Abstract
Sodium nitrite has broad antimicrobial activity at pH 6.5, including the ability to prevent biofilm growth by Pseudomonas aeruginosa on the surfaces of airway epithelial cells. Because of its antimicrobial activity, nitrite is being investigated as an inhaled agent for chronic P. aeruginosa airway infections in cystic fibrosis patients. However, the interaction between nitrite and commonly used aminoglycosides is unknown. This paper investigates the interaction between nitrite and tobramycin in liquid culture, abiotic biofilms, and a biotic biofilm model simulating the conditions in the cystic fibrosis airway. The addition of nitrite prevented killing by aminoglycosides in liquid culture, with dose dependence between 1.5 and 15 mM. The effect was not blocked by the nitric oxide scavenger CPTIO or dependent on efflux pump activity. Nitrite shifted the biofilm minimal bactericidal concentration (MBC-biofilm) from 256 μg/ml to >1,024 μg/ml in an abiotic biofilm model. In a biotic biofilm model, the addition of 50 mM nitrite decreased the antibiofilm activity of tobramycin by up to 1.2 log. Respiratory chain inhibition recapitulated the inhibition of aminoglycoside activity by nitrite, suggesting a potential mechanism of inhibition of energy-dependent aminoglycoside uptake. In summary, sodium nitrite induces resistance to both gentamicin and tobramycin in P. aeruginosa grown in liquid culture, as an abiotic biofilm, or as a biotic biofilm.
INTRODUCTION
Sodium nitrite inhibits the growth of many bacterial species, including Pseudomonas aeruginosa (1–6). This bacterium grows in highly antibiotic-resistant communities within the airways of patients with cystic fibrosis and other chronic lung diseases (7). Current treatment for P. aeruginosa airway infections involves chronic inhalation of antibiotics, such as aztreonam, colistimethate, and tobramycin (8, 9). However, drug exposure over decades leads to antimicrobial resistance and subsequent treatment failure. Sodium nitrite represents a new potential antimicrobial approach. A solution of >1 M sodium nitrite (AIR001) is currently in a phase 2b clinical trial for pulmonary hypertension and has been well tolerated to date (10). With the availability of in-human safety data, studies of nebulized nitrite as an antimicrobial agent are being designed. The >1 M concentration of AIR001 makes the achievement of the millimolar concentrations in the airway surface liquid needed to inhibit bacterial growth in humans feasible, and this agent represents a novel antimicrobial therapy.
Nitrite inhibits growth of P. aeruginosa under both aerobic and anaerobic conditions in a wide array of tested clinical isolates, with anaerobically growing, mucoid isolates being the most sensitive (2, 3, 11). The antimicrobial mechanism of action is only partially understood. Nitrite induces nitrosative stress by reacting with Fe-S-containing proteins, heme-containing proteins, free thiols, and DNA (12, 13). These chemical reactions can be mediated both through NO production and via NO-independent mechanisms, such as secondary oxidation reactions leading to nitrosation or nitration of targets (14). Because of the abundance of iron-containing proteins within the respiratory tree, nitrite inhibits bacterial oxygen uptake, and this inhibition is NO independent (3, 15). In Salmonella enterica, NO respiratory inhibition occurs at the level of cytochrome c oxidases. P. aeruginosa has a more highly branched respiratory tree, and the effects of nitrite versus NO on individual oxidases are unknown, although the overall cytochrome oxidase activity is inhibited by nitrite (15, 16). Because aminoglycoside uptake (and thus killing) requires bacterial respiration, we examined if there is an interaction between nitrite and the aminoglycosides. Determining these interactions is important for clinical use of nitrite as an antimicrobial therapy.
Aminoglycosides, such as tobramycin and gentamicin, inhibit translation by binding to the small ribosomal subunit, which increases incorporation of noncognate tRNAs and ultimately causes the creation of nonfunctional proteins (17–19). Drug uptake requires both the energy generated through the electron transport chain and a membrane potential difference. Agents that decrease the proton motive force by blocking respiration (i.e., NO and potassium cyanide) or mutations impairing production of Fe-S-containing proteins inhibit antibiotic uptake and thus render the bacterium resistant to killing by aminoglycosides (20–22). In vivo, NO produced by macrophages is sufficient to protect S. enterica from killing by aminoglycosides (16). Most studies on resistance mechanisms have been done in liquid culture, and whether this phenomenon translates to biofilms, such as those found during chronic infections in the airways, is unknown.
In this study, we tested the hypothesis that nitrite blocks aminoglycoside uptake and thus leads to decreased bacterial killing. Characterizing this interaction is obligatory for the future development of nitrite as an antimicrobial agent.
MATERIALS AND METHODS
Bacterial strains.
Pseudomonas aeruginosa strains PA14 and PAO1 (gifts from George O'Toole, Geisel School of Medicine at Dartmouth) were used in this study. ΔMexY and ΔMexXY strains were a gift from Keith Poole, Queens University, and were described previously (23, 24). Both deletions were created in-frame.
Reagents.
Tobramycin was obtained from APP Pharmaceuticals, Schaumberg, IL. Gentamicin, sodium nitrite, Luria broth (LB), LB agar, potassium cyanide, phenylalanine arginyl β-naphthylamide (PAβN), and carbonyl cyanide m-chlorophenyl hydrazone (CCCP) were obtained from Sigma, St. Louis, MO. Carboxy-PTIO (CPTIO) and PTIO were obtained from Cayman Chemicals, Ann Arbor, MI. Tobramycin Sensi-Disc test disks were obtained from BD, Sparks, MD. Fetal bovine serum was obtained from Gemini Bio-Products, West Sacramento, CA. Minimal essential medium (MEM) was obtained from Life Technologies, Carlsbad, CA.
Liquid culture experiments.
For modified time-kill assays to measure the bactericidal activity of aminoglycosides, overnight cultures were diluted in LB, pH 6.5, returned to log-phase growth at 37°C, and then treated with nitrite, potassium cyanide, CCCP, sodium azide, and/or aminoglycosides for an additional 5.5 h. The number of live bacteria was then determined by plating in a CFU assay.
Abiotic biofilm assays.
The procedure for abiotic biofilm assays was adapted from a previously published method (25). For assays of the biofilm minimal bactericidal concentration (MBC-biofilm), PAO1 was diluted 1:100 in LB, pH 6.5, and 100 μl was inoculated into each well of a 96-well polyvinyl chloride (PVC) plate (Costar 2797; Corning, NY). At 24 h, the medium was changed. At 48 h, biofilms were treated for 2 h with 16 to 1,024 μg/ml gentamicin. After treatment, the biofilms were rinsed twice with LB, and sterile LB was added to each well. The plates were incubated for 16 h, and wells were visually assessed for growth. In parallel assays, 24-h biofilms were treated for 2 h with 16 to 1,024 μg/ml gentamicin and rinsed twice, and viable bacteria were then removed by incubation with 1% Triton X-100 for 20 min. Bacteria were then counted by serial dilution. In both experiments, wells were treated with 75 mM sodium nitrite, 20 μg/ml PAβN, or 1 mM PTIO, as depicted in the figures.
Coculture experiments.
Coculture experiments were conducted as previously described (26). Briefly, the human bronchial epithelial cell line CFBE41o− complemented with wild-type CFTR was used (referred to here as CFBE-wt). Cells were grown on Transwell filters (Corning, Tewskbury, MA) at the air-liquid interface for at least 7 days. Overnight cultures of P. aeruginosa PAO1 were rinsed once, diluted in MEM to a multiplicity of infection of 25, and added to the apical surface of the epithelial cells. Biofilms were grown for 6 h and then treated with tobramycin (1 mg/ml) and/or nitrite (up to 50 mM) for 90 min. Sodium chloride was used as a tonicity control when sodium nitrite was not present. At the end of this period, biofilms were removed by use of 0.1% Triton X-100, and live bacteria were plated on LB agar for a CFU assay. To assess if nitrite treatment caused a permanent change in aminoglycoside resistance, aliquots containing 105 CFU/ml were plated on agar plates with tobramycin diffusion disks at the end of the coculture assay, and the zone of inhibition was measured the next day.
Statistics.
All data are plotted as means ± standard deviations. Data were log transformed and tested for an effect by one-way analysis of variance (ANOVA) (or two-way ANOVA, as applicable). If an effect was present, the Tukey test was used for individual comparisons, using Prism software (La Jolla, CA).
RESULTS AND DISCUSSION
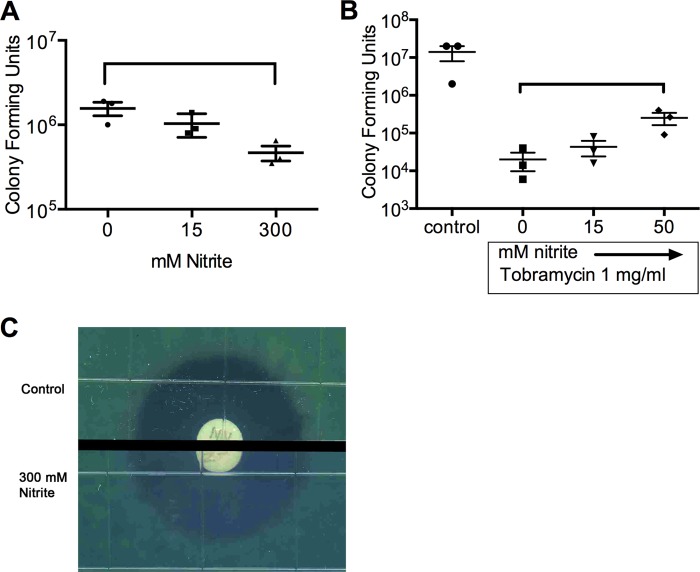
Nitric oxide blocks energy-dependent uptake of aminoglycosides and killing in Salmonella spp., Staphylococcus aureus, and Pseudomonas aeruginosa (16, 22). We tested if sodium nitrite reduces killing by aminoglycosides in liquid aerobic culture. Log-phase cultures were exposed to tobramycin (Fig. 1A) or gentamicin (Fig. 1B) at 2× the MIC for the tested lab strains, with or without nitrite. The addition of 1.5 to 15 mM nitrite caused a dose-dependent, up to 3-log increase in the number of viable bacteria for both tobramycin and gentamicin treatments. To test if nitrite also inhibited the activity of aminoglycosides against abiotic biofilms, we grew PAO1 biofilms in PVC microtiter dishes for 48 h, exposed them to gentamicin and nitrite, and then determined the sterility of the treated wells in an MBC-biofilm assay. The MBC-biofilm for PAO1 was 256 μg/ml (within 1 dilution of the previously reported MBC-biofilm of 400 μg/ml [27]). The addition of 75 mM nitrite increased the MBC-biofilm to >1,024 μg/ml (n = 4) (a representative image is shown in Fig. 1C).
FIG 1.
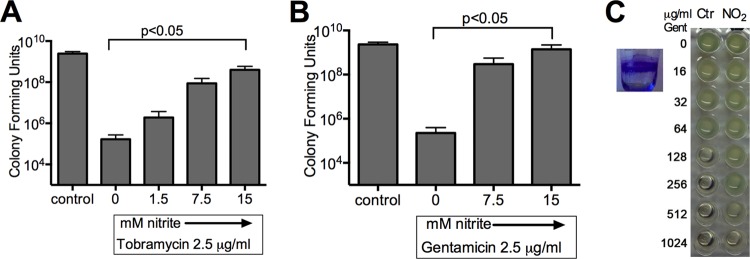
In a modified time-kill assay done in LB aerobic culture, the addition of 1.5 mM to 15 mM nitrite blocked the bactericidal activity of the aminoglycosides tobramycin (A) and gentamicin (B). Brackets indicate differences with P values of <0.05 by one-way ANOVA followed by Tukey testing (n = 4 for panel A and 3 for panel B). (C) Nitrite increases the MBC-biofilm for gentamicin. At 48 h, there was robust biofilm growth as seen by crystal violet staining (inset). Biofilms were treated for 2 h with gentamicin ± 75 mM nitrite. For control biofilms, the MBC-biofilm was 256 μg/ml, while for nitrite-treated biofilms, the MBC-biofilm was >1,024 μg/ml (n = 4; data from a representative assay are shown).
Notably, inhibition of oxygen uptake occurs within the same range, with oxygen uptake entirely arrested with 15 mM nitrite (3, 15). We next examined if inhibition of bacterial respiration, and thus energy generation, could mimic the inhibition of aminoglycoside activity by nitrite. The classic respiratory inhibitors KCN (Fig. 2A) and NaN3 (Fig. 2B) and the proton ionophore CCCP (Fig. 2C) also decreased killing by tobramycin in liquid aerobic cultures.
FIG 2.
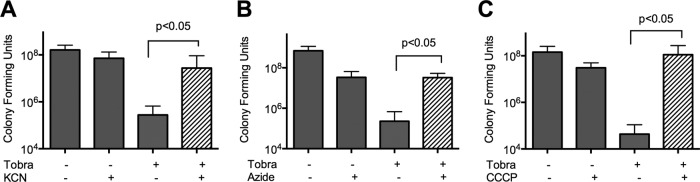
In a modified time-kill assay done in LB aerobic culture, the addition of 400 μM KCN (A), 5 mM NaN3 (B), or 50 μM CCCP (C) blocked the bactericidal activity of tobramycin. Brackets indicate differences with P values of <0.05 by one-way ANOVA followed by Tukey testing (n = 3 or 4).
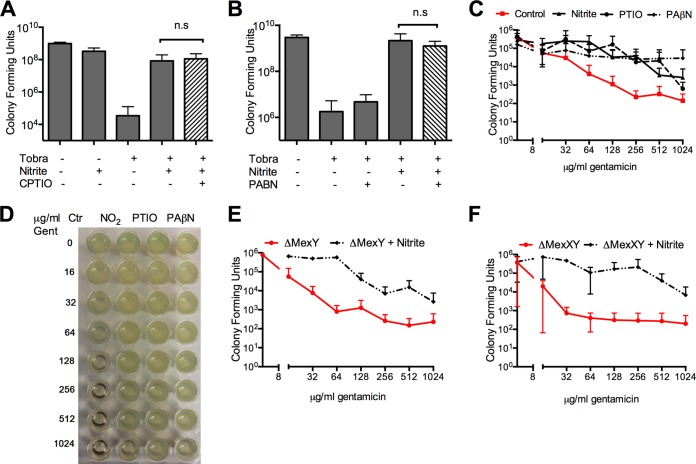
Nitrite has both NO-dependent and NO-independent biochemical reactions (3). The arrest of bacterial oxygen uptake by sodium nitrite does not require NO; therefore, we tested if the interaction between nitrite and aminoglycosides requires NO. The addition of the stoichiometric NO scavenger CPTIO did not block induction of resistance to tobramycin in liquid aerobic cultures (Fig. 3A). Similarly, the scavenger PTIO did not block induction of resistance to gentamicin in either 24-h (Fig. 3C) or 48-h (Fig. 3D) PAO1 biofilms grown on PVC. Gentamicin alone caused a decrease in the number of CFU recoverable by detergent from treated biofilms that plateaued at 256 μg/ml (Fig. 3C), which corresponds well with the absent growth seen in MBC-biofilm assays done on 48-h biofilms (Fig. 3D). The addition of nitrite prevented killing by gentamicin as measured by both recovered CFU and sterility in the MBC-biofilm assay. PTIO did not block the induction of resistance to gentamicin in abiotic biofilms as measured by the recovery of live bacteria (Fig. 3C). The MBC-biofilm for PAO1 biofilms treated with 75 mM nitrite and PTIO was >1,024 μg/ml (Fig. 3D). This observation is consistent with the hypothesis that a nitrite-induced respiratory blockade prevents killing by aminoglycosides.
FIG 3.
(A) In liquid culture, induction of resistance to tobramycin is not blocked by addition of the nitric oxide scavenger CPTIO. (B) Induction of resistance to tobramycin by nitrite is not blocked by the addition of the efflux pump inhibitor PAβN to a log-phase aerobic culture. Concentrations used were as follows: tobramycin, 2.5 μg/ml; nitrite, 15 mM; CPTIO, 1.5 mM; and PAβN, 20 μg/ml. Brackets indicate differences that are not significant (n.s) by one-way ANOVA (n = 6 for panel A and 3 for panel B). The addition of the nitric oxide scavenger PTIO or the efflux pump inhibitor PAβN did not block the induction of resistance in abiotic PAO1 biofilms grown on microtiter dishes. (C) CFU counts from 24-h biofilms treated for 2 h and then incubated with 1% Triton to recover attached bacteria (n = 4). Differences were analyzed by two-way ANOVA followed by multiple-comparison testing (P < 0.05 for differences between the control and other conditions; differences were nonsignificant for comparisons between nitrite and PTIO or PaβN). Similar results were obtained using 48-h-old biofilms in an MBC-biofilm assay. (D) Representative MBC-biofilm plate with control growth inhibited at 256 μg/ml, while the MBC-biofilm for the other conditions was >1,024 μg/ml (n = 3; a representative image is shown). To confirm that efflux pump upregulation is not required for nitrite-induced gentamicin resistance, ΔMexY (E) and ΔMexXY (F) strains were grown as biofilms on microtiter dishes for 24 h before being treated with gentamicin and 75 mM nitrite for 2 h. Attached bacteria were recovered in 1% Triton and counted. P values were <0.05 by two-way ANOVA for differences between control and nitrite treatments.
Alternatively, nitrosative stress has been reported to induce efflux pump expression (28), and aminoglycosides are potential substrates for efflux pump exclusion. To exclude upregulation of efflux pumps as a mechanism for decreased bacterial killing, we added the broad-spectrum efflux pump inhibitor PAβN to P. aeruginosa grown in liquid aerobic culture. The addition of PAβN did not block the induction of resistance to tobramycin (Fig. 3B). Additionally, PAβN did not block resistance induced by nitrite in abiotic biofilms as measured by the number of recovered live bacteria (Fig. 3C). The MBC-biofilm for PAO1 abiotic biofilms treated with nitrite and PAβN was >1,024 μg/ml (Fig. 3D). Next, because MexXY is the principle efflux pump implicated in adaptive resistance to aminoglycosides (29), we determined if deletion of MexXY would block the induction of resistance to gentamicin. In these experiments, abiotic biofilms were grown on PVC microtiter plates for 24 h, the biofilms were treated with gentamicin and nitrite, and live bacteria were recovered with detergent and counted. As expected, both ΔMexY and ΔMexXY strains were more sensitive than PAO1 to gentamicin, with gentamicin decreasing recoverable counts to <103 at 16 to 32 μg/ml rather than 256 μg/ml (Fig. 3E and F). Nitrite significantly increased the number of viable bacteria for both strains (P < 0.05 by two-way ANOVA). These data are consistent with nitrite-induced respiratory arrest leading to decreased aminoglycoside uptake and bacterial killing rather than an induction of efflux pump expression.
Biofilms grown on airway epithelial cells have much greater resistance to aminoglycosides than those on abiotic surfaces, as well as differing in their transcriptional responses to aminoglycosides (27, 30). The airway epithelial cell environment leads to very rapid biofilm formation by P. aeruginosa. Within 6 h, bacterial aggregates have formed that have downregulated motility-associated genes, such as fliC, are elaborating the exopolysaccharide matrix, and have antibiotic resistances exceeding those of 24- and 48-h biofilms grown on abiotic surfaces (27). This model allows in vitro examination of biofilms grown in an environment more similar to that found in the airway. To test if nitrite induces resistance to aminoglycosides within biotic biofilms, a coculture model was used. Note that in this model, sodium nitrite prevents biofilm growth when applied after an initial 1-h attachment period, with an effect ceiling at 50 mM, but the effect of nitrite on mature biofilms has not been studied (3). PAO1 biofilms were grown on the surface of polarized airway epithelial cells and then treated with tobramycin and/or nitrite. First, mature biofilms were treated for 90 min with nitrite at increasing concentrations. Nitrite alone caused a 0.5-log decrease in the number of CFU compared to that for untreated biofilms (Fig. 4A), which likely represents bacterial stasis or biofilm dispersal rather than killing, given the modest effect size over 90 min. When nitrite was added to 1,000 μg/ml tobramycin, which is near the maximum concentration achieved through inhalation (Fig. 4B), the addition of 15 to 50 mM nitrite caused a dose-dependent increase in the number of viable bacteria (Fig. 4B) (31). To test if the apparent change in susceptibility to tobramycin was a permanent phenotypic change, bacteria taken at the end of the assay shown in Fig. 4B were plated with a tobramycin diffusion disk. No change in the zone of inhibition was seen (Fig. 4C). In summary, nitrite decreases killing of P. aeruginosa by tobramycin in both liquid aerobic culture and a biotic biofilm model.
FIG 4.
Nitrite induces resistance to aminoglycosides within biofilms. PAO1 biofilms were grown on the surface of CFBE-wt cells for 6 h and then treated with nitrite (A) or nitrite and tobramycin (B) for 90 min. Brackets indicate differences with P values of <0.05 by one-way ANOVA followed by pairwise comparison testing. (C) The zone of inhibition is unchanged in biofilm bacteria exposed to nitrite compared to unexposed bacteria (n = 3; representative images are shown).
Conclusions.
We have demonstrated that nitrite blocks the antimicrobial activity of the aminoglycosides tobramycin and gentamicin in liquid culture, similarly to the effects of other respiratory inhibitors, such as cyanide and azide. The interaction between nitrite and aminoglycosides occurs in a dose-dependent manner over the same range as that for inhibition of oxygen uptake (3, 15). Inhibition of aminoglycoside activity is NO independent and does not rely on efflux pump induction. Importantly, inhibition of aminoglycoside activity by nitrite extends to biofilms grown on abiotic substrates as well as to biofilms growing in a nutritionally rich environment on the surface of human airway epithelial cells, so these findings may translate to what is seen with in vivo human airway biofilms.
Inhibition of aminoglycoside uptake by agents that block bacterial respiration has been described for more than 50 years, although the precise molecular mechanisms remain elusive (reviewed in reference 22). Aminoglycosides can also be effluxed by MexXY-OprM, as seen during the induction of adaptive resistance (29). Deletion of MexXY does not block aminoglycoside resistance induced by nitrite. While we did not directly show decreased uptake per se, that is the likely mechanism of action by nitrite.
This study contributes three valuable findings. First, nitrite has not been studied specifically in regard to blocking aminoglycoside uptake. Nitrite is unique as a bacterial respiratory inhibitor in that it can be nebulized safely at high concentrations by humans (10). Nitrite has been proposed as an inhaled antimicrobial agent for P. aeruginosa colonization in cystic fibrosis, and inhaled tobramycin is the most established agent used in this population (26). Given the data in this paper, the use of these two agents together would be ill advised without further study.
Second, this study distinguishes additional NO-dependent and -independent effects of nitrite on bacterial physiology (3). For these experiments, the scavenger CPTIO was added in vast excess of the nanomolar concentration of NO that is produced from nitrite (2, 3). We have previously shown that nitrite-induced inhibition of respiration is NO independent, and the nitrite-aminoglycoside interaction is likely just an extension of this observation. Inhibition of eukaryotic respiration by nitrite is typically an NO-dependent effect, through nitrosation of NADH dehydrogenase (mitochondrial complex 1) and inhibition of cytochrome c oxidase activity by NO (reviewed in reference 32). Additionally, aconitase is very sensitive to damage by reactive nitrogen species (reviewed in reference 33). Given the highly branched respiratory chain of P. aeruginosa and the ability of bacteria to subsist on diverse metabolic substrates, parsing out which molecular targets are NO dependent and NO independent remains an area of active research.
Finally, inhibition of aminoglycoside activity by respiratory inhibitors has not previously been shown in a biotic biofilm community. Within biofilms, many bacteria have a low metabolic rate and subsist through denitrification (34). The oxygen tension in our system is low, but P. aeruginosa can respire on as little as 0.4% oxygen; thus, we cannot exclude the possibility of aerobic respiration within this model (35). Previous studies have shown that anaerobic growth within biofilms may contribute to baseline resistance to aminoglycosides (36). However, nitrite induced further resistance to the very high concentration of tobramycin used in this study to simulate aerosol delivery. The data derived from biotic biofilm models are more directly applicable to what is found within the patient's infected airway.
In conclusion, nitrite induces resistance to aminoglycosides both within liquid culture and in biofilms growing on abiotic surfaces and on the surface of airway epithelial cells. Nitrite itself prevents biotic biofilm formation by P. aeruginosa and has antimicrobial activity against organisms for which we do not currently have effective inhaled treatments, including colistin-resistant strains of P. aeruginosa, the Burkholderia cepacia complex, the Achromobacter complex, and Stenotrophomonas maltophilia (3). While initial trials with human subjects will exclude the use of other antibiotics simultaneously, understanding the interactions between nitrite and existing antimicrobial agents is important for nitrite's clinical use. We previously identified a cooperative interaction between the inhaled polymyxin colistimethate and nitrite against biotic biofilms. Therefore, given the contrasting effects of nitrite on antimicrobial susceptibility, additional studies investigating the interaction between nitrite and other antimicrobial agents is warranted for the development of nitrite as an antimicrobial.
ACKNOWLEDGMENTS
We thank Lauren Lashua for providing technical assistance. We thank Keith Poole for supplying strains used in this work.
J.M.B. was funded by NIH grants R00HL098342 and P30DK072506 and by a Breathe Pennsylvania research grant. A.C.Z. was funded by Cystic Fibrosis Foundation Clinical Training Fellowship ZEMKE14DO and NIH grant T32HL007563. M.T.G. was funded by NIH grants 2R01HL098032, 1R01HL125886, P01HL103455, T32 HL110849, and T32 HL007563 and by the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania.
M.T.G. is listed as a coinventor on an NIH government patent for the use of nitrite salts in cardiovascular diseases, and he consults with Aires/MAST Pharmaceuticals on the development of a phase II proof-of-concept trial using inhaled nitrite for pulmonary arterial hypertension.
REFERENCES
- 1.Yoon SS, Karabulut AC, Lipscomb JD, Hennigan RF, Lymar SV, Groce SL, Herr AB, Howell ML, Kiley PJ, Schurr MJ, Gaston B, Choi KH, Schweizer HP, Hassett DJ. 2007. Two-pronged survival strategy for the major cystic fibrosis pathogen, Pseudomonas aeruginosa, lacking the capacity to degrade nitric oxide during anaerobic respiration. EMBO J 26:3662–3672. doi: 10.1038/sj.emboj.7601787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoon SS, Coakley R, Lau GW, Lymar SV, Gaston B, Karabulut AC, Hennigan RF, Hwang SH, Buettner G, Schurr MJ, Mortensen JE, Burns JL, Speert D, Boucher RC, Hassett DJ. 2006. Anaerobic killing of mucoid Pseudomonas aeruginosa by acidified nitrite derivatives under cystic fibrosis airway conditions. J Clin Invest 116:436–446. doi: 10.1172/JCI24684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zemke AC, Shiva S, Burns JL, Moskowitz SM, Pilewski JM, Gladwin MT, Bomberger JM. 2014. Nitrite modulates bacterial antibiotic susceptibility and biofilm formation in association with airway epithelial cells. Free Radic Biol Med 77:307–316. doi: 10.1016/j.freeradbiomed.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunningham R, Mustoe E, Spiller L, Lewis S, Benjamin N. 2014. Acidified nitrite: a host defence against colonization with C. difficile spores? J Hosp Infect 86:155–157. doi: 10.1016/j.jhin.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Dykhuizen RS, Fraser A, McKenzie H, Golden M, Leifert C, Benjamin N. 1998. Helicobacter pylori is killed by nitrite under acidic conditions. Gut 42:334–337. doi: 10.1136/gut.42.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlag S, Nerz C, Birkenstock TA, Altenberend F, Gotz F. 2007. Inhibition of staphylococcal biofilm formation by nitrite. J Bacteriol 189:7911–7919. doi: 10.1128/JB.00598-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams HD, Davies JC. 2012. Basic science for the chest physician: Pseudomonas aeruginosa and the cystic fibrosis airway. Thorax 67:465–467. doi: 10.1136/thoraxjnl-2011-201498. [DOI] [PubMed] [Google Scholar]
- 8.Assael BM, Pressler T, Bilton D, Fayon M, Fischer R, Chiron R, Larosa M, Knoop C, McElvaney N, Lewis SA, Bresnik M, Montgomery AB, Oermann CM. 2013. Inhaled aztreonam lysine vs. inhaled tobramycin in cystic fibrosis: a comparative efficacy trial. J Cyst Fibros 12:130–140. doi: 10.1016/j.jcf.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Schuster A, Haliburn C, Doring G, Goldman MH. 2013. Safety, efficacy and convenience of colistimethate sodium dry powder for inhalation (Colobreathe DPI) in patients with cystic fibrosis: a randomised study. Thorax 68:344–350. doi: 10.1136/thoraxjnl-2012-202059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rix PJ, Vick A, Attkins NJ, Barker GE, Bott AW, Alcorn H, Gladwin MT, Shiva S, Bradley S, Hussaini A, Hoye WL, Parsley EL, Masamune H. 2015. Pharmacokinetics, pharmacodynamics, safety, and tolerability of nebulized sodium nitrite (AIR001) following repeat-dose inhalation in healthy subjects. Clin Pharmacokinet 54:261–272. doi: 10.1007/s40262-014-0201-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Major TA, Panmanee W, Mortensen JE, Gray LD, Hoglen N, Hassett DJ. 2010. Sodium nitrite-mediated killing of the major cystic fibrosis pathogens Pseudomonas aeruginosa, Staphylococcus aureus, and Burkholderia cepacia under anaerobic planktonic and biofilm conditions. Antimicrob Agents Chemother 54:4671–4677. doi: 10.1128/AAC.00379-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourret TJ, Boylan JA, Lawrence KA, Gherardini FC. 2011. Nitrosative damage to free and zinc-bound cysteine thiols underlies nitric oxide toxicity in wild-type Borrelia burgdorferi. Mol Microbiol 81:259–273. doi: 10.1111/j.1365-2958.2011.07691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowman LA, McLean S, Poole RK, Fukuto JM. 2011. The diversity of microbial responses to nitric oxide and agents of nitrosative stress close cousins but not identical twins. Adv Microb Physiol 59:135–219. doi: 10.1016/B978-0-12-387661-4.00006-9. [DOI] [PubMed] [Google Scholar]
- 14.Kim-Shapiro DB, Gladwin MT. 2014. Mechanisms of nitrite bioactivation. Nitric Oxide 38:58–68. doi: 10.1016/j.niox.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowe JJ, Yarborough JM, Rake JB, Eagon RG. 1979. Nitrite inhibition of aerobic bacteria. Curr Microbiol 2:51–54. doi: 10.1007/BF02601735. [DOI] [Google Scholar]
- 16.McCollister BD, Hoffman M, Husain M, Vazquez-Torres A. 2011. Nitric oxide protects bacteria from aminoglycosides by blocking the energy-dependent phases of drug uptake. Antimicrob Agents Chemother 55:2189–2196. doi: 10.1128/AAC.01203-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edelmann P, Gallant J. 1977. Mistranslation in E. coli. Cell 10:131–137. doi: 10.1016/0092-8674(77)90147-7. [DOI] [PubMed] [Google Scholar]
- 18.Davies J, Gorini L, Davis BD. 1965. Misreading of RNA codewords induced by aminoglycoside antibiotics. Mol Pharmacol 1:93–106. [PubMed] [Google Scholar]
- 19.Ling J, Cho C, Guo L-T, Aerni HR, Rinehart J, Söll D. 2012. Protein aggregation caused by aminoglycoside action is prevented by a hydrogen peroxide scavenger. Mol Cell 48:713–722. doi: 10.1016/j.molcel.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bryan LE, Kowand SK, Van Den Elzen HM. 1979. Mechanism of aminoglycoside antibiotic resistance in anaerobic bacteria: Clostridium perfringens and Bacteroides fragilis. Antimicrob Agents Chemother 15:7–13. doi: 10.1128/AAC.15.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ezraty B, Vergnes A, Banzhaf M, Duverger Y, Huguenot A, Brochado AR, Su S-Y, Espinosa L, Loiseau L, Py B, Typas A, Barras F. 2013. Fe-S cluster biosynthesis controls uptake of aminoglycosides in a ROS-less death pathway. Science 340:1583–1587. doi: 10.1126/science.1238328. [DOI] [PubMed] [Google Scholar]
- 22.Taber HW, Mueller JP, Miller PF, Arrow AS. 1987. Bacterial uptake of aminoglycoside antibiotics. Microbiol Rev 51:439–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau CH-F, Hughes D, Poole K. 2014. MexY-promoted aminoglycoside resistance in Pseudomonas aeruginosa: involvement of a putative proximal binding pocket in aminoglycoside recognition. mBio 5:e01068. doi: 10.1128/mBio.01068-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dean CR, Visalli MA, Projan SJ, Sum P-E, Bradford PA. 2003. Efflux-mediated resistance to tigecycline (GAR-936) in Pseudomonas aeruginosa PAO1. Antimicrob Agents Chemother 47:972–978. doi: 10.1128/AAC.47.3.972-978.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mah TF. 2014. Establishing the minimal bactericidal concentration of an antimicrobial agent for planktonic cells (MBC-P) and biofilm cells (MBC-B). J Vis Exp 2014:e50854. doi: 10.3791/50854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moskowitz SM, Silva SJ, Mayer-Hamblett N, Pasta DJ, Mink DR, Mabie JA, Konstan MW, Wagener JS. 2008. Shifting patterns of inhaled antibiotic use in cystic fibrosis. Pediatr Pulmonol 43:874–881. doi: 10.1002/ppul.20873. [DOI] [PubMed] [Google Scholar]
- 27.Moreau-Marquis S, Bomberger JM, Anderson GG, Swiatecka-Urban A, Ye S, O'Toole GA, Stanton BA. 2008. The DeltaF508-CFTR mutation results in increased biofilm formation by Pseudomonas aeruginosa by increasing iron availability. Am J Physiol Lung Cell Mol Physiol 295:L25–L37. doi: 10.1152/ajplung.00391.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fetar H, Gilmour C, Klinoski R, Daigle DM, Dean CR, Poole K. 2011. mexEF-oprN multidrug efflux operon of Pseudomonas aeruginosa: regulation by the MexT activator in response to nitrosative stress and chloramphenicol. Antimicrob Agents Chemother 55:508–514. doi: 10.1128/AAC.00830-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hocquet D, Vogne C, El Garch F, Vejux A, Gotoh N, Lee A, Lomovskaya O, Plésiat P. 2003. MexXY-OprM efflux pump is necessary for adaptive resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob Agents Chemother 47:1371–1375. doi: 10.1128/AAC.47.4.1371-1375.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson GG, Moreau-Marquis S, Stanton BA, O'Toole GA. 2008. In vitro analysis of tobramycin-treated Pseudomonas aeruginosa biofilms on cystic fibrosis-derived airway epithelial cells. Infect Immun 76:1423–1433. doi: 10.1128/IAI.01373-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geller DE, Konstan MW, Smith J, Noonberg SB, Conrad C. 2007. Novel tobramycin inhalation powder in cystic fibrosis subjects: pharmacokinetics and safety. Pediatr Pulmonol 42:307–313. doi: 10.1002/ppul.20594. [DOI] [PubMed] [Google Scholar]
- 32.Bueno M, Wang J, Mora AL, Gladwin MT. 2013. Nitrite signaling in pulmonary hypertension: mechanisms of bioactivation, signaling, and therapeutics. Antioxid Redox Signal 18:1797–1809. doi: 10.1089/ars.2012.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radi R, Cassina A, Hodara R. 2002. Nitric oxide and peroxynitrite interactions with mitochondria. Biol Chem 383:401–409. doi: 10.1515/BC.2002.044. [DOI] [PubMed] [Google Scholar]
- 34.Yoon SS, Hennigan RF, Hilliard GM, Ochsner UA, Parvatiyar K, Kamani MC, Allen HL, DeKievit TR, Gardner PR, Schwab U, Rowe JJ, Iglewski BH, McDermott TR, Mason RP, Wozniak DJ, Hancock RE, Parsek MR, Noah TL, Boucher RC, Hassett DJ. 2002. Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev Cell 3:593–603. doi: 10.1016/S1534-5807(02)00295-2. [DOI] [PubMed] [Google Scholar]
- 35.Alvarez-Ortega C, Harwood CS. 2007. Responses of Pseudomonas aeruginosa to low oxygen indicate that growth in the cystic fibrosis lung is by aerobic respiration. Mol Microbiol 65:153–165. doi: 10.1111/j.1365-2958.2007.05772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borriello G, Richards L, Ehrlich GD, Stewart PS. 2006. Arginine or nitrate enhances antibiotic susceptibility of Pseudomonas aeruginosa in biofilms. Antimicrob Agents Chemother 50:382–384. doi: 10.1128/AAC.50.1.382-384.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]