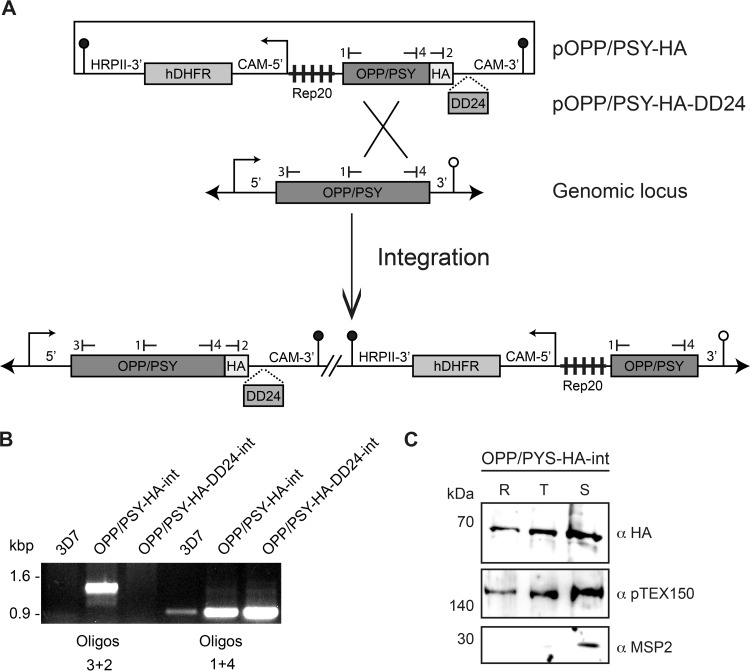
In order to obtain genetic proof that the biosynthesis of phytoene actually is essential for P. falciparum, we attempted to generate a transgenic parasite line where expression of the bifunctional enzyme OPP/PSY could be regulated (Fig. 3). To this end, 3D7 parasites were transfected with the vector pOPP/PSY-HA-DD24 and cultured in the presence of Shld-1. After the expected integration into the genomic locus (Fig. 3A), OPP/PSY should be expressed in fusion at its C terminus with a triple repeat of the HA epitope, which is used for detection by Western blotting, and the destabilization domain (DD24). Expression would be dependent on the ligand Shld-1, permitting the regulation of OPP/PSY levels. After three independent transfections, parasites with the plasmid integrated were never recovered (Fig. 3B), suggesting that OPP/PSY does not retain its physiological function when in fusion with a 15-kDa tag or that its expression levels were too low even in the presence of Shld-1.
FIG 3.
OPP/PSY is constitutively expressed and essential during the intraerythrocytic cycle. (A) Outline of the HA and HA-DD24 targeting vectors and their predicted integration into the wild-type OPP/PSY genomic locus. (B) Diagnostic PCR of integration using genomic DNA from the wild-type 3D7 strain and the transfected lines and oligonucleotides specific to the integrated lines (3 + 2) or common to all lines (1 + 4). Binding sites for the oligonucleotides used are shown in panel A. (C) Expression levels of OPP/PSY during the three intraerythrocytic stages of OPP/PSY-HA-int parasites were determined by Western blotting using α-HA antibody. Antibodies for a constitutively expressed protein (PTEX150) and a schizont-expressed protein (MSP2) were used as synchronization controls. R, ring; T, trophozoite; S, schizont.
To confirm that the OPP/PSY genomic locus was not refractory to DNA integration, we generated a similar transgenic line, except that after integration the bifunctional enzyme should be expressed at endogenous levels in fusion with the significantly smaller 3-kDa HA tag (Fig. 3A). Parasites with the plasmid integrated were detected by PCR (data not shown) and cloned by limiting dilution, generating the clonal line OPP/PSY-HA-int, in which the integration was confirmed by PCR (Fig. 3B).
After plasmid integration, the gene encoding the fusion OPP/PSY-HA is supposed to be under the control of the endogenous promoter and only one copy expressed, making this line suitable to determine the expression profile of OPP/PSY. Protein samples were extracted from OPP/PSY-HA-int parasites synchronized in the three main stages (ring, trophozoite, and schizont) and detected with a monoclonal antibody against HA (Fig. 3C). As controls of parasite synchronization, antibodies that recognize the constitutively expressed protein pTEX150 (37) and the schizont-specific protein MSP2 (38) were used. The results indicate that OPP/PSY is constitutively expressed in all stages during the asexual intraerythrocytic cycle of P. falciparum, with its levels peaking at schizont stage. OPP/PSY-HA-int growth was similar to that of 3D7 parasites, and expression was stable after several months in culture, suggesting that neither the 3-kDa HA tag nor the integrated plasmid affected the function of this enzyme (data not shown).
OPP/PSY overexpression.
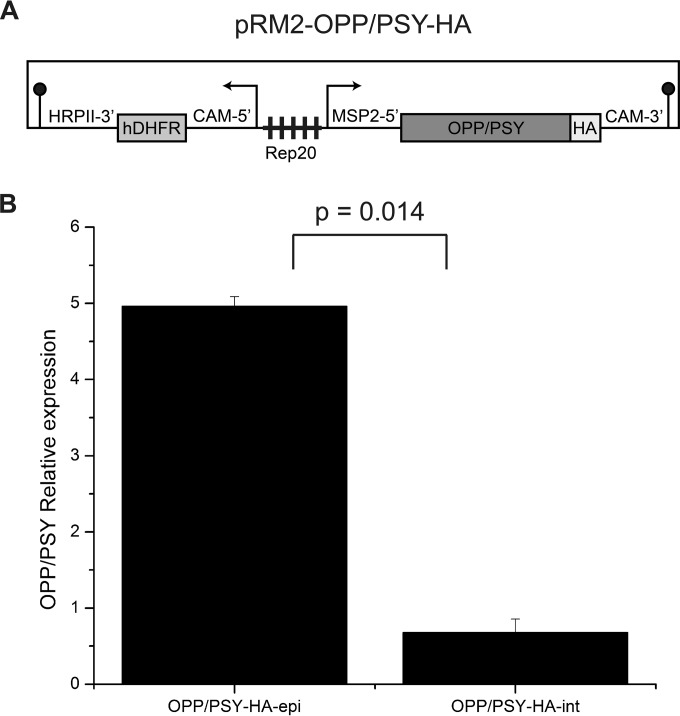
To gather further proof that phytoene is essential and that PSY is the main target of squalestatin in the parasites, we attempted to overexpress OPP/PSY using a pEF-based vector (26), from which the gene would be under the control of the constitutive ef1-α promoter (39, 40). It was expected that OPP/PSY would be overexpressed in the transfected parasites, since plasmids usually are maintained as concatemeric episomes of multiple copies. However, we were never able to generate transfected parasites, suggesting constitutive overexpression is toxic (data not shown). To attenuate any possible toxic effect of OPP/PSY overexpression, the ef1-α promoter was replaced with the schizont-specific MSP2 promoter (38), and the resulting plasmid pRM2-OPP/PSY-HA was successfully transfected in 3D7 parasites, generating the transgenic line OPP/PSY-HA-epi (Fig. 4A). In order to compare OPP/PSY expression in the transgenic lines OPP/PSY-HA-epi and OPP/PSY-HA-int to that in wild-type 3D7 parasites, real-time PCR was applied. Although OPP/PSY is constitutively expressed, MSP2 is a stage-specific promoter; therefore, transcript levels were analyzed in schizont-stage parasites. While expression in the integrated line is similar to that of 3D7, as expected, it is about 5-fold higher in OPP/PSY-HA-epi parasites (Fig. 4B).
FIG 4.
OPP/PSY overexpression in P. falciparum. (A) Schematic diagram of the vector used to overexpress OPP/PSY in P. falciparum. (B) OPP/PSY expression in the integrated HA-tagged line OPP/PSY-HA-int and in transfected parasites where the overexpression vector is episomally maintained (OPP/PSY-HA-epi) was accessed by real-time PCR. The transcript levels of the gene encoding OPP/PSY were normalized by the control gene K1 and are represented relative to levels for 3D7. Statistical significance was determined by one-way ANOVA, and the P value is indicated.
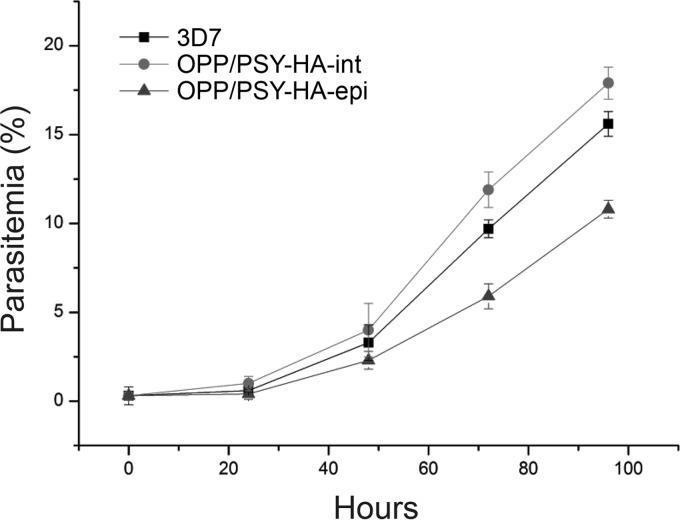
The failure to constitutively overexpress OPP/PSY suggested that an excess of this enzyme was toxic for the parasite. To investigate whether the OPP/PSY-HA-epi line had reduced fitness, parasite growth was compared to that of wild-type 3D7 and the integrated line (OPP/PSY-HA-int) for 96 h during the erythrocytic cycle. OPP/PSY-overexpressing parasites grew at a rate slower than those of both the wild-type 3D7 strain and the transfected line with the plasmid integrated (Fig. 5). We expected that the OPP/PSY-HA-epi line was unstable and would lose the plasmid or the transgene expression over time due to toxic OPP/PSY overexpression. In order to avoid that, the following experiments always were performed with freshly transfected parasites no more than 4 weeks after the reestablishment of growth.
FIG 5.
OPP/PSY overexpression negatively affects parasite growth. Growth of wild-type 3D7 parasites and the HA-tagged lines, either integrated (OPP/PSY-HA-int) or episomal (OPP/PSY-HA-epi). Parasitemia was determined by microscopic examination of smears stained with Giemsa. The points refer to the averages from triplicate experiments, and the error bars show the standard deviations.
Effect of OPP/PSY overexpression on carotenoid biosynthesis.
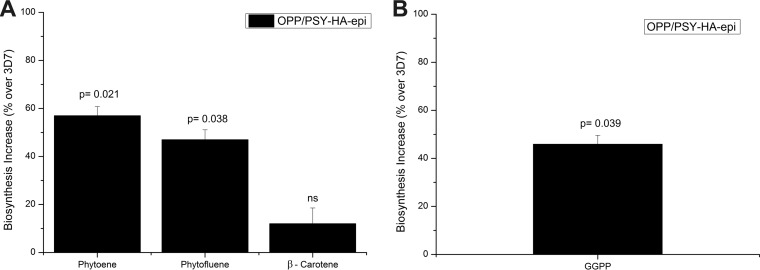
To determine whether the products of OPP and PSY would be biosynthesized at a higher rate in the overexpressing transgenic line, OPP/PSY-HA-epi and 3D7 parasites were metabolically labeled and the metabolites analyzed by RP-HPLC (Fig. 6A). Biosynthesis of the direct product of PSY, phytoene, and of the product of the subsequent reaction, phytofluene, was increased 57.3% ± 3% and 47% ± 5%, respectively. In contrast, biosynthesis of β-carotene, a downstream product in the carotenoid pathway, was not affected. Biosynthesis of GGPP, the substrate of PSY, and one of the intermediate products of OPP was increased 46.2% ± 5% (Fig. 6B).
FIG 6.
Effect of OPP/PSY overexpression on carotenoid and GGPP biosynthesis. (A) Phytoene, phytofluene, and β-carotene biosynthesis in OPP/PSY-HA-epi strain compared to those of 3D7. Carotenes were extracted from [1-(n)-3H]GGPP-labeled schizonts and analyzed by HPLC. The radioactive peaks corresponding to the retention time of phytoene, phytofluene, and β-carotene were plotted. (B) GGPP was extracted from [1-(n)-3H]FPP-labeled schizonts of OPP/PSY-HA-epi and 3D7 wild-type parasites and analyzed by HPLC. The radioactive peaks corresponding to the retention time of GGPP were plotted. Statistical significance was determined by one-way ANOVA. P values of significant results (P < 0.05) are shown, and ns denotes nonsignificant associations (P > 0.05).
OPP/PSY-overexpressing parasites are more resistant to squalestatin.
The correlation between OPP/PSY overexpression and increased biosynthesis of its products suggested these parasites can tolerate enzyme activity inhibition at higher levels than parasites expressing OPP/PSY at standard levels. To test that hypothesis, the inhibitory effect of squalestatin on P. falciparum growth was assessed by culturing the wild-type (3D7), the integrated (OPP/PSY-HA-int), and the overexpressing (OPP/PSY-HA-epi) lines in the absence or in the presence of increasing concentrations of the drug (Fig. 7). Parasite growth was inhibited in a dose-dependent manner and was correlated with OPP/PSY expression. Specifically, while the line transfected with the integrated plasmid was as sensitive to squalestatin as the wild-type parasites, the squalestatin IC50 in the overexpressing line was about 5.5-fold higher. This not only demonstrates that the coding DNA sequences from the plasmid, such as the human dhfr gene or the HA tag per se, are not able to confer a resistant phenotype but also that the overexpression of OPP/PSY was responsible for the increase in the observed IC50.
FIG 7.
Parasites overexpressing OPP/PSY are more resistant to squalestatin. (A) Parasites of the wild-type 3D7 strain and the transgenic lines OPP-PSY-HA-int and OPP-PSY-HA-epi were cultured for 2 days in the presence of various concentrations of squalestatin. Growth is represented relative to that of control parasites cultured in the presence of the solvent control. (B) The IC50s represent the means and error bars (95% confidence intervals) from 3 experiments: 3D7, 5.2 μM (3.0 to 8.8 μM); OPP/PSY-HA-int, 5.3 μM (3.5 to 7.8 μM); OPP/PSY-HA-epi, 28.7 μM (27.1 to 30.4 μM).
DISCUSSION
The discovery of new antimalarials is a key aspect for the success of malaria control. High-throughput drug screening studies allow the identification of new potential hits, but those are expensive and time-consuming until new compounds can be produced in large scale and applied for malaria treatment. In contrast, the identification of parasite pathways that already can be targeted by known licensed drugs, either commercially available or in the pipeline for mass production, might eventually speed up the process.
Squalestatin is a potential cholesterol-lowering drug and is expected to produce fewer side effects, since it inhibits the first specific step in sterol biosynthesis. In contrast, the standard treatment based on statins inhibits the enzyme 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, from which the product mevalonate is a precursor of several other metabolites. Reduction of serum cholesterol in rodents has been achieved successfully with squalestatin, suggesting it can be used in vivo in mammals (19). Further studies are required to determine its safety and efficacy for humans, as well as large-scale production optimizations. As for use as an antimalarial, in vivo tests also are required to determine its potency against Plasmodium infection.
In this report, we applied reverse genetic strategies in order to investigate the importance of P. falciparum OPP/PSY during the intraerythrocytic cycle. The possibility of altering the parasite genome only when OPP/PSY would be expressed in fusion with a 3-kDa HA tag indicated the genomic locus is amenable to knock-in experiments. However, the failure to generate transgenic lines where OPP/PSY would be expressed in fusion with the 15-kDa HA-DD24 tag suggested the protein levels expressed are insufficient, even in the presence of the stabilizing ligand Shld-1, or that its physiological function is lost when in fusion with the larger tag. In a previous study, it was shown that DD24 decreases the amount of fused proteins to a significant extent, which is not fully reversed by the presence of Shld-1 (26). We also attempted to generate a transgenic line expressing OPP/PSY in fusion with a 30-kDa GFP-HA tag, which should not affect its expression, but also failed (data not shown). Taken together, these experiments suggest OPP/PSY plays an important and possibly essential role during the intraerythrocytic cycle, and it cannot fully perform its physiological function when in fusion with large tags at its C-terminal region. PSY from plants usually is associated with membranes and with other enzymes; therefore, a reasonably free C-terminal region may be required for some of those interactions to happen (41).
Once the likely essential role for this bifunctional enzyme had been proposed from the knock-in experiments, gene overexpression and treatment with squalestatin were carried out to investigate the importance of its PSY activity and, consequently, of carotenoid biosynthesis. The squalestatin-mediated inhibition of phytoene biosynthesis, but not of other carotenoids, and the resistance phenotype presented by the overexpressing parasites suggest PSY is the main target of this drug in the parasites, and that phytoene is essential during the intraerythrocytic cycle.
It is uncertain whether phytoene is the only carotenoid required for parasite development or if its derived products also play important roles. A way to investigate this issue would be to inhibit the reactions involved in the biosynthesis of the derived carotenoids. However, the genes encoding these enzymes have not been identified in Plasmodium; therefore, it is not yet possible to target them either by reverse genetics or to test inhibitors in vitro.
Rather than a general toxic effect, squalestatin affected mainly parasites at the schizont stage before segmentation (data not shown). Although OPP/PSY is constitutively expressed, its products are detected mostly in mature-stage parasites (13, 15), suggesting its activity is limited by the availability of some of the precursors, which could explain the failure to generate transgenic parasites where OPP/PSY would be constitutively overexpressed. Toxic effects of PSY overexpression have been reported both in bacteria and plants, which was caused mainly by the depletion of its substrate, GGPP, inducing a decrease of other products of the isoprenoid pathway (23, 42). In the overexpressing parasite line, OPP also is overexpressed, and GGPP is one of its intermediate products; therefore, other isoprenoids precursors, such as FPP, GPP, IPP, or DMAPP (dimethylallyl pyrophosphate), could be the ones being depleted. Since these metabolites are biosynthesized mostly in mature parasites (30 h postinfection), early-stage parasites could be even more sensitive to their consumption caused by a constitutive overexpression.
The resistance to squalestatin in parasites overexpressing OPP/PSY under the control of the MSP2 schizont-specific promoter also points to a specific function during this part of the life cycle which needs to be further investigated. However, since the overexpression is limited to schizont-stage parasites, it is not possible to investigate if there is a function for this enzyme in ring- and/or trophozoite-stage parasites. It is possible that carotenoids act as antioxidants during hemoglobin digestion or even as precursors of signaling molecules. Parasites live in a prooxidant environment containing oxygen and iron, which are the key players for the formation of reactive oxygen species. As a consequence, P. falciparum is heavily dependent on efficient antioxidant systems (43–46).
An indication that carotenoids are involved in the parasite antioxidant system has been demonstrated previously (13). During studies to determine the free radical scavenger capacity in terms of the electron donor mechanism and the deactivation of singlet oxygen, phytoene and phytofluene presented a higher antioxidant capacity than expected considering the small number of conjugated double bonds (47). Other probable functions essential for the parasite may be taken into account, such as coordination of plastid and nuclear gene expression (48) and/or membrane structure modification (49).
In conclusion, we demonstrated through biochemical and reverse genetics approaches that phytoene synthase is essential for the parasite and apparently the main target of squalestatin. The data presented here suggest that carotenoid biosynthesis is a target for the development of new antimalarials and squalestatin, or that derived compounds can be further exploited as potential antiplasmodial drugs.
ACKNOWLEDGMENTS
This work was supported by grants from CNPq and FAPESP. H.B.G. is the recipient of a postgraduate fellowship from FAPESP.
We thank S. Wendel (Blood Bank at the Sírio Libanês Hospital) for providing the erythrocytes.
REFERENCES
- 1.WHO. 2013. World malaria report. Report 9789241564694 World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh B, Daneshvar C. 2013. Human infections and detection of Plasmodium knowlesi. Clin Microbiol Rev 26:165–184. doi: 10.1128/CMR.00079-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakrabarti D, Da Silva T, Barger J, Paquette S, Patel H, Patterson S, Allen CM. 2002. Protein farnesyltransferase and protein prenylation in Plasmodium falciparum. J Biol Chem 277:42066–42073. doi: 10.1074/jbc.M202860200. [DOI] [PubMed] [Google Scholar]
- 5.Borrmann S, Issifou S, Esser G, Adegnika AA, Ramharter M, Matsiegui PB, Oyakhirome S, Mawili-Mboumba DP, Missinou MA, Kun JF, Jomaa H, Kremsner PG. 2004. Fosmidomycin-clindamycin for the treatment of Plasmodium falciparum malaria. J Infect Dis 190:1534–1540. doi: 10.1086/424603. [DOI] [PubMed] [Google Scholar]
- 6.Moura IC, Wunderlich G, Uhrig ML, Couto AS, Peres VJ, Katzin AM, Kimura EA. 2001. Limonene arrests parasite development and inhibits isoprenylation of proteins in Plasmodium falciparum. Antimicrob Agents Chemother 45:2553–2558. doi: 10.1128/AAC.45.9.2553-2558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jomaa H, Wiesner J, Sanderbrand S, Altincicek B, Weidemeyer C, Hintz M, Turbachova I, Eberl M, Zeidler J, Lichtenthaler HK, Soldati D, Beck E. 1999. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science 285:1573–1576. doi: 10.1126/science.285.5433.1573. [DOI] [PubMed] [Google Scholar]
- 8.Cassera MB, Gozzo FC, D'Alexandri FL, Merino EF, del Portillo HA, Peres VJ, Almeida IC, Eberlin MN, Wunderlich G, Wiesner J, Jomaa H, Kimura EA, Katzin AM. 2004. The methylerythritol phosphate pathway is functionally active in all intraerythrocytic stages of Plasmodium falciparum. J Biol Chem 279:51749–51759. doi: 10.1074/jbc.M408360200. [DOI] [PubMed] [Google Scholar]
- 9.Flesch G, Rohmer M. 1988. Prokaryotic hopanoids: the biosynthesis of the bacteriohopane skeleton. Formation of isoprenic units from two distinct acetate pools and a novel type of carbon/carbon linkage between a triterpene and D-ribose. Eur J Biochem 175:405–411. [DOI] [PubMed] [Google Scholar]
- 10.Eisenreich W, Bacher A, Arigoni D, Rohdich F. 2004. Biosynthesis of isoprenoids via the non-mevalonate pathway. Cell Mol Life Sci 61:1401–1426. doi: 10.1007/s00018-004-3381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein JL, Brown MS. 1990. Regulation of the mevalonate pathway. Nature 343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 12.Luo S, Marchesini N, Moreno SN, Docampo R. 1999. A plant-like vacuolar H(+)-pyrophosphatase in Plasmodium falciparum. FEBS Lett 460:217–220. doi: 10.1016/S0014-5793(99)01353-8. [DOI] [PubMed] [Google Scholar]
- 13.Tonhosolo R, D'Alexandri FL, de Rosso VV, Gazarini ML, Matsumura MY, Peres VJ, Merino EF, Carlton JM, Wunderlich G, Mercadante AZ, Kimura EA, Katzin AM. 2009. Carotenoid biosynthesis in intraerythrocytic stages of Plasmodium falciparum. J Biol Chem 284:9974–9985. doi: 10.1074/jbc.M807464200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mijts BN, Schmidt-Dannert C. 2003. Engineering of secondary metabolite pathways. Curr Opin Biotechnol 14:597–602. doi: 10.1016/j.copbio.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Tonhosolo R, D'Alexandri FL, Genta FA, Wunderlich G, Gozzo FC, Eberlin MN, Peres VJ, Kimura EA, Katzin AM. 2005. Identification, molecular cloning and functional characterization of an octaprenyl pyrophosphate synthase in intra-erythrocytic stages of Plasmodium falciparum. Biochem J 392:117–126. doi: 10.1042/BJ20050441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandmann G. 2002. Combinatorial biosynthesis of carotenoids in a heterologous host: a powerful approach for the biosynthesis of novel structures. Chembiochem 3:629–635. doi:. [DOI] [PubMed] [Google Scholar]
- 17.Sato S, Clough B, Coates L, Wilson RJ. 2004. Enzymes for heme biosynthesis are found in both the mitochondrion and plastid of the malaria parasite Plasmodium falciparum. Protist 155:117–125. doi: 10.1078/1434461000169. [DOI] [PubMed] [Google Scholar]
- 18.Bergstrom JD, Kurtz MM, Rew DJ, Amend AM, Karkas JD, Bostedor RG, Bansal VS, Dufresne C, Van Middlesworth FL, Hensens OD. 1993. Zaragozic acids: a family of fungal metabolites that are picomolar competitive inhibitors of squalene synthase. Proc Natl Acad Sci U S A 90:80–84. doi: 10.1073/pnas.90.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baxter A, Fitzgerald BJ, Hutson JL, McCarthy AD, Motteram JM, Ross BC, Sapra M, Snowden MA, Watson NS, Williams RJ, Wright C. 1992. Squalestatin 1, a potent inhibitor of squalene synthase, which lowers serum cholesterol in vivo. J Biol Chem 267:11705–11708. [PubMed] [Google Scholar]
- 20.Sidebottom PJ, Highcock RM, Lane SJ, Procopiou PA, Watson NS. 1992. The squalestatins, novel inhibitors of squalene synthase produced by a species of Phoma. II. Structure elucidation. J Antibiot 45:648–658. [DOI] [PubMed] [Google Scholar]
- 21.Dawson MJ, Farthing JE, Marshall PS, Middleton RF, O'Neill MJ, Shuttleworth A, Stylli C, Tait RM, Taylor PM, Wildman HG, Buss AD, Langley D, Hayes MV. 1992. The squalestatins, novel inhibitors of squalene synthase produced by a species of Phoma. I. Taxonomy, fermentation, isolation, physico-chemical properties and biological activity. J Antibiot 45:639–647. [DOI] [PubMed] [Google Scholar]
- 22.Haeuptle MA, Welti M, Troxler H, Hulsmeier AJ, Imbach T, Hennet T. 2011. Improvement of dolichol-linked oligosaccharide biosynthesis by the squalene synthase inhibitor zaragozic acid. J Biol Chem 286:6085–6091. doi: 10.1074/jbc.M110.165795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neudert U, Martinez-Ferez IM, Fraser PD, Sandmann G. 1998. Expression of an active phytoene synthase from Erwinia uredovora and biochemical properties of the enzyme. Biochim Biophys Acta 1392:51–58. doi: 10.1016/S0005-2760(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 24.Goodwin TW. 1983. Developments in carotenoid biochemistry over 40 years. The third Morton lecture. Biochem Soc Trans 11:473–483. [DOI] [PubMed] [Google Scholar]
- 25.Vial HJ, Philippot JR, Wallach DF. 1984. A reevaluation of the status of cholesterol in erythrocytes infected by Plasmodium knowlesi and P. falciparum. Mol Biochem Parasitol 13:53–65. doi: 10.1016/0166-6851(84)90101-4. [DOI] [PubMed] [Google Scholar]
- 26.de Azevedo MF, Gilson PR, Gabriel HB, Simoes RF, Angrisano F, Baum J, Crabb BS, Wunderlich G. 2012. Systematic analysis of FKBP inducible degradation domain tagging strategies for the human malaria parasite Plasmodium falciparum. PLoS One 7:e40981. doi: 10.1371/journal.pone.0040981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trager W, Jensen JB. 1976. Human malaria parasites in continuous culture. Science 193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 28.Trang DT, Huy NT, Kariu T, Tajima K, Kamei K. 2004. One-step concentration of malarial parasite-infected red blood cells and removal of contaminating white blood cells. Malar J 3:7. doi: 10.1186/1475-2875-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Y, Sifri CD, Lei HH, Su XZ, Wellems TE. 1995. Transfection of Plasmodium falciparum within human red blood cells. Proc Natl Acad Sci U S A 92:973–977. doi: 10.1073/pnas.92.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fidock DA, Wellems TE. 1997. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc Natl Acad Sci U S A 94:10931–10936. doi: 10.1073/pnas.94.20.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salanti A, Staalsoe T, Lavstsen T, Jensen AT, Sowa MP, Arnot DE, Hviid L, Theander TG. 2003. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol Microbiol 49:179–191. doi: 10.1046/j.1365-2958.2003.03570.x. [DOI] [PubMed] [Google Scholar]
- 32.Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother 16:710–718. doi: 10.1128/AAC.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moneriz C, Marin-Garcia P, Bautista JM, Diez A, Puyet A. 2009. Haemoglobin interference and increased sensitivity of fluorimetric assays for quantification of low-parasitaemia Plasmodium infected erythrocytes. Malar J 8:279. doi: 10.1186/1475-2875-8-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Rosso VV, Mercadante AZ. 2007. Identification and quantification of carotenoids, by HPLC-PDA-MS/MS, from Amazonian fruits. J Agric Food Chem 55:5062–5072. doi: 10.1021/jf0705421. [DOI] [PubMed] [Google Scholar]
- 35.Bullen HE, Tonkin CJ, O'Donnell RA, Tham WH, Papenfuss AT, Gould S, Cowman AF, Crabb BS, Gilson PR. 2009. A novel family of Apicomplexan glideosome-associated proteins with an inner membrane-anchoring role. J Biol Chem 284:25353–25363. doi: 10.1074/jbc.M109.036772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawthorne PL, Trenholme KR, Skinner-Adams TS, Spielmann T, Fischer K, Dixon MW, Ortega MR, Anderson KL, Kemp DJ, Gardiner DL. 2004. A novel Plasmodium falciparum ring stage protein, REX, is located in Maurer's clefts. Mol Biochem Parasitol 136:181–189. doi: 10.1016/j.molbiopara.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 37.de Koning-Ward TF, Gilson PR. 2009. Keeping it simple: an easy method for manipulating the expression levels of malaria proteins. Trends Parasitol 25:4–7. doi: 10.1016/j.pt.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Wickham ME, Thompson JK, Cowman AF. 2003. Characterisation of the merozoite surface protein-2 promoter using stable and transient transfection in Plasmodium falciparum. Mol Biochem Parasitol 129:147–156. doi: 10.1016/S0166-6851(03)00118-X. [DOI] [PubMed] [Google Scholar]
- 39.de Koning-Ward TF, Speranca MA, Waters AP, Janse CJ. 1999. Analysis of stage specificity of promoters in Plasmodium berghei using luciferase as a reporter. Mol Biochem Parasitol 100:141–146. doi: 10.1016/S0166-6851(99)00042-0. [DOI] [PubMed] [Google Scholar]
- 40.Fernandez-Becerra C, de Azevedo MF, Yamamoto MM, del Portillo HA. 2003. Plasmodium falciparum: new vector with bi-directional promoter activity to stably express transgenes. Exp Parasitol 103:88–91. doi: 10.1016/S0014-4894(03)00065-1. [DOI] [PubMed] [Google Scholar]
- 41.Cunningham FX, Gantt E. 1998. Genes and enzymes of carotenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 49:557–583. doi: 10.1146/annurev.arplant.49.1.557. [DOI] [PubMed] [Google Scholar]
- 42.Fray RG, Wallace A, Fraser PD, Valero D, Hedden P, Bramley PM, Grierson D. 1995. Constitutive expression of a fruit phytoene synthase gene in transgenic tomatoes causes dwarfism by redirecting metabolites from the gibberellin pathway. Plant J 8:693–701. doi: 10.1046/j.1365-313X.1995.08050693.x. [DOI] [Google Scholar]
- 43.Sies H. 1993. Strategies of antioxidant defense. Eur J Biochem 215:213–219. doi: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]
- 44.Sies H. 1997. Oxidative stress: oxidants and antioxidants. Exp Physiol 82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 45.Muller S, Liebau E, Walter RD, Krauth-Siegel RL. 2003. Thiol-based redox metabolism of protozoan parasites. Trends Parasitol 19:320–328. doi: 10.1016/S1471-4922(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 46.Becker K, Tilley L, Vennerstrom JL, Roberts D, Rogerson S, Ginsburg H. 2004. Oxidative stress in malaria parasite-infected erythrocytes: host-parasite interactions. Int J Parasitol 34:163–189. doi: 10.1016/j.ijpara.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 47.Martinez A, Stinco CM, Melendez-Martinez AJ. 2014. Free radical scavenging properties of phytofluene and phytoene isomers as compared to lycopene: a combined experimental and theoretical study. J Phys Chem B 118:9819–9825. doi: 10.1021/jp503227j. [DOI] [PubMed] [Google Scholar]
- 48.Gray JC, Sullivan JA, Wang JH, Jerome CA, MacLean D. 2003. Coordination of plastid and nuclear gene expression. Philos Trans R Soc Lond B Biol Sci 358:135–144. doi: 10.1098/rstb.2002.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gruszecki WI, Strzalka K. 2005. Carotenoids as modulators of lipid membrane physical properties. Biochim Biophys Acta 1740:108–115. doi: 10.1016/j.bbadis.2004.11.015. [DOI] [PubMed] [Google Scholar]